Review of Laser Raman Spectroscopy for Surgical Breast Cancer Detection: Stochastic Backpropagation Neural Networks
Abstract
:1. Introduction
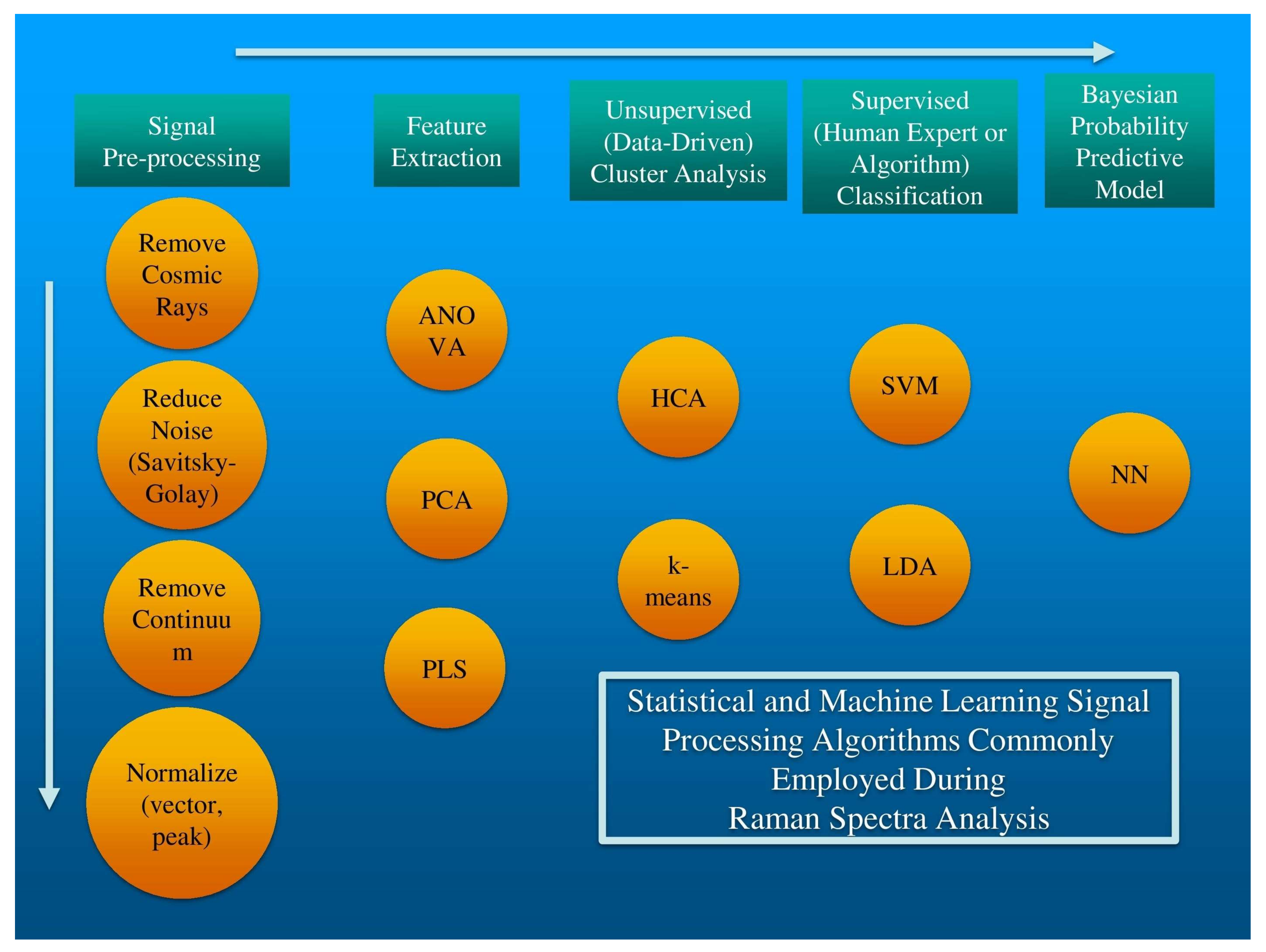
2. Statistical and Machine Learning Methods
2.1. Data Pre-Processing
2.2. Data Optimization and Dimension Reduction
2.3. Unsupervised, Autonomous Data Exploration
2.4. Supervised Data Classification
2.5. Bayesian Probabilities of Correct Classification—Stochastic Neural Networks
3. Review of Major Research Advancements
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- DeSantis, C.E.; Ma, J.; Gaudet, M.M.; Newman, L.A.; Miller, K.D.; Goding Sauer, A.; Jemal, A.; Siegel, R.L. Breast cancer statistics, 2019. CA A Cancer J. Clin. 2019, 69, 438–451. [Google Scholar] [CrossRef] [PubMed]
- Apantaku, L.M. Breast-conserving surgery for breast cancer. Am. Fam Physician 2002, 66, 2271–2278. [Google Scholar] [PubMed]
- Fisher, B.; Anderson, S.; Bryant, J.; Margolese, R.G.; Deutsch, M.; Fisher, E.R.; Jeong, J.H.; Wolmark, N. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N. Engl. J. Med. 2002, 347, 1233–1241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maloney, B.W.; McClatchy, D.M.; Pogue, B.W.; Paulsen, K.D.; Wells, W.A.; Barth, R.J. Review of methods for intraoperative margin detection for breast conserving surgery. J. Biomed. Opt. 2018, 23, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Pleijhuis, R.G.; Graafland, M.; De Vries, J.; Bart, J.; De Jong, J.S.; Van Dam, G.M. Obtaining Adequate Surgical Margins in Breast-Conserving Therapy for Patients with Early-Stage Breast Cancer: Current Modalities and Future Directions. Ann. Surg. Oncol. 2009, 16, 2717–2730. [Google Scholar] [CrossRef] [Green Version]
- Thill, M. MarginProbe: Intraoperative margin assessment during breast conserving surgery by using radiofrequency spectroscopy. Expert Rev. Med. Devices 2013, 10, 301–315. [Google Scholar] [CrossRef] [PubMed]
- Dixon, J.M.; Renshaw, L.; Young, O.; Kulkarni, D.; Saleem, T.; Sarfaty, M.; Sreenivasan, R.; Kusnick, C.; Thomas, J.; Williams, L.J. Intra-operative assessment of excised breast tumour margins using ClearEdge imaging device. Eur. J. Surg. Oncol. 2016, 42, 1834–1840. [Google Scholar] [CrossRef]
- Piras, D.; Xia, W.; Steenbergen, W.; Leeuwen, T.G.V.; Manohar, S. Photoacoustic Imaging of the Breast Using the Twente Photoacoustic Mammoscope: Present Status and Future Perspectives. IEEE J. Sel. Top. Quantum. Electron. 2010, 16, 730–739. [Google Scholar]
- Laughney, A.M.; Krishnaswamy, V.; Rizzo, E.J.; Schwab, M.C.; Barth, R.J., Jr.; Cuccia, D.J.; Tromberg, B.J.; Paulsen, K.D.; Pogue, B.W.; Wells, W.A. Spectral discrimination of breast pathologies in situ using spatial frequency domain imaging. Breast Cancer Res. 2013, 15, R61. [Google Scholar] [CrossRef] [Green Version]
- Tummers, Q.R.; Verbeek, F.P.; Schaafsma, B.E.; Boonstra, M.C.; Van der Vorst, J.R.; Liefers, G.J.; Van de Velde, C.J.; Frangioni, J.V.; Vahrmeijer, A.L. Real-time intraoperative detection of breast cancer using near-infrared fluorescence imaging and Methylene Blue. Eur. J. Surg Oncol. 2014, 40, 850–858. [Google Scholar] [CrossRef] [Green Version]
