Noninvasive Electromagnetic Wave Sensing of Glucose
Abstract
:1. Introduction
2. Performance Evaluation
3. Methodologies for Noninvasive Glucose Sensing Utilizing Electromagnetic Waves
3.1. Infrared (IR) Spectroscopy
3.1.1. Near-Infrared (NIR) Spectroscopy
3.1.2. Mid-Infrared (MIR) Spectroscopy
3.2. Photoacoustic Spectroscopy
3.3. Fluorescence Spectroscopy
3.4. Raman Spectroscopy
3.5. Optical Coherence Tomography (OCT)
3.6. Terahertz Spectroscopy
3.7. Microwave Sensing
4. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gardner, D.G.; Shoback, D.; Greenspan, F.S. Greenspan’s Basic & Clinical Endocrinology; McGraw-Hill Medical: New York, NY, USA, 2007. [Google Scholar]
- Whiting, D.R.; Guariguata, L.; Weil, C.; Shaw, J. Idf Diabetes Atlas: Global Estimates of the Prevalence of Diabetes for 2011 and 2030. Diabetes Res. Clin. Pract. 2011, 94, 311–321. [Google Scholar] [CrossRef] [PubMed]
- National Diabetes Statistics Report. 2017. Available online: https://www.cdc.gov/features/diabetes-statistic-report/index.html (accessed on 8 February 2019).
- American Diabetes Association. Standards of Medical Care in Diabetes. Diabetes Care 2007, 30, S74–S76. [Google Scholar]
- Concannon, P.; Rich, S.S.; Nepom, G.T. Genetics of Type 1a Diabetes. New Engl. J. Med. 2009, 360, 1646–1654. [Google Scholar] [CrossRef] [PubMed]
- Daneman, D. Type 1 Diabetes. The Lancet 2006, 367, 847–858. [Google Scholar] [CrossRef]
- Atkinson, M.A.; Eisenbarth, G.S.; Michels, A.W. Type 1 Diabetes. The Lancet 2014, 383, 69–82. [Google Scholar] [CrossRef]
- International Diabetes Federation. IDF Diabetes Atlas, 7th ed.; International Diabetes Federation: Brussels, Belgium, 2015. [Google Scholar]
- Risérus, U.; Willett, W.C.; Hu, F.B. Dietary Fats and Prevention of Type 2 Diabetes. Prog. Lipid Res. 2009, 48, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Weyer, C.; Funahashi, T.; Tanaka, S.; Hotta, K.; Matsuzawa, Y.; Pratley, R.E.; Tataranni, P.A. Hypoadiponectinemia in Obesity and Type 2 Diabetes: Close Association with Insulin Resistance and Hyperinsulinemia. J. Clin. Endocrinol. Metab. 2001, 86, 1930–1935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vijan, S. Type 2 Diabetes. Annals Int. Med. 2010, 152, ITC3-1-1. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Khunti, K.; Davies, M.J. Type 2 Diabetes. The Lancet 2017, 389, 2239–2251. [Google Scholar] [CrossRef]
- Kim, C.; Newton, K.M.; Knopp, R.H. Gestational Diabetes and the Incidence of Type 2 Diabetes: A Systematic Review. Diabetes Care 2002, 25, 1862–1868. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Gestational Diabetes Mellitus. Diabetes Care 2004, 27, S88. [Google Scholar] [CrossRef]
- Umpierrez, G.E.; Isaacs, S.D.; Bazargan, N.; You, X.; Thaler, L.M.; Kitabchi, A.E. Hyperglycemia: An Independent Marker of in-Hospital Mortality in Patients with Undiagnosed Diabetes. J. Clin. Endocrinol. Metab. 2002, 87, 978–982. [Google Scholar] [CrossRef] [PubMed]
- Cryer, P.E. Hypoglycemia, Functional Brain Failure, and Brain Death. J. Clin. Invest. 2007, 117, 868–870. [Google Scholar] [CrossRef] [PubMed]
- Bonora, E.; Formentini, G.; Calcaterra, F.; Lombardi, S.; Marini, F.; Zenari, L.; Saggiani, F.; Poli, M.; Perbellini, S.; Raffaelli, A. Homa-Estimated Insulin Resistance Is an Independent Predictor of Cardiovascular Disease in Type 2 Diabetic Subjects: Prospective Data from the Verona Diabetes Complications Study. Diabetes Care 2002, 25, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- Morrish, N.J.; Wang, S.L.; Stevens, L.K.; Fuller, J.H.; Keen, H.; WHO Multinational Study Group. Mortality and Causes of Death in the Who Multinational Study of Vascular Disease in Diabetes. Diabetologia 2001, 44, S14. [Google Scholar] [CrossRef] [PubMed]
