An LC Wireless Microfluidic Sensor Based on Low Temperature Co-Fired Ceramic (LTCC) Technology
Abstract
:1. Introduction
2. Sensor Design
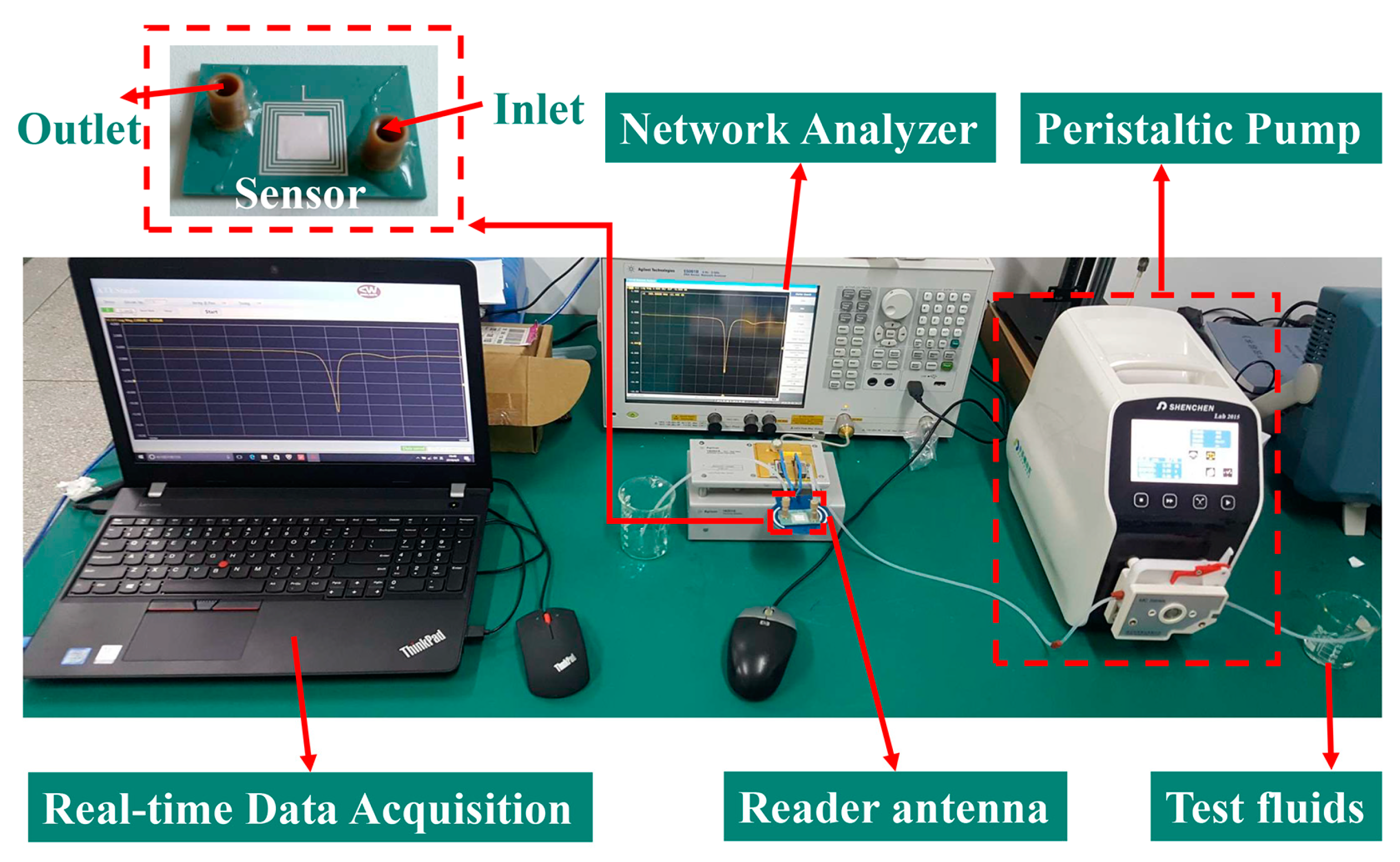
3. Sensor Fabrication and Test
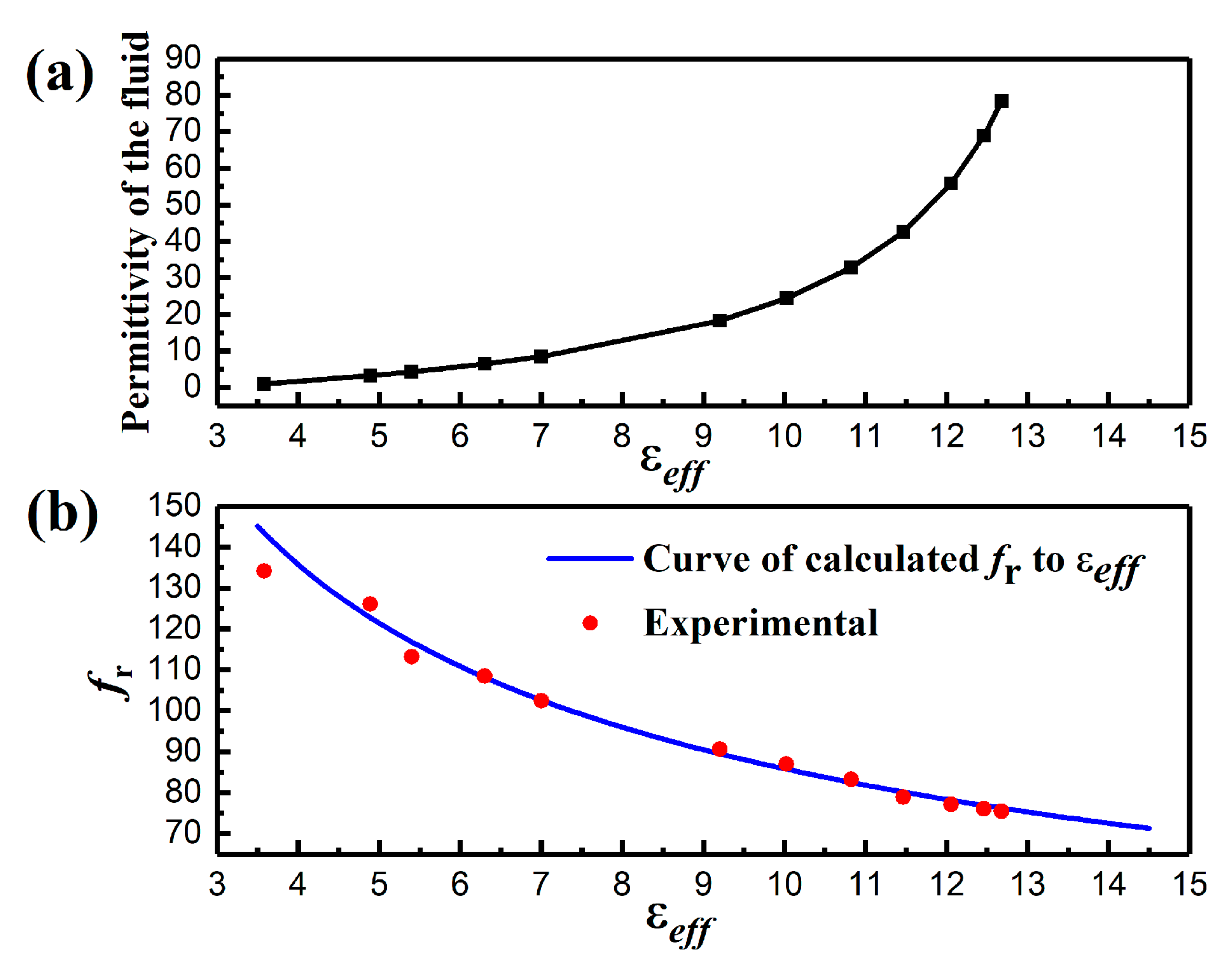
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Nge, P.N.; Rogers, C.I.; Woolley, A.T. Advances in microfluidic materials, functions, integration, and applications. Chem. Rev. 2013, 113, 2550–2583. [Google Scholar] [CrossRef] [PubMed]
- Hassan, U.; Ghonge, T.; Reddy, B., Jr.; Patel, M.; Rappleye, M.; Taneja, I.; Tanna, A.; Healey, R.; Manusry, N.; Price, Z.; et al. A point-of-care microfluidic biochip for quantification of CD64 expression from whole blood for sepsis stratification. Nat. Commun. 2017, 8, 15949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandey, C.M.; Augustine, S.; Kumar, S.; Kumar, S.; Nara, S.; Srivastava, S.; Malhotra, B.D. Microfluidics Based Point-of-Care Diagnostics. Biotechnol. J. 2018, 13, 1700047. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Li, J.; Song, Y.; Zhou, L.; Zhu, Z.; Yang, C.J. Distance-based microfluidic quantitative detection methods for point-of-care testing. Lab Chip 2016, 16, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Yager, P.; Edwards, T.; Fu, E.; Helton, K.; Nelson, K.; Tam, M.R.; Weigl, B.H. Microfluidic diagnostic technologies for global public health. Nature 2006, 442, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Zarei, M. Advances in point-of-care technologies for molecular diagnostics. Biosens. Bioelectron. 2017, 98, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ding, B.; Chen, Q.; Feng, Q.; Lin, L.; Sun, J. Point-of-care-testing of nucleic acids by microfluidics. TrAC Trends Anal. Chem. 2017, 94, 106–116. [Google Scholar] [CrossRef]
- Khan, R.S.; Khurshid, Z.; Yahya Ibrahim Asiri, F. Advancing Point-of-Care (PoC) Testing Using Human Saliva as Liquid Biopsy. Diagnostics 2017, 7, 39. [Google Scholar] [CrossRef]
- Chretiennot, T.; Dubuc, D.; Grenier, K. Microwave-Based Microfluidic Sensor for Non-Destructive and Quantitative Glucose Monitoring in Aqueous Solution. Sensors 2016, 16, 1733. [Google Scholar] [CrossRef]
- Liu, C.-F.; Wang, M.-H.; Jang, L.-S. Microfluidics-based hairpin resonator biosensor for biological cell detection. Sens. Actuators B Chem. 2018, 263, 129–136. [Google Scholar] [CrossRef]