- Troyan, S.L.; Kianzad, V.; Gibbs-Strauss, S.L.; Gioux, S.; Matsui, A.; Oketokoun, R.; Ngo, L.; Khamene, A.; Azar, F.; Frangioni, J.V. The FLARE intraoperative near-infrared fluorescence imaging system: A first-in-human clinical trial in breast cancer sentinel lymph node mapping. Ann. Surg. Oncol. 2009, 16, 2943–2952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, K.S.; Chicken, D.W.; Pickard, D.C.; Lee, A.C.; Briggs, G.; Falzon, M.; Bigio, I.J.; Keshtgar, M.R.; Bown, S.G. Elastic scattering spectroscopy for intraoperative determination of sentinel lymph node status in the breast. J. Biomed. Opt. 2004, 9, 1122–1128. [Google Scholar] [PubMed] [Green Version]
- Fereidouni, F.; Harmany, Z.T.; Tian, M.; Todd, A.; Kintner, J.A.; McPherson, J.D.; Borowsky, A.D.; Bishop, J.; Lechpammer, M.; Demos, S.G.; et al. Microscopy with ultraviolet surface excitation for rapid slide-free histology. Nat. Biomed. Eng. 2017, 1, 957–966. [Google Scholar]
- Glaser, A.K.; Reder, N.P.; Chen, Y.; McCarty, E.F.; Yin, C.; Wei, L.; Wang, Y.; True, L.D.; Liu, J.T.C. Light-sheet microscopy for slide-free non-destructive pathology of large clinical specimens. Nat. Biomed. Eng. 2017, 1, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.K.; Shen, D.; Sheikine, Y.; Ahsen, O.O.; Wang, H.H.; Schmolze, D.B.; Johnson, N.B.; Brooker, J.S.; Cable, A.E.; Connolly, J.L.; et al. Assessment of breast pathologies using nonlinear microscopy. Proc. Natl. Acad. Sci. USA 2014, 111, 15304–15309. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, F.T.; Zysk, A.M.; Chaney, E.J.; Kotynek, J.G.; Oliphant, U.J.; Bellafiore, F.J.; Rowland, K.M.; Johnson, P.A.; Boppart, S.A. Intraoperative evaluation of breast tumor margins with optical coherence tomography. Cancer Res. 2009, 69, 8790–8796. [Google Scholar] [CrossRef] [Green Version]
- Haka, A.S.; Volynskaya, Z.; Gardecki, J.A.; Nazemi, J.; Lyons, J.; Hicks, D.; Fitzmaurice, M.; Dasari, R.R.; Crowe, J.P.; Feld, M.S. In vivo margin assessment during partial mastectomy breast surgery using raman spectroscopy. Cancer Res. 2006, 66, 3317–3322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haka, A.S.; Shafer-Peltier, K.E.; Fitzmaurice, M.; Crowe, J.; Dasari, R.R.; Feld, M.S. Diagnosing breast cancer by using Raman spectroscopy. Proc. Natl. Acad. Sci. USA 2005, 102, 12371–12376. [Google Scholar] [CrossRef] [Green Version]
- Haka, A.S.; Shafer-Peltier, K.E.; Fitzmaurice, M.; Crowe, J.; Dasari, R.R.; Feld, M.S. Identifying microcalcifications in benign and malignant breast lesions by probing differences in their chemical composition using Raman spectroscopy. Cancer Res. 2002, 62, 5375–5380. [Google Scholar]
- Haka, A.S.; Volynskaya, Z.; Gardecki, J.A.; Nazemi, J.; Shenk, R.; Wang, N.; Dasari, R.R.; Fitzmaurice, M.; Feld, M.S. Diagnosing breast cancer using Raman spectroscopy: Prospective analysis. J. Biomed. Opt. 2009, 14, 054023. [Google Scholar] [CrossRef] [Green Version]
- Shafer-Peltier, K.E.; Haka, A.S.; Fitzmaurice, M.; Crowe, J.; Myles, J.; Dasari, R.R.; Feld, M.S. Raman microspectroscopic model of human breast tissue: Implications for breast cancer diagnosis in vivo. J. Raman. Spectrosc. 2002, 33, 552–563. [Google Scholar] [CrossRef]
- Hanlon, E.B.; Manoharan, R.; Koo, T.-W.; Shafer, K.E.; Motz, J.T.; Fitzmaurice, M.; Kramer, J.R.; Itzkan, I.; Dasari, R.R.; Feld, M.S. Procpets for in vivo Raman spectroscopy. Phys. Eng. Med. Biol. 1999, 45, R1–R59. [Google Scholar]
- Stokes, G.G. XXX. On the change of refrangibility of light. Philos. Trans. R. Soc. Lond. 1852, 142, 463–562. [Google Scholar]