- Dyck, P.J.; Kratz, K.M.; Karnes, J.L.; Litchy, W.J.; Klein, R.; Pach, J.M.; Wilson, D.M.; O’brien, P.C.; Melton, L., Jr. The Prevalence by Staged Severity of Various Types of Diabetic Neuropathy, Retinopathy, and Nephropathy in a Population-Based Cohort the Rochester Diabetic Neuropathy Study. Neurology 1993, 43, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Gross, J.L.; De Azevedo, M.J.; Silveiro, S.P.; Canani, L.H.; Caramori, M.L.; Zelmanovitz, T. Diabetic Nephropathy: Diagnosis, Prevention, and Treatment. Diabetes Care 2005, 28, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Klein, B.E.K.; Moss, S.E.; Davis, M.D.; DeMets, D.L. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: Ii. Prevalence and Risk of Diabetic Retinopathy When Age at Diagnosis Is Less Than 30 Years. Arch. Ophthalmol. 1984, 102, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Baena-Díez, J.M.; Peñafiel, J.; Subirana, I.; Ramos, R.; Elosua, R.; Marín-Ibañez, A.; Guembe, M.J.; Rigo, F.; Tormo-Díaz, M.J.; Moreno-Iribas, C. Risk of Cause-Specific Death in Individuals with Diabetes: A Competing Risks Analysis. Diabetes Care 2016, 39, 1987–1995. [Google Scholar] [CrossRef] [PubMed]
- McEwen, L.N.; Kim, C.; Karter, A.J.; Haan, M.N.; Ghosh, D.; Lantz, P.M.; Mangione, C.M.; Thompson, T.J.; Herman, W.H. Risk Factors for Mortality among Patients with Diabetes: The Translating Research into Action for Diabetes (Triad) Study. Diabetes Care 2007, 30, 1736–1741. [Google Scholar] [CrossRef] [PubMed]
- Buse, J.B.; Caprio, S.; Cefalu, W.T.; Ceriello, A.; Del Prato, S.; Inzucchi, S.E.; McLaughlin, S.; Phillips, G.L.; Robertson, R.P.; Rubino, F. How Do We Define Cure of Diabetes? Diabetes Care 2009, 32, 2133–2135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, L.C.; Lyons, C. Electrode Systems for Continuous Monitoring in Cardiovascular Surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Andreea, C.; Hernández, C.; Simó, R. Non-Invasive Methods of Glucose Measurement: Current Status and Future Perspectives. Curr. Diabetes Rev. 2012, 8, 48–54. [Google Scholar]
- Tonyushkina, K.; Nichols, J.H. Glucose Meters: A Review of Technical Challenges to Obtaining Accurate Results. J. Diabetes Sci. Technol. 2009, 3, 971–980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharon, M.G. Infection Transmission Associated with Point of Care Testing and the Laboratory’s Role in Risk Reduction. EJIFCC 2014, 25, 188–194. [Google Scholar] [PubMed]
- Inayat, F.; Rai, A.B.S. Acute Hepatitis C Virus Infection Related to Capillary Blood Glucose Meter. Saudi Med. J. 2016, 37, 93–95. [Google Scholar] [CrossRef] [PubMed]
- Yeaw, J.; Lee, W.C.; Aagren, M.; Christensen, T. Cost of Self-Monitoring of Blood Glucose in the United States among Patients on an Insulin Regimen for Diabetes. J. Manag. Care Pharm. 2012, 18, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.L. The Pursuit of Noninvasive Glucose: Hunting the Deceitful Turkey. Available online: https://www.researchgate.net/profile/John_Smith155/publication/215519631_The_Pursuit_of_Noninvasive_Glucose_Hunting_the_Deceitful_Turkey/links/047f0180f0cf2751e6eee084/The-Pursuit-of-Noninvasive-Glucose-Hunting-the-Deceitful-Turkey.pdf (accessed on 8 February 2019).
- Vashist, S.K. Non-Invasive Glucose Monitoring Technology in Diabetes Management: A Review. Analytica Chim. Acta 2012, 750, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Ishimaru, A. Electromagnetic Wave Propagation, Radiation, and Scattering: From Fundamentals to Applications; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Yadav, J.; Rani, A.; Singh, V.; Murari, B.M. Prospects and Limitations of Non-Invasive Blood Glucose Monitoring Using near-Infrared Spectroscopy. Biomed. Sign. Process. Cont. 2015, 18, 214–227. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, X.; Li, Z.; Zhu, Z.; Qian, S.; Flewitt, A.J. Current and Emerging Technology for Continuous Glucose Monitoring. Sensors 2017, 17, 182. [Google Scholar] [CrossRef] [PubMed]
- Bruen, D.; Delaney, C.; Florea, L.; Diamond, D. Glucose Sensing for Diabetes Monitoring: Recent Developments. Sensors 2017, 17, 1866. [Google Scholar] [CrossRef] [PubMed]
- Kovatchev, B.P.; King, C.; Breton, M.; Anderson, S.; Clarke, W. Clinical Assessment and Mathematical Modeling of the Accuracy of Continuous Glucose Sensors (Cgs). In Proceedings of the 28th Annual International Conference of Engineering in Medicine and Biology Society, New York, NY, USA, 30 August–3 September 2006. [Google Scholar]
