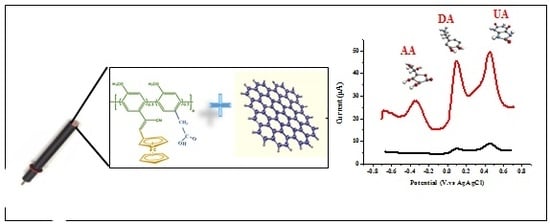
Nanocomposite Based on Poly (para-phenylene)/Chemical Reduced Graphene Oxide as a Platform for Simultaneous Detection of Ascorbic Acid, Dopamine and Uric Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Instrumentation
2.2. Synthesis of Chemical Reduced Graphene Oxide (CRGO)
2.3. Nanocomposite Formation CRGO/Fc-ac-PPP
3. Results and Discussions
3.1. Characterization of CRGO/Fc-ac-PPP Nanocomposite
3.2. Electrochemical Characterizations
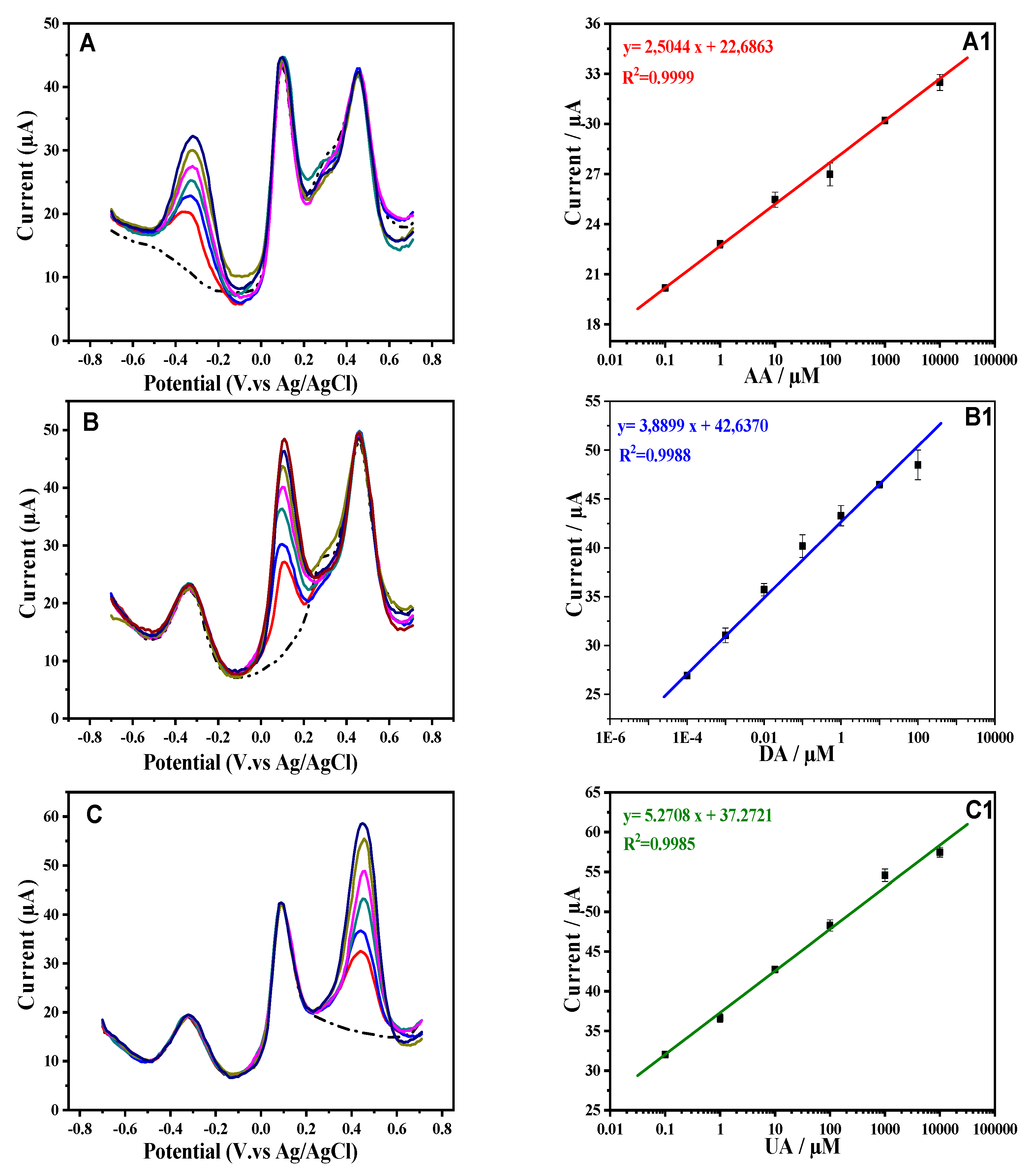
3.3. Electrochemical Activities of AA, DA and UA on Modified Surfaces
3.4. Analytical Performance of Au/CRGO/Fc-ac-PPP Sensor
3.5. Interferences, Stability and Reproducibility
3.6. Determination of DA in Biological Fluids
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lin, L.; Chen, J.; Yao, H.; Chen, Y.; Zheng, Y.; Lin, X. Simultaneous determination of dopamine, ascorbic acid and uric acid at poly (Evans Blue) modified glassy carbon electrode. Bioelectrochemistry 2008, 73, 11–17. [Google Scholar] [CrossRef]
- Huang, J.; Liu, Y.; Hou, H.; You, T. Simultaneous electrochemical determination of dopamine, uric acid and ascorbic acid using palladium nanoparticle-loaded carbon nanofibers modified electrode. Biosens. Bioelectron. 2008, 24, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Miao, Y.; Ji, S.; Tjiu, W.W.; Liu, T. Electrospun Carbon Nanofibers Decorated with Ag−Pt Bimetallic Nanoparticles for Selective Detection of Dopamine. ACS Appl. Mater. Interfaces 2014, 6, 12449–12456. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.R.; Kim, T.H.; Hong, S.H.; Kim, H.J. Direct Detection of Tetrahydrobiopterin (BH4) and Dopamine in Rat Brain Using Liquid Chromatography Coupled Electrospray Tandem Mass Spectrometry. Biochem. Biophys. Res. Commun. 2012, 419, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Habibi, B.; Jahanbakhshi, M.; Azar, M.H.P. Simultaneous Determination of Acetaminophen and Dopamine Using SWCNT Modified Carbon-Ceramic Electrode by Differential Pulse Voltammetry. Electrochim. Acta 2011, 56, 2888–2894. [Google Scholar] [CrossRef]