- Gautam, R.; Vanga, S.; Ariese, F.; Umapathy, S. Review of multidimensional data processing approaches for Raman and infrared spectroscopy. EPJ Tech. Instrum. 2015, 2, 1–38. [Google Scholar] [CrossRef] [Green Version]
- Savitzky, A.; Golay, M.J.F. Smoothing and differentiation of data by simplified least squares procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Camerlingo, C.; Zenone, F.; Gaeta, G.M.; Riccio, R.; Lepore, M. Wavelet data processing of micro-Raman spectra of biological samples. Meas. Sci. Technol. 2006, 17, 298–303. [Google Scholar] [CrossRef]
- Mosier-Boss, P.A.; Lieberman, S.H.; Newbery, R. Fluorescence rejection in Raman-spectroscopy by shifted-Spectra, edge-detection, and FFT filtering techniques. Appl. Spectrosc. 1995, 49, 630–638. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, J. A general-purpose signal processing algorithm for biological profiles using only first-order derivative information. BMC Bioinform. 2019, 20, 611. [Google Scholar] [CrossRef]
- Cadusch, P.J.; Hlaing, M.M.; Wade, S.A.; McArthur, S.L.; Stoddart, P.R. Improved methods for fluorescence background subtraction from Raman spectra. J. Raman Spectrosc. 2013, 44. [Google Scholar] [CrossRef] [Green Version]
- Mendes, T.d.O.; Pinto, L.P.; Santos, L.D.; Tippavajhala, V.K.; Soto, C.A.T.L.; Martin, A.A.O. Statistical strategies to reveal potential vibrational markers for in vivo analysis by confocal Raman spectroscopy. J. Biomed. Opt. 2016, 21, 075010. [Google Scholar] [CrossRef]
- Baek, S.-J.; Park, A.; Ahn, Y.-J.; Choo, J. Baseline correction using asymmetrically reweighted penalized least squares smoothing. Analyst 2015, 140, 250–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beattie, J.R.; Glenn, J.V.; Boulton, M.E.; Stitt, A.W.; McGarvey, J.J. Effect of signal intensity normalization on the multivariate analysis of spectral data in complex ‘real-world’ datasets. J. Raman Spectrosc. 2008, 40. [Google Scholar] [CrossRef]
- Murtagh, F.; Heck, A. Multivariate Data Analysis; Springer Science & Business Media: Dordrecht, The Netherlands, 1986. [Google Scholar]
- Wold, S.; Sjöströma, M.; Erikssonb, L. PLS-regression: A basic tool of chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Revelle, W. Hierarchical cluster analysis and the internal structure of tests. Multivar. Behav. Res. 1979, 14, 57–74. [Google Scholar] [CrossRef] [PubMed]
- Woese, C.R.; Kandler, O.; Wheelis, M.L. Towards a natural system of organisms: Proposal for the domains Archaea, Bacteria, and Eucarya. Proc. Natl. Acad. Sci. USA 1990, 87, 4576–4579. [Google Scholar] [CrossRef] [Green Version]
- MacQueen, J.B. Some methods for classification and analysis of multivariate observations. In Proceedings of the Fifth Symposium on Mathematical Statistics and Probability, Berkeley, CA, USA, 21 June–18 July 1965; University of California Press: Berkeley, CA, USA; pp. 281–297. [Google Scholar]
- Fisher, R.A. The use of multiple measurements in taxonomic problems. Ann. Eugen. 1936, 7, 179–188. [Google Scholar] [CrossRef]
- Boser, B.E.; Guyon, I.M.; Vapnik, V.N. A training algorithm for optimal margin classifiers. In Proceedings of the Fifth Annual Workshop on Computational Learning Theory–COLT ‘92, Pittsburgh, PA, USA, July 1992; Association for Computing Machinery: New York, NY, USA, 1992; p. 144. [Google Scholar]
- Cortes, C.; Vapnik, V. Support-vector networks. Mach. Learn. 1995, 20, 273–297. [Google Scholar] [CrossRef]