- Bland, J.M.; Altman, D.G. Statistical Methods for Assessing Agreement between Two Methods of Clinical Measurement. The Lancet 1986, 327, 307–310. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Comparing Methods of Measurement: Why Plotting Difference against Standard Method Is Misleading. The Lancet 1995, 346, 1085–1087. [Google Scholar] [CrossRef]
- Clarke, W.L.; Cox, D.; Gonder-Frederick, L.A.; Carter, W.; Pohl, S.L. Evaluating Clinical Accuracy of Systems for Self-Monitoring of Blood Glucose. Diabetes Care 1987, 10, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Stuart, B. Infrared Spectroscopy; Wiley Online Library: Hoboken, NJ, USA, 2005. [Google Scholar]
- Wilson, E.B.; Decius, J.C.; Cross, P.C. Molecular Vibrations: The Theory of Infrared and Raman Vibrational Spectra; Courier Corporation: North Chelmsford, MA, USA, 1955. [Google Scholar]
- Siesler, H.W.; Ozaki, Y.; Kawata, S.; Heise, H.M. Near-Infrared Spectroscopy: Principles, Instruments, Applications; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Seddon, A.B.; Napier, B.; Lindsay, I.; Lamrini, S.; Moselund, P.M.; Stone, N.; Bang, O. Mid-Infrared Spectroscopy/Bioimaging: Moving toward Mir Optical Biopsy. Laser Focus World 2016, 52, 50–53. [Google Scholar]
- Griffiths, P.R.; Homes, C.C. Instrumentation for Far-Infrared Spectroscopy. In Handbook of Vibrational Spectroscopy; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar] [Green Version]
- Herschel, W. Xiii. Investigation of the Powers of the Prismatic Colours to Heat and Illuminate Objects; with Remarks, that Prove the Different Refrangibility of Radiant Heat. To Which Is Added, an Inquiry into the Method of Viewing the Sun Advantageously, with Telescopes of Large Apertures and High Magnifying Powers. Philos. Trans. R. Soc. London 1800, 90, 255–283. [Google Scholar]
- Abney, W.; Festing, E.R. Near Infrared Spectral of Organic Liquids. Philos. Trans. R. Soc. 1881, 172, 887. [Google Scholar]
- Ozaki, Y. Near-Infrared Spectroscopy—Its Versatility in Analytical Chemistry. Analyt. Sci. 2012, 28, 545–563. [Google Scholar] [CrossRef]
- Burns, D.A.; Ciurczak, E.W. Handbook of near-Infrared Analysis; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Perrey, S. Non-Invasive Nir Spectroscopy of Human Brain Function During Exercise. Methods 2008, 45, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Braverman, I.M. The Cutaneous Microcirculation: Ultrastructure and Microanatomical Organization. Microcirculation 1997, 4, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Shore, A.C. Capillaroscopy and the Measurement of Capillary Pressure. Br. J. Clin. Pharmacol. 2000, 50, 501–513. [Google Scholar] [CrossRef] [PubMed]
- McCall, R.E.; Tankersley, C.M. Phlebotomy Essentials; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008. [Google Scholar]
- McClure, W.F. Near-Infrared Spectroscopy the Giant Is Running Strong. Analyt. Chem. 1994, 66, 42A–53A. [Google Scholar] [CrossRef]
- Weyer, L.; Workman, J., Jr. Practical Guide to Interpretive near-Infrared Spectroscopy; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Khalil, O.S. Spectroscopic and Clinical Aspects of Noninvasive Glucose Measurements. Clin. Chem. 1999, 45, 165–177. [Google Scholar] [PubMed]
- Yang, W.; Liao, N.; Cheng, H.; Li, Y.; Bai, X.; Deng, C. Determination of Nir Informative Wavebands for Transmission Non-Invasive Blood Glucose Measurement Using a Fourier Transform Spectrometer. AIP Adv. 2018, 8, 035216. [Google Scholar] [CrossRef]
- Golic, M.; Walsh, K.; Lawson, P. Short-Wavelength near-Infrared Spectra of Sucrose, Glucose, and Fructose with Respect to Sugar Concentration and Temperature. Appl. Spectrosc. 2003, 57, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Osborne, B.G.; Fearn, T.; Hindle, P.H. Practical Nir Spectroscopy with Applications in Food and Beverage Analysis; Longman Scientific and Technical: Harlow, UK, 1993. [Google Scholar]
- Šašić, S.; Ozaki, Y. Short-Wave near-Infrared Spectroscopy of Biological Fluids. 1. Quantitative Analysis of Fat, Protein, and Lactose in Raw Milk by Partial Least-Squares Regression and Band Assignment. Analyt. Chem. 2001, 73, 64–71. [Google Scholar] [CrossRef]
- Swinehart, D.F. The Beer-Lambert Law. J. Chem. Edu. 1962, 39, 333. [Google Scholar] [CrossRef]