- Xue, C.; Han, Q.; Wang, Y.; Wu, J.; Wen, T.; Wang, R.; Hong, J.; Zhou, X.; Jiang, H. Amperometric Detection of Dopamine in Human Serum by Electrochemical Sensor Based on Gold Nanoparticles Doped Molecularly Imprinted Polymers. Biosens. Bioelectron. 2013, 49, 199–203. [Google Scholar] [CrossRef]
- Zhu, Z.; Qu, L.; Guo, Y.; Zeng, Y.; Sun, W.; Huang, X. Electrochemical Detection of Dopamine on a Ni/Al Layered Double Hydroxide Modified Carbon Ionic Liquid Electrode. Sens. Actuators B 2010, 151, 146–152. [Google Scholar] [CrossRef]
- Kannan, P.; John, S.A. Determination of nanomolar uric and ascorbic acids using enlarged gold nanoparticles modified electrode. Anal. Biochem. 2009, 386, 65–72. [Google Scholar] [CrossRef]
- Tian, X.; Cheng, C.; Yuan, H.; Du, J.; Xiao, D.; Xie, S.; Choi, M.M.F. Simultaneous determination of l-ascorbic acid, dopamine and uric acid with gold nanoparticles-β-cyclodextrin–graphene-modified electrode by square wave voltammetry. Talanta 2012, 93, 79–85. [Google Scholar] [CrossRef]
- Bilal, S.; Akbar, A.; Shah, A.-H.A. Highly Selective and Reproducible Electrochemical Sensing of Ascorbic Acid Through a Conductive Polymer Coated Electrode. Polymers 2019, 11, 1346. [Google Scholar] [CrossRef] [Green Version]
- Pakapongpan, S.; Mensing, J.P.; Phokharatkul, D.; Lomas, T.; Tuantranont, A. Highly selective electrochemical sensor for ascorbic acid based on a novel hybrid graphene-copper phthalocyanine-polyaniline nanocomposites. Electrochim. Acta 2014, 133, 294–301. [Google Scholar] [CrossRef]
- Ali, M.; Khalid, M.A.U.; Shah, I.; Kim, S.W.; Kim, Y.S.; Lim, J.H.; Choi, K.H. Paper-based selective and quantitative detection of uric acid using citrate-capped Pt nanoparticles (PtNPs) as a colorimetric sensing probe through a simple and remote-based device. New J. Chem. 2019, 43, 7636–7645. [Google Scholar] [CrossRef]
- Fabregat, G.; Armelin, G.; Alemań, C. Selective Detection of Dopamine Combining Multilayers of Conducting Polymers with Gold Nanoparticles. J. Phys. Chem. B 2014, 118, 4669–4682. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Y.C.; Ma, L.X. One-pot facile fabrication of graphene-zinc oxide composite and its enhanced sensitivity for simultaneous electrochemical detection of ascorbic acid, dopamine and uric acid. Sens. Actuators B 2016, 227, 488–496. [Google Scholar] [CrossRef]