- Murty, M.N.; Raghava, R. Kernel-based SVM. In Support Vector Machines and Perceptrons; SpringerBriefs in Computer Science; Springer: Cham, Germany, 2016. [Google Scholar]
- Bayes, T. An Essay towards Solving a Problem in the Doctrine of Chances. Philos. Trans. R. Soc. Lond. 1763, 53, 370–418. [Google Scholar] [CrossRef]
- Kohonen, T. Self-Organization and Associative Memory; Springer: New York, NY, USA, 1977. [Google Scholar]
- Hopfield, J.J.; Tank, D.W. Computing with neural circuits: A model. Science 1986, 233, 625–633. [Google Scholar] [CrossRef] [Green Version]
- Rumelhart, D.E.; Hinton, G.E.; Williams, R.J. Learning representations by back-propagating errors. Nature 1986, 323, 533–536. [Google Scholar] [CrossRef]
- Adorf, H.M. Connectionism in neural networks. In Knowledge-Based Systems in Astronomy; Heck, A., Murtagh, F., Eds.; Springer: New York, NY, USA, 1989; pp. 215–245. [Google Scholar]
- Hinton, G.E. Connectionist Symbol Processing; MIT Press: Cambridge, MA, USA, 1991. [Google Scholar]
- Richard, M.D.; Lippmann, R.P. Neural network classifiers estimate Bayesian a-posteriori probabilities. Neural Comput. 1991, 3, 461–483. [Google Scholar] [CrossRef]
- Storrie-Lombardi, M.C.; Lahav, O.; Sodre, L.; Storrie-Lombardi, L.J. Morphological classification of galaxies by artificial neural networks. Mon. Not. R. Astron. Soc. 1992, 259, 8–12. [Google Scholar] [CrossRef] [Green Version]
- Hinton, G.E.; Osindero, S.; Teh, Y.W. A Fast Learning Algorithm for Deep Belief Nets. Neural Comput. 2006, 18, 1527–1554. [Google Scholar] [CrossRef] [PubMed]
- Polyak, K. Heterogeneity in breast cancer. J. Clin. Investig. 2011, 121, 3786–3788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kothari, R.; Jones, V.; Mena, D.; Bermúdez Reyes, V.; Shon, Y.; Smith, J.P.; Schmolze, D.; Cha, P.D.; Lai, L.; Fong, Y.; et al. Raman Spectroscopy and Artificial Intelligence to Predict the Bayesian Probability of Breast Cancer. Sci. Rep. 2020. under review. [Google Scholar] [CrossRef]
- Movasaghi, Z.; Rehman, S.; Rehman, I.U. Raman Spectroscopy of Biological Tissues. Appl. Spectrosc. Rev. 2007, 42, 493–541. [Google Scholar] [CrossRef]
- Werbos, P.J. An overview of neural networks for control. IEEE Control. Syst. Mag. 1991, 11, 40–41. [Google Scholar]
- Parker, D.B. Learning-Logic: Casting the Cortex of the Human Brain in Silicon. Technical Report No.47; Center for Computational Research in Economics and Management Science. MIT: Cambridge, MA, USA, 1985; pp. 1–47. [Google Scholar]
- Mohs, A.M.; Mancini, M.C.; Singhal, S.; Provenzale, J.M.; Leyland-Jones, B.; Wang, M.D.; Nie, S. Hand-held Spectroscopic Device for In Vivo and Intraoperative Tumor Detection: Contrast Enhancement, Detection Sensitivity, and Tissue Penetration. Anal. Chem. 2010, 82, 9058–9065. [Google Scholar] [CrossRef] [Green Version]
- Keller, M.; Vargis, E.; Mahadevan-Jansen, A.; De Matos Granja, N.; Wilson, R.; Mycek, M.-A.; Kelley, M. Development of a spatially offset Raman spectroscopy probe for breast tumor surgical margin evaluation. J. Biomed. Opt. 2011, 16, 077006. [Google Scholar] [CrossRef] [Green Version]
- Brożek-Płuska, B.; Placek, I.; Kurczewski, K.; Morawiec, Z.; Tazbir, M.; Abramczyk, H. Breast cancer diagnostics by Raman spectroscopy. J. Mol. Liq. 2008, 141, 145–148. [Google Scholar] [CrossRef]
- Abramczyk, H.; Surmacki, J.; Brożek-Płuska, B.; Morawiec, Z.; Tazbir, M. The hallmarks of breast cancer by Raman spectroscopy. J. Mol. Struct. 2009, 924–926, 175–182. [Google Scholar] [CrossRef]