- Kocsis, L.; Herman, P.; Eke, A. The Modified Beer–Lambert Law Revisited. Phys. Med. Biology 2006, 51, N91. [Google Scholar] [CrossRef] [PubMed]
- Cirovic, D.A. Feed-Forward Artificial Neural Networks: Applications to Spectroscopy. TrAC Trends Analyt. Chem. 1997, 16, 148–155. [Google Scholar] [CrossRef]
- Jagemann, K.; Fischbacher, C.; Danzer, K.; Mueller, U.A.; Mertes, B. Application of near-Infrared Spectroscopy for Non-Invasive Determination of Blood/Tissue Glucose Using Neural Networks. Zeitschrift für Physikalische Chemie 1995, 191, 179–190. [Google Scholar] [CrossRef]
- Fischbacher, C.; Jagemann, K.U.; Danzer, K.; Müller, U.A.; Papenkordt, L.; Schüler, J. Enhancing Calibration Models for Non-Invasive near-Infrared Spectroscopical Blood Glucose Determination. Fresenius’ J. Analyt. Chem. 1997, 359, 78–82. [Google Scholar] [CrossRef]
- Mouazen, A.M.; Kuang, B.; De Baerdemaeker, J.; Ramon, H. Comparison among Principal Component, Partial Least Squares and Back Propagation Neural Network Analyses for Accuracy of Measurement of Selected Soil Properties with Visible and near Infrared Spectroscopy. Geoderma 2010, 158, 23–31. [Google Scholar] [CrossRef]
- Bhandare, P.; Mendelson, Y.; Peura, R.A.; Janatsch, G.; Kruse-Jarres, J.D.; Marbach, R.; Heise, H.M. Multivariate Determination of Glucose in Whole Blood Using Partial Least-Squares and Artificial Neural Networks Based on Mid-Infrared Spectroscopy. Appl. Spectrosc. 1993, 47, 1214–1221. [Google Scholar] [CrossRef]
- Uwadaira, Y.; Ikehata, A.; Momose, A.; Miura, M. Identification of Informative Bands in the Short-Wavelength Nir Region for Non-Invasive Blood Glucose Measurement. Biomedical Opt. express 2016, 7, 2729–2737. [Google Scholar] [CrossRef] [PubMed]
- Jintao, X.; Ye, L.; Liu, Y.; Li, C.; Chen, H. Noninvasive and Fast Measurement of Blood Glucose in Vivo by near Infrared (Nir) Spectroscopy. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2017, 179, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Khalil, O.S. Non-Invasive Glucose Measurement Technologies: An Update from 1999 to the Dawn of the New Millennium. Diabetes Technol. Therapeut. 2004, 6, 660–697. [Google Scholar] [CrossRef] [PubMed]
- Vasko, P.D.; Blackwell, J.; Koenig, J.L. Infrared and Raman Spectroscopy of Carbohydrates: Part I: Identification of O-H and C-H-Related Vibrational Modes for D-Glucose, Maltose, Cellobiose, and Dextran by Deuterium-Substitution Methods. Carbohydr. Res. 1971, 19, 297–310. [Google Scholar] [CrossRef]
- Vasko, P.D.; Blackwell, J.; Koenig, J.L. Infrared and Raman Spectroscopy of Carbohydrates: Part II: Normal Coordinate Analysis of A-D-Glucose. Carbohydr. Res. 1972, 23, 407–416. [Google Scholar] [CrossRef]
- Tuchin, V.V. Handbook of Optical Sensing of Glucose in Biological Fluids and Tissues; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Pleitez, M.A.; Lieblein, T.; Bauer, A.; Hertzberg, O.; von Lilienfeld-Toal, H.; Mäntele, W. In Vivo Noninvasive Monitoring of Glucose Concentration in Human Epidermis by Mid-Infrared Pulsed Photoacoustic Spectroscopy. Analyt. Chem. 2012, 85, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; Alaam, M.; El-Haes, H.; Jalbout, A.F.; de Leon, A. Analysis of the Structure and Vibrational Spectra of Glucose and Fructose. Ecletica Quim. 2006, 31, 15–21. [Google Scholar] [CrossRef]
- Kino, S.; Omori, S.; Katagiri, T.; Matsuura, Y. Hollow Optical-Fiber Based Infrared Spectroscopy for Measurement of Blood Glucose Level by Using Multi-Reflection Prism. Biomed. Opt. Express 2016, 7, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Pleitez, M.; von Lilienfeld-Toal, H.; Mäntele, W. Infrared Spectroscopic Analysis of Human Interstitial Fluid in Vitro and in Vivo Using Ft-Ir Spectroscopy and Pulsed Quantum Cascade Lasers (Qcl): Establishing a New Approach to Non Invasive Glucose Measurement. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2012, 85, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Petibois, C.; Rigalleau, V.; Melin, A.; Perromat, A.; Cazorla, G.; Gin, H.; Déléris, G. Determination of Glucose in Dried Serum Samples by Fourier-Transform Infrared Spectroscopy. Clin. Chem. 1999, 45, 1530–1535. [Google Scholar] [PubMed]