- Narang, R.; Mohammadi, S.; Ashani, M.M.; Sadabadi, H.; Hejazi, H.; Zarifi, M.H.; Sanati-Nezhad, A. Sensitive, Real-time and Non-Intrusive Detection of Concentration and Growth of Pathogenic Bacteria using Microfluidic-Microwave Ring Resonator Biosensor. Sci. Rep. 2018, 8, 15807. [Google Scholar] [CrossRef] [PubMed]
- Salim, A.; Lim, S. Complementary Split-Ring Resonator-Loaded Microfluidic Ethanol Chemical Sensor. Sensors 2016, 16, 1802. [Google Scholar] [CrossRef] [PubMed]
- Zarifi, M.H.; Daneshmand, M. Liquid sensing in aquatic environment using high quality planar microwave resonator. Sens. Actuators B Chem. 2016, 225, 517–521. [Google Scholar] [CrossRef]
- Zarifi, M.H.; Sadabadi, H.; Hejazi, S.H.; Daneshmand, M.; Sanati-Nezhad, A. Noncontact and Nonintrusive Microwave-Microfluidic Flow Sensor for Energy and Biomedical Engineering. Sci. Rep. 2018, 8, 139. [Google Scholar] [CrossRef]
- Yang, K.; Peretz-Soroka, H.; Liu, Y.; Lin, F. Novel developments in mobile sensing based on the integration of microfluidic devices and smartphones. Lab Chip 2016, 16, 943–958. [Google Scholar] [CrossRef]
- Huang, X.; Liu, Y.; Chen, K.; Shin, W.J.; Lu, C.J.; Kong, G.W.; Patnaik, D.; Lee, S.H.; Cortes, J.F.; Rogers, J.A. Stretchable, wireless sensors and functional substrates for epidermal characterization of sweat. Small 2014, 10, 3083–3090. [Google Scholar] [CrossRef]
- Kim, S.B.; Lee, K.; Raj, M.S.; Lee, B.; Reeder, J.T.; Koo, J.; Hourlier-Fargette, A.; Bandodkar, A.J.; Won, S.M.; Sekine, Y.; et al. Soft, Skin-Interfaced Microfluidic Systems with Wireless, Battery-Free Electronics for Digital, Real-Time Tracking of Sweat Loss and Electrolyte Composition. Small 2018, 14, e1802876. [Google Scholar] [CrossRef]
- Boutry, C.M.; Beker, L.; Kaizawa, Y.; Vassos, C.; Tran, H.; Hinckley, A.C.; Pfattner, R.; Niu, S.; Li, J.; Claverie, J.; et al. Biodegradable and flexible arterial-pulse sensor for the wireless monitoring of blood flow. Nat. Biomed. Eng. 2019, 3, 47–57. [Google Scholar] [CrossRef]
- Nowak, D.; Dziedzic, A. LTCC package for high temperature applications. Microelectron. Reliab. 2011, 51, 1241–1244. [Google Scholar] [CrossRef]
- Baluta, S.; Malecha, K.; Zając, D.; Sołoducho, J.; Cabaj, J. Dopamine sensing with fluorescence strategy based on low temperature co-fired ceramic technology modified with conducting polymers. Sens. Actuators B Chem. 2017, 252, 803–812. [Google Scholar] [CrossRef]