- Shipp, D.W.; Rakha, E.A.; Koloydenko, A.A.; Macmillan, R.D.; Ellis, I.O.; Notingher, I. Intra-operative spectroscopic assessment of surgical margins during breast conserving surgery. Breast Cancer Res. 2018, 20, 69. [Google Scholar] [CrossRef] [Green Version]
- García-Flores, A.F.; Raniero, L.; Canevari, R.A.; Jalkanen, K.J.; Bitar, R.; Martinho, H.D.S. High-wavenumber FT-Raman spectroscopy for in vivo and ex vivo measurements of breast cancer. Theor. Chem. Acc. 2011, 130, 1231–1238. [Google Scholar] [CrossRef]
- Zúñiga, W.C.; Jones, V.; Anderson, S.M.; Echevarria, A.; Miller, N.L.; Stashko, C.; Schmolze, D.; Cha, P.D.; Kothari, R.; Fong, Y.; et al. Raman spectroscopy for rapid evaluation of surgical margins during breast cancer lumpectomy. Sci. Rep. 2019, 9, 14639. [Google Scholar] [CrossRef] [Green Version]
- Barman, I.; Dingari, N.C.; Saha, A.; McGee, S.; Galindo, L.H.; Liu, W.; Plecha, D.; Klein, N.; Dasari, R.R.; Fitzmaurice, M. Application of Raman spectroscopy to identify microcalcifications and underlying breast lesions at stereotactic core needle biopsy. Cancer Res. 2013, 73, 3206–3215. [Google Scholar] [CrossRef] [Green Version]
- Lyng, F.M.; Traynor, D.; Nguyen TN, Q.; Meade, A.D.; Rakib, F.; Al-Saady, R.; Goormaghtigh, E.; Al-Saad, K.; Ali, M.H. Discrimination of breast cancer from benign tumours using Raman spectroscopy. PLoS ONE 2019, 14, e0212376. [Google Scholar] [CrossRef]
- Shang, L.; Ma, D.; Fu, J.; Lu, Y.; Zhao, Y.; Xu, X.; Yin, J. Fluorescence imaging and Raman spectroscopy applied for the accurate diagnosis of breast cancer with deep learning algorithms. Biomed. Opt. Express 2020, 11, 3673–3683. [Google Scholar] [CrossRef]
- Koya, S.K.; Brusatori, M.; Yurgelevic, S.; Huang, C.; Werner, C.W.; Kast, R.E.; Shanley, J.; Sherman, M.; Honn, K.V.; Maddipati, K.R.; et al. Accurate identification of breast cancer margins in microenvironments of ex-vivo basal and luminal breast cancer tissues using Raman spectroscopy. Prostaglandins Other Lipid Mediat. 2020, 151, 106475. [Google Scholar] [CrossRef]
- Keller, M.D.; Majumder, S.K.; Mahadevan-Jansen, A. Spatially offset Raman spectroscopy of layered soft tissues. Opt. Lett. 2009, 34, 926–928. [Google Scholar] [CrossRef]
- Matousek, P.; Clark, I.P.; Draper, E.R.C.; Morris, M.D.; Goodship, A.E.; Everall, N.; Towrie, M.; Finney, W.F.; Parker, A.W. Subsurface Probing in Diffusely Scattering Media Using Spatially Offset Raman Spectroscopy. Appl. Spectrosc. 2005, 59, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Kong, K.; Rowlands, C.J.; Varma, S.; Perkins, W.; Leach, I.H.; Koloydenko, A.A.; Williams, H.C.; Notingher, I. Diagnosis of tumors during tissue-conserving surgery with integrated autofluorescence and Raman scattering microscopy. Proc. Natl. Acad. Sci. USA 2013, 110, 15189–15194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanter, E.M.; Vargis, E.; Majumder, S.; Keller, M.D.; Woeste, E.; Rao, G.G.; Mahadevan-Jansen, A. Application of Raman spectroscopy for cervical dysplasia diagnosis. J. Biophotonics 2009, 2, 81–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanter, E.M.; Majumder, S.; Kanter, G.J.; Woeste, E.M.; Mahadevan-Jansen, A. Effect of hormonal variation on Raman spectra for cervical disease detection. Am. J. Obstet. Gynecol. 2009, 200, 512.e1–512.e5. [Google Scholar] [CrossRef] [Green Version]