- Waynant, R.W.; Chenault, V.M. Overview of Non-Invasive Fluid Glucose Measurement Using Optical Techniques to Maintain Glucose Control in Diabetes Mellitus. LEOS Newslett. 1998, 12, 3–6. [Google Scholar]
- Elsner, P. Skin Bioengineering: Techniques and Applications in Dermatology and Cosmetology; Karger Medical and Scientific Publishers: Basel, Switzerland, 1998. [Google Scholar]
- Von Lilienfeld-Toal, H.; Weidenmüller, M.; Xhelaj, A.; Mäntele, W. A Novel Approach to Non-Invasive Glucose Measurement by Mid-Infrared Spectroscopy: The Combination of Quantum Cascade Lasers (Qcl) and Photoacoustic Detection. Vib. Spectrosc. 2005, 38, 209–215. [Google Scholar] [CrossRef]
- Pfohl, M.; Pfeiffer, A.; Schaltz, H. Spot Glucose Measurement in Epidermal Interstitial Fluid—an Alternative to Capillary Blood Glucose Estimation? Exp. Clin. Endocrinol. Diabetes 2000, 108, 1–4. [Google Scholar] [CrossRef] [PubMed]
- O’brien, P.C.; Wise, S.D.; Ness, S.; LeBlanc, S.M. Dermal Interstitial Glucose as an Indicator of Ambient Glycemia. Diabetes Care 1997, 20, 1426–1429. [Google Scholar]
- Gebhart, S.; Faupel, M.; Fowler, R.; Kapsner, C.; Lincoln, D.; McGee, V.; Pasqua, J.; Steed, L.; Wangsness, M.; Xu, F. Glucose Sensing in Transdermal Body Fluid Collected under Continuous Vacuum Pressure Via Micropores in the Stratum Corneum. Diabetes Technol. Therapeut. 2003, 5, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Thennadil, S.N.; Rennert, J.L.; Wenzel, B.J.; Hazen, K.H.; Ruchti, T.L.; Block, M.B. Comparison of Glucose Concentration in Interstitial Fluid, and Capillary and Venous Blood During Rapid Changes in Blood Glucose Levels. Diabetes Technol. Therapeut. 2001, 3, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Liakat, S.; Bors, K.A.; Xu, L.; Woods, C.M.; Doyle, J.; Gmachl, C.F. Noninvasive in Vivo Glucose Sensing on Human Subjects Using Mid-Infrared Light. Biomed. Opt. Express 2014, 5, 2397–2404. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, R.; Kino, S.; Soyama, S.; Matsuura, Y. Noninvasive Glucose Monitoring Using Mid-Infrared Absorption Spectroscopy Based on a Few Wavenumbers. Biomed. Opt. Express 2018, 9, 289–302. [Google Scholar] [CrossRef] [PubMed]
- Liakat, S.; Bors, K.A.; Xu, L.; Woods, C.M.; Doyle, J.; Gmachl, C.F. Mid-Infrared Noninvasive in Vivo Glucose Detection in Healthy Human Subjects. In Proceedings of the 2014 Conference on Lasers and Electro-Optics (CLEO) - Laser Science to Photonic Applications, San Jose, CA, USA, 8–13 June 2014. [Google Scholar]
- Liakat, S. Development of a Noninvasive in Vivo Glucose Sensor Based on Mid-Infrared Quantum Cascade Laser Spectroscopy; Princeton University: Princeton, NJ, USA, 2015. [Google Scholar]
- Werth, A.; Liakat, S.; Dong, A.; Woods, C.M.; Gmachl, C.F. Implementation of an Integrating Sphere for the Enhancement of Noninvasive Glucose Detection Using Quantum Cascade Laser Spectroscopy. Appl. Phys. B 2018, 124, 75. [Google Scholar] [CrossRef]
- Gao, F.; Feng, X.; Zheng, Y.; Ohl, C.D. Photoacoustic Resonance Spectroscopy for Biological Tissue Characterization. J. Biomed. Opt. 2014, 19, 067006. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Gao, F.; Feng, X.; Liu, S.; Ding, R.; Zheng, Y. Photoacoustic Resonance Imaging. IEEE J. Sel. Top. Quantum Electron. 2019, 25, 1–7. [Google Scholar] [CrossRef]
- Wang, L.V. Tutorial on Photoacoustic Microscopy and Computed Tomography. IEEE J. Sel. Top. Quantum Electron. 2018, 24, 171–179. [Google Scholar] [CrossRef]
- Diebold, G.J.; Sun, T. Properties of Photoacoustic Waves in One, Two, and Three Dimensions. Acta Acustica United Acustica 1994, 80, 339–351. [Google Scholar]
- Zhao, S.; Tao, W.; He, Q.; Zhao, H.; Cao, W. A Non-Invasive Photoacoustic and Ultrasonic Method for the Measurement of Glucose Solution Concentration. AIP Adv. 2017, 7, 035313. [Google Scholar] [CrossRef]
- Zhao, Z. Pulsed Photoacoustic Techniques and Glucose Determination in Human Blood and Tissue; University of Oulu Finland: Oulu, Finland, 2002. [Google Scholar]