- Bartsch, H.; Welker, T.; Welker, K.; Witte, H.; Müller, J. LTCC based bioreactors for cell cultivation. IOP Conf. Ser. Mater. Sci. Eng. 2016, 104, 012001. [Google Scholar] [CrossRef] [Green Version]
- Halonen, N.; Kilpijarvi, J.; Sobocinski, M.; Datta-Chaudhuri, T.; Hassinen, A.; Prakash, S.B.; Moller, P.; Abshire, P.; Kellokumpu, S.; Lloyd Spetz, A. Low temperature co-fired ceramic packaging of CMOS capacitive sensor chip towards cell viability monitoring. Beilstein J. Nanotechnol. 2016, 7, 1871–1877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasudev, A.; Kaushik, A.; Jones, K.; Bhansali, S. Prospects of low temperature co-fired ceramic (LTCC) based microfluidic systems for point-of-care biosensing and environmental sensing. Microfluid. Nanofluid. 2012, 14, 683–702. [Google Scholar] [CrossRef]
- Lei, D.; Li-Feng, W.; Qing-An, H. Implementation of Multiparameter Monitoring by an LC-Type Passive Wireless Sensor Through Specific Winding Stacked Inductors. IEEE Internet Things J. 2015, 2, 168–174. [Google Scholar] [CrossRef]
- Ma, M.; Khan, H.; Shan, W.; Wang, Y.; Ou, J.Z.; Liu, Z.; Kalantar-zadeh, K.; Li, Y. A novel wireless gas sensor based on LTCC technology. Sens. Actuators B Chem. 2017, 239, 711–717. [Google Scholar] [CrossRef]
- Bergman, D.J. The dielectric constant of a composite material—A problem in classical physics. Phys. Rep. 1978, 43, 377–407. [Google Scholar] [CrossRef]
- Karkkainen, K.K.; Sihvola, A.H.; Nikoskinen, K.I. Effective permittivity of mixtures: Numerical validation by the FDTD method. IEEE Trans. Geosci. Remote Sens. 2000, 38, 1303–1308. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Y.; Li, Y. Combinatorial study of ceramic tape-casting slurries. ACS Comb. Sci. 2012, 14, 205–210. [Google Scholar] [CrossRef]
- Lin, L.; Ma, M.; Zhang, F.; Liu, F.; Liu, Z.; Li, Y. Fabrications and Performance of Wireless LC Pressure Sensors through LTCC Technology. Sensors 2018, 18, 340. [Google Scholar] [CrossRef]
- Marsland, T.P.; Evans, S. Dielectric measurements with an open-ended coaxial probe. IEEE Proc. H Microw. Antennas Propag. 1987, 134, 341–349. [Google Scholar] [CrossRef]
- Abduljabar, A.A.; Rowe, D.J.; Porch, A.; Barrow, D.A. Novel Microwave Microfluidic Sensor Using a Microstrip Split-Ring Resonator. IEEE Trans. Microw. Theory Tech. 2014, 62, 679–688. [Google Scholar] [CrossRef]