- Cubeddu, R.; D’Andrea, C.; Pifferi, A.; Taroni, P.; Torricelli, A.; Valentini, G. Effects of the menstrual cycle on the red and near-infrared optical properties of the human breast. Photochem. Photobiol. 2000, 72, 383–391. [Google Scholar] [PubMed]
- Shah, N.; Cerussi, A.E.; Jakubowski, D.; Hsiang, D.; Butler, J.; Tromberg, B.J. The role of diffuse optical spectroscopy in the clinical management of breast cancer. Dis. Markers 2003, 19, 95–105. [Google Scholar] [CrossRef] [Green Version]
- Hollon, T.C.; Pandian, B.; Adapa, A.R.; Urias, E.; Save, A.V.; Khalsa SS, S.; Eichberg, D.G.; D’Amico, R.S.; Farooq, Z.U.; Lewis, S.; et al. Near real-time intraoperative brain tumor diagnosis using stimulated Raman histology and deep neural networks. Nat. Med. 2020, 26, 52–58. [Google Scholar] [CrossRef]
- Desroches, J.; Lemoine, E.; Pinto, M.; Marple, E.; Urmey, K.; Diaz, R.; Guiot, M.C.; Wilson, B.C.; Petrecca, K.; Leblond, F. Development and first in-human use of a Raman spectroscopy guidance system integrated with a brain biopsy needle. J. Biophotonics 2019, 12, e201800396. [Google Scholar] [CrossRef]
- Desroches, J.; Jermyn, M.; Pinto, M.; Picot, F.; Tremblay, M.A.; Obaid, S.; Marple, E.; Urmey, K.; Trudel, D.; Soulez, G.; et al. A new method using Raman spectroscopy for in vivo targeted brain cancer tissue biopsy. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Desroches, J.; Jermyn, M.; Mok, K.; Lemieux-Leduc, C.; Mercier, J.; St-Arnaud, K.; Urmey, K.; Guiot, M.C.; Marple, E.; Petrecca, K.; et al. Characterization of a Raman spectroscopy probe system for intraoperative brain tissue classification. Biomed. Opt. Express 2015, 6, 2380–2397. [Google Scholar] [CrossRef] [Green Version]
- Iyengar, N.M.; Zhou, X.K.; Gucalp, A.; Morris, P.G.; Howe, L.R.; Giri, D.D.; Morrow, M.; Wang, H.; Pollak, M.; Jones, L.W.; et al. Systemic correlates of white adipose tissue inflammation in early-stage breast cancer. Clin. Cancer Res. 2016, 22, 2283–2289. [Google Scholar] [CrossRef] [Green Version]
- Cinti, S.; Mitchell, G.; Barbatelli, G.; Murano, I.; Ceresi, E.; Faloia, E.; Wang, S.; Fortier, M.; Greenberg, A.S.; Obin, M.S. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 2005, 46, 2347–2355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haka, A.S.; Sue, E.; Chi Zhang, P.B.; Sterling, J.; Carpenter, C.; Leonard, M.; Manzoor, M.; Walker, J.; Aleman, J.O.; Gareau, D.; et al. Noninvasive Detection of Inflammatory Changes in White Adipose Tissue by Label-Free Raman Spectroscopy. Anal. Chem. 2016, 88, 2140–2148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poggio, T.; Liao, Q.; Banburski, A. Complexity control by gradient descent in deep networks. Nat. Commun. 2020, 11, 1027. [Google Scholar] [CrossRef] [PubMed]
- Goldfeld, Z.; Berg, E.V.D.; Greenewald, K.; Melnyk, I.; Nguyen, N.; Kingsbury, B.; Polyanskiy, Y. Estimating information flow in deep neural networks. In Proceedings of the thirty-sixth International Conference on Machine Learning, Long Beach, CA, USA, 9–15 June 2019; PMLR 97: Long Beach, CA, USA. [Google Scholar]
- Poggio, T.; Mhaskar, H.; Rosasco, L.; Miranda, B.; Liao, Q. Why and When Can Deep-but Not Shallow-networks Avoid the Curse of Dimensionality: A Review. Int. J. Autom. Comput. 2017, 14, 503–519. [Google Scholar] [CrossRef] [Green Version]


| Region of Interest | Raman Shift (cm−1) | Biomolecular Assignment * [53] |
|---|---|---|