- Harman-Boehm, I.; Gal, A.; Raykhman, A.M.; Zahn, J.D.; Naidis, E.; Mayzel, Y. Noninvasive Glucose Monitoring: A Novel Approach. J. Diabetes Sci. Technol. 2009, 3, 253–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kottmann, J.; Rey, J.M.; Luginbühl, J.; Reichmann, E.; Sigrist, M.W. Glucose Sensing in Human Epidermis Using Mid-Infrared Photoacoustic Detection. Biomed. Opt. Express 2012, 3, 667–680. [Google Scholar] [CrossRef] [PubMed]
- Pai, P.P.; Sanki, P.K.; Banerjee, S. A Photoacoustics Based Continuous Non-Invasive Blood Glucose Monitoring System. In Proceedings of the 2015 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Torino, Italy, 7–9 May 2015. [Google Scholar]
- Sim, J.Y.; Ahn, C.-G.; Jeong, E.-J.; Kim, B.K. In Vivo Microscopic Photoacoustic Spectroscopy for Non-Invasive Glucose Monitoring Invulnerable to Skin Secretion Products. Sci. Rep. 2018, 8, 1059. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Gao, F.; Feng, X.; Liu, S.; Kishor, R.; Luo, Y.; Zheng, Y. Noninvasive Photoacoustic Measurement of Glucose by Data Fusion. Analyst 2017, 142, 2892–2896. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Gao, F.; Feng, X.; Jin, H.; Zhang, S.; Liu, S.; Luo, Y.; Xing, B.; Zheng, Y. “Guide Star” Assisted Noninvasive Photoacoustic Measurement of Glucose. ACS Sensors 2018, 3, 2550–2557. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S. Fluorescence Spectroscopy of Single Biomolecules. Science 1999, 283, 1676–1683. [Google Scholar] [CrossRef] [PubMed]
- Barone, P.W.; Parker, R.S.; Strano, M.S. In Vivo Fluorescence Detection of Glucose Using a Single-Walled Carbon Nanotube Optical Sensor: Design, Fluorophore Properties, Advantages, and Disadvantages. Anal. Chem. 2005, 77, 7556–7562. [Google Scholar] [CrossRef] [PubMed]
- Tiangco, C.; Fon, D.; Sardesai, N.; Kostov, Y.; Sevilla, F., III; Rao, G.; Tolosa, L. Fiber Optic Biosensor for Transdermal Glucose Based on the Glucose Binding Protein. Sensors Actuat. B: Chem. 2017, 242, 569–576. [Google Scholar] [CrossRef]
- Su, F.; Zhang, L.; Kong, X.; Lee, F.; Tian, Y.; Meldrum, D.R. Ratiometric Glucose Sensing Based on Fluorescent Oxygen Films and Glucose Oxidase. Sensing Bio-Sensing Res. 2017, 14, 1–6. [Google Scholar] [CrossRef]
- Raman, C.V.; Krishnan, K.S. A New Type of Secondary Radiation. Nature 1928, 121, 501. [Google Scholar] [CrossRef]
- Dong, J.; Tao, Q.; Guo, M.; Yan, T.; Qian, W. Glucose-Responsive Multifunctional Acupuncture Needle: A Universal Sers Detection Strategy of Small Biomolecules in Vivo. Analyt. Methods 2012, 4, 3879–3883. [Google Scholar] [CrossRef]
- Enejder, A.M.K.; Scecina, T.G.; Oh, J.; Hunter, M.; Shih, W.; Sasic, S.; Horowitz, G.L.; Feld, M.S. Raman Spectroscopy for Noninvasive Glucose Measurements. J. Biomed. Opt. 2005, 10, 031114. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhu, X.; Wang, Z.; Hou, Z.; Gao, F.; Nie, R.; Cui, X.; She, J.; Peng, B. Noninvasive Blood Glucose Detection Using a Miniature Wearable Raman Spectroscopy System. Chin. Opt. Lett. 2017, 15, 083001. [Google Scholar] [CrossRef]
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A. Optical Coherence Tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef] [PubMed]
- Larin, K.V.; Motamedi, M.; Ashitkov, T.V.; Esenaliev, R.O. Specificity of Noninvasive Blood Glucose Sensing Using Optical Coherence Tomography Technique: A Pilot Study. Phys. Med. Biol. 2003, 48, 1371. [Google Scholar] [CrossRef] [PubMed]
- De Pretto, L.R.; Yoshimura, T.M.; Ribeiro, M.S.; de Freitas, A.Z. Optical Coherence Tomography for Blood Glucose Monitoring in Vitro through Spatial and Temporal Approaches. J. Biomed. Opt. 2016, 21, 086007. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.T.; Kuang, Y.P.; Zhou, L.P.; Wu, G.Y.; Gu, P.C.; Wei, H.J.; Chen, K. Noninvasive Monitoring of Blood Glucose Concentration in Diabetic Patients with Optical Coherence Tomography. Laser Phys. Lett. 2017, 14, 035603. [Google Scholar] [CrossRef]
- Siegel, P.H. Terahertz Technology. IEEE Trans. Microwave Theor. Tech. 2002, 50, 910–928. [Google Scholar] [CrossRef]
- Tonouchi, M. Cutting-Edge Terahertz Technology. Nature Phot. 2007, 1, 97. [Google Scholar] [CrossRef]
- Gallot, G.; Grischkowsky, D. Electro-Optic Detection of Terahertz Radiation. JOSA B 1999, 16, 1204–1212. [Google Scholar] [CrossRef]