- Wessel, J.; Schmalz, K.; Scheytt, J.C.; Cahill, B.; Gastrock, G. Microwave Biosensor for Characterization of Compartments in Teflon Capillaries. In Proceedings of the 2012 42nd European Microwave Conference (EuMC), Amsterdam, The Netherlands, 29 October–1 November 2012; pp. 534–537. [Google Scholar] [CrossRef]
- Cook, B.S.; Cooper, J.R.; Tentzeris, M.M. An Inkjet-Printed Microfluidic RFID-Enabled Platform for Wireless Lab-on-Chip Applications. IEEE Trans. Microw. Theory Tech. 2013, 61, 4714–4723. [Google Scholar] [CrossRef]
- Ambrozkiewicz, M.; Jacob, A.F. Substrate Integrated Resonant Near-Field Sensor for Material Characterization. In Proceedings of the 2010 IEEE MTT-S International Microwave Symposium, Anaheim, CA, USA, 23–28 May 2010; pp. 628–631. [Google Scholar] [CrossRef]
- Haase, N.; Jacob, A.F. Substrate-Integrated Half-Mode Resonant Near-Field Sensor for Liquid Characterization. In Proceedings of the 2013 European Microwave Conference, Nuremberg, Germany, 6–10 October 2013; pp. 310–313. [Google Scholar] [CrossRef]
- Silavwe, E.; Somjit, N.; Robertson, I.D. Microwave Microlitre Lab-on-Substrate Liquid Characterisation based on SIW Slot Antenna. In Proceedings of the 2016 46th European Microwave Conference (EuMC), London, UK, 4–6 October 2016; pp. 261–264. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Withayachumnankul, W.; Al-Sarawi, S.F.; Abbott, D. Microwave microfluidic sensor for determination of glucose concentration in water. In Proceedings of the 2015 IEEE 15th Mediterranean Microwave Symposium (MMS), Lecce, Italy, 3 November–2 December 2015; pp. 1–3. [Google Scholar] [CrossRef]
- Vasudev, A.; Kaushik, A.; Tomizawa, Y.; Norena, N.; Bhansali, S. An LTCC-based microfluidic system for label-free, electrochemical detection of cortisol. Sens. Actuators B Chem. 2013, 182, 139–146. [Google Scholar] [CrossRef]
- Remiszewska, E.; Malecha, K.; Kruk, J.; Jankowska-Śliwińska, J.; Torbicz, W.; Samluk, A.; Pluta, K.D.; Pijanowska, D.G. Enzymatic method of urea determination in LTCC microfluidic system based on absorption photometry. Sens. Actuators B Chem. 2019, 285, 375–384. [Google Scholar] [CrossRef]