| v1 | 780–810 | O—P—O stretching DNA, ring breathing mode U, T, C bases of RNA and DNA |
| v2 | 815–825 | C—C stretch of proline and hydroxyproline, out of plane ring breathing of tyrosine |
| v3 | 1302 | assigned to CH2 twisting of lipids in healthy; in tumor assigned to CH2 twisting in proteins or amide III (protein) |
| v4 | 1441 | CH2 bending mode in lipids |
| v4 | 2853 | CH2 symmetric stretch of lipids |
| v4 | 2903 | CH2 asymmetric stretch of lipids and proteins |
| Author | Tissue Type, Number of Patients, Number of Spectra | LRS System | Algorithm | Prediction Statistics | Findings |
|---|---|---|---|---|---|
| Shafer-Peltier et al. [21] | Ex vivo normal, benign and malignant tissue; For each basis spectra: 60–80 spectra from 5–6 patients; 60 Raman images | 830 nm; Raman confocal microscope | Non-Negative Least squares fitting, PCA | N/A | Nine basis spectra; Raman micro spectroscopic model compared to H&E findings |
| Haka et al. [19] | Ex vivo microcalcifications 11 patients | 830 nm | PCA | Sensitivity = 88% specificity = 93% for determining microcalcifications in malignant and benign ducts | Type ΙΙ microcalcifications in benign ducts have more calcium carbonate and less protein than type ΙΙ microcalcifications in malignant ducts |
| Haka et al. [18] | Ex vivo normal, fibrocystic change, fibroadenoma, and infiltrating carcinoma; 58 patients; 130 Raman spectra | 830 nm | Linear combination of basis spectra, logistic regression, diagnostic algorithm based on fat and collagen content | Sensitivity = 94% specificity = 96% for infiltrating carcinoma | 9 basis spectra; Fit coefficients for each basis spectra highlight chemical and morphological features of the macroscopic spectra |
| Haka et al. [17] | In vivo breast tissue during partial mastectomy; nine patients; 31 spectra | 830 nm | Linear combination of basis spectra, logistic regression, diagnostic algorithm based on fat and collagen content | Sensitivity and specificity = 100% for carcinoma, only one malignant spectrum | In vivo spectra collected in 1 s; if malignant spectrum was taken into account during initial surgery second surgery could have been avoided |
| Mohs et al. [56] | In vivo mouse model; injected with 4T1 tumor cell line and ICG and SERS contrast agents; 14 in vivo spectra | SpectroPen at 785 nm | Linear regression model | N/A Validated using bright field and bioluminescence images of mouse | Descriptive development of hand-held SpectroPen compared to normal 785 LRS system |
| Keller et al. [57] | 35 in vitro tissue samples | Spatially offset 785 nm LRS system; probe design discussed | Sparse multinomial logistic regression | Sensitivity = 95% specificity = 100% for discerning positive and negative margins | SORS allows collection of photons from deeper within the sample |
| Brozek-Pluska et al. [58] | Ex vivo breast tissue; 44 patients; 321 spectra | 514 nm | Least squares fitting, PCA | Sensitivity = 72% for malignant tissue; sensitivity = 62% for benign tissue | Specific band and band ratio differences in malignant, normal and benign tissue discussed, malignant spectra has more autofluorescence |
| Abramczyk et al. [59] | Ex vivo breast tissue; 99 patients; 1100 spectra | 514 nm | Least squares fitting, PCA | Sensitivity = 72% for malignant tissue; sensitivity = 62% for benign tissue; specificity = 83% for normal tissue | Same as Brozek-Pluska above |