- Pickwell, E.; Wallace, V.P. Biomedical Applications of Terahertz Technology. J. Phys. D Appl. Phys. 2006, 39, R301. [Google Scholar] [CrossRef]
- Oh, S.J.; Kim, S.-H.; Jeong, K.; Park, Y.; Huh, Y.-M.; Son, J.-H.; Suh, J.-S. Measurement Depth Enhancement in Terahertz Imaging of Biological Tissues. Opt. Express 2013, 21, 21299–21305. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chen, X.; Ma, S.; Wu, X.; Yang, W.; Zhang, W.; Li, X. Quantify Glucose Level in Freshly Diabetic’s Blood by Terahertz Time-Domain Spectroscopy. J. Infrared Millimeter Terahertz Waves 2018, 39, 399–408. [Google Scholar] [CrossRef]
- Cherkasova, O.; Nazarov, M.; Shkurinov, A. Noninvasive Blood Glucose Monitoring in the Terahertz Frequency Range. Opt. Quantum Electron. 2016, 48, 217. [Google Scholar] [CrossRef]
- Fuchs, J.; Herrling, T.; Groth, N. Detection of Free Radicals in Skin: A Review of the Literature and New Developments. In Oxidants and Antioxidants in Cutaneous Biology; Karger Publisher: Basel, Switzerland, 2001. [Google Scholar]
- Zhadobov, M.; Chahat, N.; Sauleau, R.; Quement, C.L.; Drean, Y.L. Millimeter-Wave Interactions with the Human Body: State of Knowledge and Recent Advances. Int. J. Microwave Wirel. Technol. 2011, 3, 237–247. [Google Scholar] [CrossRef]
- Alekseev, S.I.; Ziskin, M.C. Human Skin Permittivity Determined by Millimeter Wave Reflection Measurements. Bioelectromagn. J.Bioelectromagn Soc. Soc. Phys. Regul. Biol. Med Eur. Bioelectromagn. Assoc. 2007, 28, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Kim, C.-S.; Choi, B.-C.; Ham, K.-Y. The Correlation of the Complex Dielectric Constant and Blood Glucose at Low Frequency. Biosens. Bioelectron. 2003, 19, 321–324. [Google Scholar] [CrossRef]
- Caduff, A.; Hirt, E.; Feldman, Y.; Ali, Z.; Heinemann, L. First Human Experiments with a Novel Non-Invasive, Non-Optical Continuous Glucose Monitoring System. Biosens. Bioelectron. 2003, 19, 209–217. [Google Scholar] [CrossRef]
- Hayashi, Y.; Livshits, L.; Caduff, A.; Feldman, Y. Dielectric Spectroscopy Study of Specific Glucose Influence on Human Erythrocyte Membranes. J. Phys. D Appl. Phys. 2003, 36, 369. [Google Scholar] [CrossRef]
- Cole, K.S.; Cole, R.H. Dispersion and Absorption in Dielectrics I. Alternating Current Characteristics. J. Chem. Phys. 1941, 9, 341–351. [Google Scholar] [CrossRef]
- Turgul, V.; Kale, I. Characterization of the Complex Permittivity of Glucose/Water Solutions for Noninvasive Rf/Microwave Blood Glucose Sensing. In Proceedings of the 2016 IEEE International Instrumentation and Measurement Technology Conference Proceedings (I2MTC), Taipei, Taiwan, 23–26 May 2016. [Google Scholar]
- Buford, R.J.; Green, E.C.; McClung, M.J. A Microwave Frequency Sensor for Non-Invasive Blood-Glucose Measurement. In Proceedings of the 2008 Sensors Applications Symposium, Atlanta, GA, USA, 12–14 February 2008. [Google Scholar]
- Chien, J.; Yeh, E.-C.; Lee, L.P.; Anwar, M.; Niknejad, A.M. A Microwave Reconfigurable Dielectric-Based Glucose Sensor with 20 Mg/Dl Sensitivity at Sub-Nl Sensing Volume in Cmos. In Proceedings of the 2015 IEEE MTT-S International Microwave Symposium, Phoenix, AZ, USA, 17–22 May 2015. [Google Scholar]
- Bababjanyan, A.; Melikyan, H.; Kim, S.; Kim, J.; Lee, K.; Friedman, B. Real-Time Noninvasive Measurement of Glucose Concentration Using a Microwave Biosensor. J. Sens. 2010, 2010, 452163. [Google Scholar] [CrossRef]
- Xiao, X.; Li, Q. A Non-Invasive Measurement of Blood Glucose Concentration by Uwb Microwave Spectrum. IEEE Antennas Wirel. Propag. Lett. 2017, 16, 1040–1043. [Google Scholar] [CrossRef]
- Kim, J.; Babajanyan, A.; Hovsepyan, A.; Lee, K.; Friedman, B. Microwave Dielectric Resonator Biosensor for Aqueous Glucose Solution. Rev. Sci. Instrum. 2008, 79, 086107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Qu, Z.; Jin, H.; Liu, S.; Luo, Y.; Zheng, Y. Noninvasive Glucose Measurement by Microwave Biosensor with Accuracy Enhancement. In Proceedings of the 2018 IEEE International Symposium on Circuits and Systems (ISCAS), Florence, Italy, 27–30 May 2018. [Google Scholar]