- Malecha, K.; Jasińska, L.; Grytsko, A.; Drzozga, K.; Słobodzian, P.; Cabaj, J. Monolithic Microwave-Microfluidic Sensors Made with Low Temperature Co-Fired Ceramic (LTCC) Technology. Sensors 2019, 19, 577. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Dubuc, D.; Grenier, K. Resonant-based Microwave Biosensor for Physiological Liquid Identification. In Proceedings of the 2012 42nd European Microwave Conference (EuMC), Amsterdam, The Netherlands, 29 October–1 November 2012; pp. 420–422. [Google Scholar] [CrossRef]
- Ciobanu, R.; Schreiner, C.; Drug, V.; Schreiner, T.; Antal, D. Sensors in LTCC-technology with embedded microfluidic features, for medical applications. In Proceedings of the IEEE 2015 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Turin, Italy, 7–9 May 2015; pp. 407–410. [Google Scholar] [CrossRef]





| Symbol | Designed Value (mm) |
|---|---|
| a | 9.6 |
| d0 | 16.0 |
| di | 10.4 |
| s | 0.40 |
| w | 0.40 |
| t1 | 0.22 |
| t2 | 0.86 |
| t3 | 0.04 |
| t4 | 0.39 |
| d | 1.60 |
| Mole Fraction of Glucose | ||
|---|---|---|
| 0 | 78.11 | 0.0757 |
| 0.005 | 77.11 | 0.1207 |
| 0.010 | 75.56 | 0.1418 |
| 0.015 | 74.43 | 0.165 |
| 0.020 | 73.05 | 0.1821 |
| 0.025 | 71.85 | 0.2014 |
| 0.030 | 71.01 | 0.2306 |
| 0.035 | 70.13 | 0.2573 |
| 0.040 | 69.26 | 0.2852 |
| 0.045 | 68.34 | 0.313 |
| 0.050 | 67.05 | 0.3338 |
| 0.055 | 66.79 | 0.3816 |
| 0.060 | 65.23 | 0.4008 |
| 0.065 | 63.64 | 0.4284 |
| 0.070 | 63.46 | 0.4452 |
| 0.075 | 62.43 | 0.465 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Y.; Ma, M.; Zhang, F.; Liu, F.; Liu, Z.; Wang, D.; Li, Y. An LC Wireless Microfluidic Sensor Based on Low Temperature Co-Fired Ceramic (LTCC) Technology. Sensors 2019, 19, 1189. https://doi.org/10.3390/s19051189
Liang Y, Ma M, Zhang F, Liu F, Liu Z, Wang D, Li Y. An LC Wireless Microfluidic Sensor Based on Low Temperature Co-Fired Ceramic (LTCC) Technology. Sensors. 2019; 19(5):1189. https://doi.org/10.3390/s19051189
Chicago/Turabian StyleLiang, Yongyuan, Mingsheng Ma, Faqiang Zhang, Feng Liu, Zhifu Liu, Dong Wang, and Yongxiang Li. 2019. "An LC Wireless Microfluidic Sensor Based on Low Temperature Co-Fired Ceramic (LTCC) Technology" Sensors 19, no. 5: 1189. https://doi.org/10.3390/s19051189
APA StyleLiang, Y., Ma, M., Zhang, F., Liu, F., Liu, Z., Wang, D., & Li, Y. (2019). An LC Wireless Microfluidic Sensor Based on Low Temperature Co-Fired Ceramic (LTCC) Technology. Sensors, 19(5), 1189. https://doi.org/10.3390/s19051189