| Shipp et al. [60] | 51 fresh whole BCS specimens | 405 nm confocal microscope for autofluoresence (AF) images, 785 nm system for Raman spectra | Unsupervised algorithm to detect segments in AF images; K-means, LDA | Sensitivity = 100% and specificity is at least 80% for multimodal spectral histopathology for 51 BCS surfaces | Results were obtained within an intraoperative timescale (12–24 min), diagnosis model trained on smaller mastectomy samples with sensitivity = 91% and specificity = 83% |
| Garcia-Flores et al. [61] | Ex vivo and in vivo breast tissue of rats | High-frequency (HF) Fourier transform LRS system at 1064 nm | PCA, LDA | Discrimination accuracy of 77.2%, 82.3% and 100% for in vivo transcutaneous, in vivo skin-removed and ex vivo spectra respectively | HF Raman spectra has a shorter acquisition time due to more intense signal in this region, HF region has no interfering signal from optical fiber |
| Zúñiga et al. [62] | Ex vivo breast tissue; six patients; 164 spectra | 785 nm and 1064 commercially available systems | PCA, LDA | Sensitivity = 90% specificity = 86% with 785 nm system without microscope | Systematic comparison of 1064 and 785 nm systems with and without microscope; discussion of importance of high wavenumber signals |
| Barman et al. [63] | Ex vivo breast tissue; 33 patients undergoing stereotactic core needle breast biopsy procedures; 146 tumor sites | 830 nm | SVM | Sensitivity = 62.5% specificity = 100% | SVM and LRS have been used to identify normal tissue, fibrocystic change (FCC), fibroadenoma (FA) and breast cancer, in the absence and presence of microcalcifications |
| Lyng et al. [64] | Ex vivo benign lesions (fibrocystic, fibroadenoma, intraductal papilloma) and cancer (invasive ductal carcinoma and lobular carcinoma); 20 patients | 532 nm | PCA, LDA, QDA (quadratic discriminant analysis), SVM, Partial least squares discriminant analysis (PLSDA) | Sensitivity = 83% and specificity = 80% (PCA-LDA and PCA-QDA); Sensitivity = 82% and specificity = 84% (PLSDA) | Study also included immunohistochemical staining for ER and HER2 receptor |
| Shang et al. [65] | Ex vivo breast tissue; 14 patients | 785 nm | CNN on autofluorescence images, BP-NN on Raman spectra, PLS | Discrimination accuracy of 95.33% and 98.67% respectively for collagen and lipid BP-NNs | Auto florescence images and Raman spectra fed into PLS model to achieve 100% accuracy |
| Koya et al. [66] | Ex vivo basal and luminal breast cancer samples | 785 nm | CNN with one hidden layer | Sensitivity = 88.8% and specificity = 90.8% for discriminating cancerous and normal breast tissue | Specific band differences discussed |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kothari, R.; Fong, Y.; Storrie-Lombardi, M.C. Review of Laser Raman Spectroscopy for Surgical Breast Cancer Detection: Stochastic Backpropagation Neural Networks. Sensors 2020, 20, 6260. https://doi.org/10.3390/s20216260
Kothari R, Fong Y, Storrie-Lombardi MC. Review of Laser Raman Spectroscopy for Surgical Breast Cancer Detection: Stochastic Backpropagation Neural Networks. Sensors. 2020; 20(21):6260. https://doi.org/10.3390/s20216260
Chicago/Turabian StyleKothari, Ragini, Yuman Fong, and Michael C. Storrie-Lombardi. 2020. "Review of Laser Raman Spectroscopy for Surgical Breast Cancer Detection: Stochastic Backpropagation Neural Networks" Sensors 20, no. 21: 6260. https://doi.org/10.3390/s20216260