- Abedeen, Z.; Agarwal, P. Microwave Sensing Technique Based Label-Free and Real-Time Planar Glucose Analyzer Fabricated on Fr4. Sens. Actuators A Phys. 2018, 279, 132–139. [Google Scholar] [CrossRef]
- Choi, H.; Naylon, J.; Luzio, S.; Beutler, J.; Birchall, J.; Martin, C.; Porch, A. Design and in Vitro Interference Test of Microwave Noninvasive Blood Glucose Monitoring Sensor. System 2015, 19, 20. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Cano-Garcia, H.; Sotiriou, I.; Lipscombe, O.; Gouzouasis, I.; Koutsoupidou, M.; Palikaras, G.; Mackenzie, R.; Reeve, T.; Kosmas, P. A Glucose Sensing System Based on Transmission Measurements at Millimetre Waves Using Micro Strip Patch Antennas. Sci. Rep. 2017, 7, 6855. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Nylon, J.; Luzio, S.; Beutler, J.; Porch, A. Design of Continuous Non-Invasive Blood Glucose Monitoring Sensor Based on a Microwave Split Ring Resonator. In Proceedings of the 2014 IEEE MTT-S International Microwave Workshop Series on RF and Wireless Technologies for Biomedical and Healthcare Applications (IMWS-Bio2014), London, UK, 8–10 December 2014. [Google Scholar]

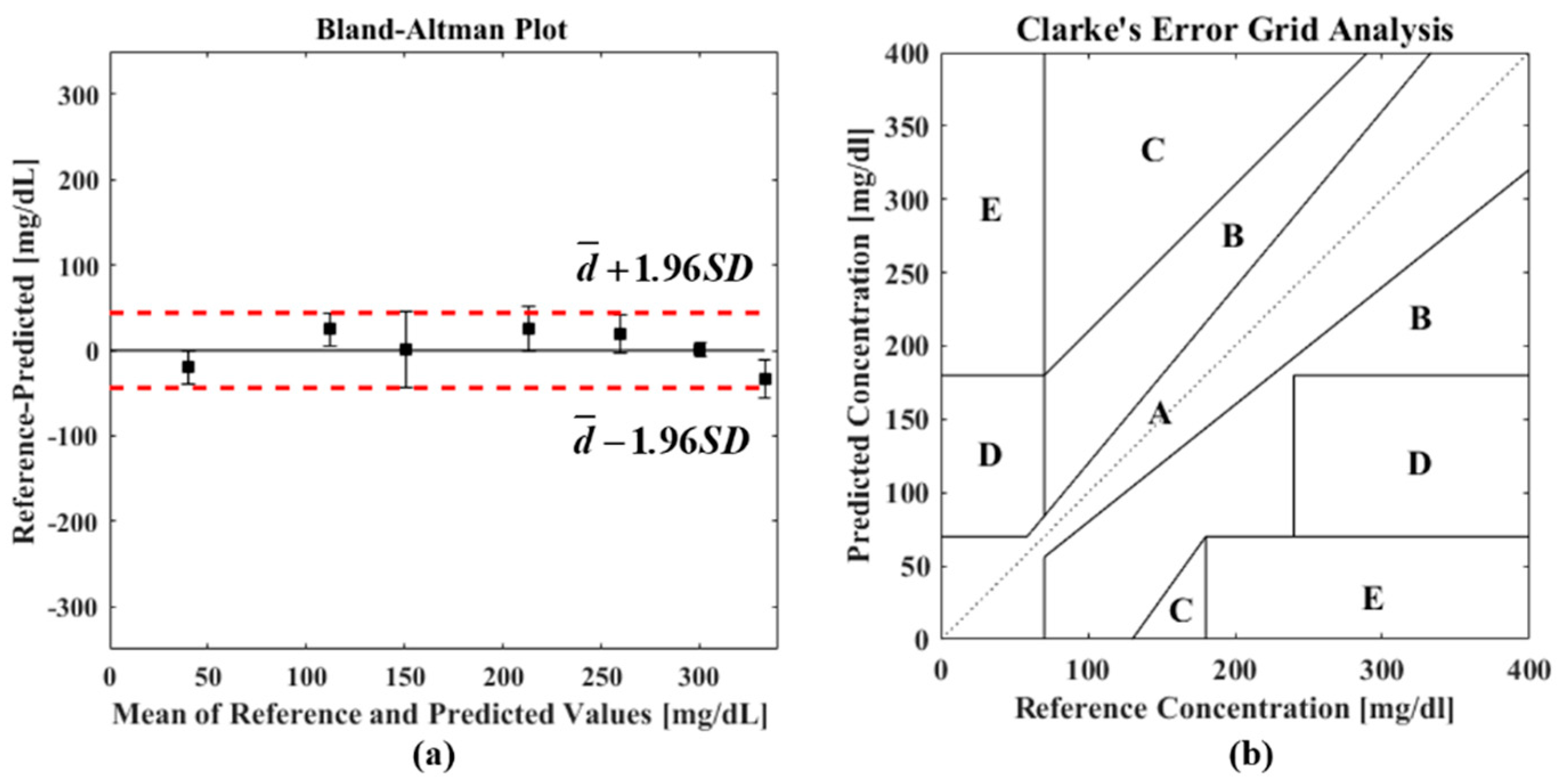



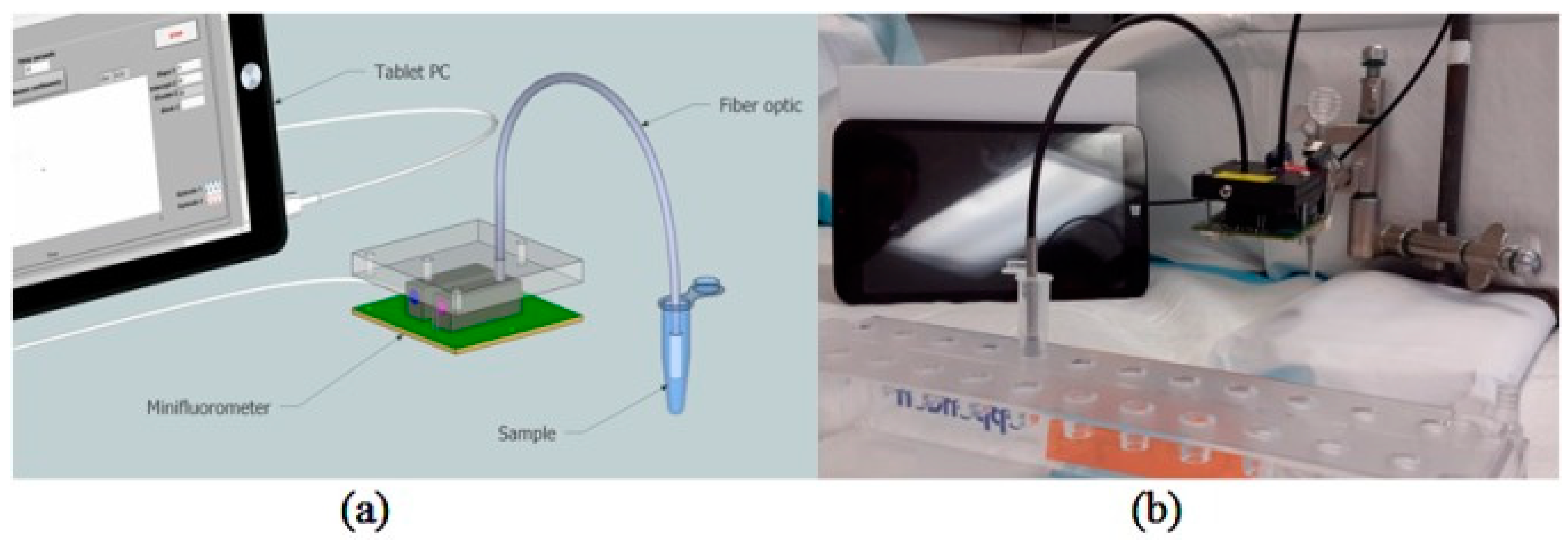
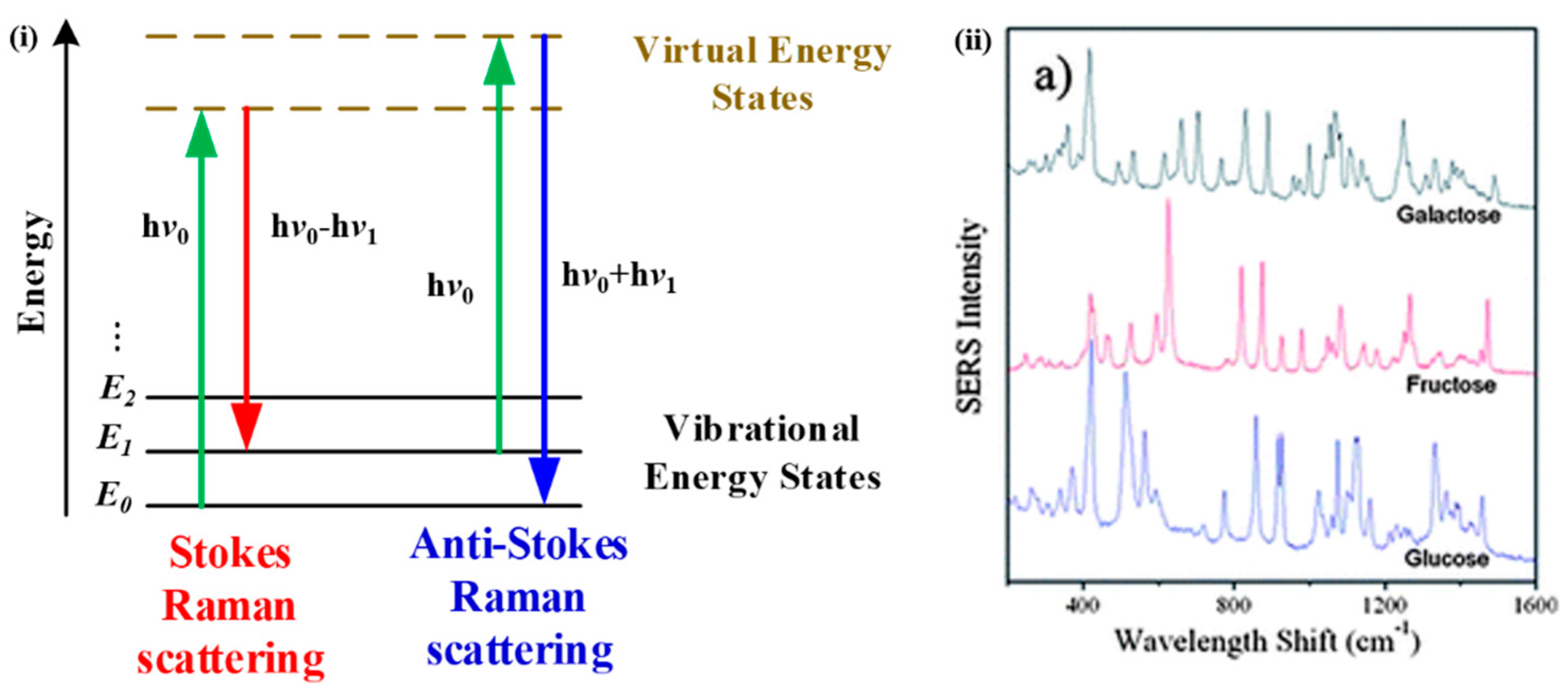
| No. | Wavelength (nm) | Functional Group |
|---|---|---|
| 1 | 2273 | Combination of O-H/C-O stretching [55] |
| 2 | 2261 | νCH + νCCH [56] |
| 3 | 1688 | 2νCH [56] |
| 4 | 1638 | First overtone [57] |
| 5 | 1536 | νOH + νCH [56] |
| 6 | 1408 | 2νOH [56] |
| 7 | 1126 | 3νCH [56] |
| 8 | 1042 | Combination of νCH [58,59] |
| 9 | 1018 | Combination of νCH [60] |
| 10 | 939 | 3νOH [56] |
| 11 | 930 | 3νCH2 [58] |
| 12 | 910 | 4νCH [58,59] |
| No. | Wavelength (nm) | Functional Group |
|---|---|---|
| 1 | 8000 | C-H bending vibrations [70,71,72,73] |
| 2 | 8244 | [74] |
| 3 | 8658 | Pyranose ring [75,76] |
| 4 | 8680 | [77] |
| 5 | 9290 | C-H bending vibrations [70,71,72,73,74,77] |
| 6 | 9551 | C-H bending vibrations [70,71,72,73] |
| 7 | 9680 | ν(C–O–H) or ν(C–O–C) vibration [72,73,74,77,78] |
| 8 | 9746 | C–O–H bending vibration [70,72,73] |
| Technology | Penetration | Target | Sensitivity | Selectivity | System Size | Cost |
|---|---|---|---|---|---|---|
| NIR | >1 mm | ISF, blood | High | Good | Portable | Low |
| MIR | Several μm | ISF | High | Good, better than NIR spectroscopy | Large | High |
| PA | Better than IR spectroscopy | ISF, blood | High | Good | Portable | Low |
| Fluorescence | <1 mm | Tear, ISF, blood | High | Excellent | Portable | Low |
| Raman | <1 mm | ISF, tears | Low | Excellent | Portable | Medium |
| OCT | <1 mm | ISF | High | Poor | Portable | Medium |
| THz | ~100 μm | ISF | High | Good | Large | High |
| Microwave | >1 mm | Blood, ISF | High | Poor | Portable/wearable | Very Low |
| Technology | Experiment | Number of Points | CEG | Sensitivity | R2 | MAE | LOD | |||
|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | |||||||
| NIR [68] | In vivo | 2737 | 94.2% | 5.7% | 0% | 0.1% | - | - | 0.65 mM | - |
| MIR [76] | In vivo | 14 | 100% | 0% | 0% | 0% | - | - | 0.67 mM | - |
| PA [100] | In vivo | 76 | 70% | 30% | 0% | 0% | - | - | 1.03 mM | - |
| Fluorescence [105] | In vitro | 8 | - | - | - | - | 0.1296 μM−1 | 0.99 | - | 2 μM |
| Raman [110] | In vivo | 34 | - | - | - | - | - | 0.84 | 0.37 mM | - |
| OCT [114] | In vivo | 81 | - | - | - | - | 5.78% mM−1 | 0.91 | - | - |
| THz [120] | Ex vivo | 20 | - | - | - | - | - | 0.97 | 0.25 mM | - |
| Microwave [138] | In vivo | 89 | - | - | - | - | 0.0235 dB*mM−1 | - | - | 1.33 mM |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Liu, S.; Jin, H.; Luo, Y.; Zheng, Z.; Gao, F.; Zheng, Y. Noninvasive Electromagnetic Wave Sensing of Glucose. Sensors 2019, 19, 1151. https://doi.org/10.3390/s19051151
Zhang R, Liu S, Jin H, Luo Y, Zheng Z, Gao F, Zheng Y. Noninvasive Electromagnetic Wave Sensing of Glucose. Sensors. 2019; 19(5):1151. https://doi.org/10.3390/s19051151
Chicago/Turabian StyleZhang, Ruochong, Siyu Liu, Haoran Jin, Yunqi Luo, Zesheng Zheng, Fei Gao, and Yuanjin Zheng. 2019. "Noninvasive Electromagnetic Wave Sensing of Glucose" Sensors 19, no. 5: 1151. https://doi.org/10.3390/s19051151