- Prasad, B.B.; Jauhari, D.; Tiwari, M.P. A dual-template imprinted polymer-modified carbon ceramic electrode for ultra trace simultaneous analysis of ascorbic acid and dopamine. Biosens. Bioelectron. 2013, 50, 19–27. [Google Scholar] [CrossRef]
- Tang, Y.; Pan, K.; Wang, X.; Liu, C.; Luo, S. Electrochemical synthesis of polyaniline in surface-attached poly(acrylic acid) network, and its application to the electrocatalytic oxidation of ascorbic acid. Microchim. Acta 2010, 168, 231–237. [Google Scholar] [CrossRef]
- Zheng, X.; Zhou, X.; Ji, X.; Lin, X.; Lin, W. Simultaneous determination of ascorbic acid, dopamine and uric acid using poly(4-aminobutyric acid) modified glassy carbon electrode. Sens. Actuators B 2013, 178, 359–365. [Google Scholar] [CrossRef]
- Liu, M.; Chen, Q.; Lai, C.; Zhang, Y.; Deng, J.; Li, H.; Yao, S. A double signal amplification platform for ultrasensitive and simultaneous detection of ascorbic acid, dopamine, uric acid and acetaminophen based on a nanocomposite of ferrocene thiolate stabilized Fe3O4@Au nanoparticles with graphene sheet. Biosens. Bioelectron. 2013, 48, 75–81. [Google Scholar] [CrossRef]
- Miodek, A.; Le, H.Q.A.; Dorizon, H.S.; Youssoufi, H.K. Streptavidin-polypyrrole Film as Platform for BiotinylatedRedox Probe Immobilization for Electrochemical Immunosensor Application. Electroanalysis 2016, 28, 1–10. [Google Scholar] [CrossRef]
- Sun, C.L.; Lee, H.H.; Yang, J.M.; Wu, C.C. The simultaneous electrochemical detection of ascorbic acid, dopamine, and uric acid using graphene/size-selected Pt nanocomposites. Biosens. Bioelectron. 2011, 26, 3450–3455. [Google Scholar] [CrossRef]
- Su, C.-H.; Sun, C.-L.; Liao, Y.-C. Printed Combinatorial Sensors for Simultaneous Detection of Ascorbic Acid, Uric Acid, Dopamine, and Nitrite. ACS Omega 2017, 2, 4245–4252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.-H.; Luo, S.-C. Tuning Surface Charge and Morphology for the Efficient Detection of Dopamine under the Interferences of Uric Acid, Ascorbic Acid, and Protein Adsorption. ACS Appl. Mater. Interfaces 2015, 7, 21931–21938. [Google Scholar] [CrossRef] [PubMed]
- Fei Huang, P.; Wang, L.; Yue Bai, J.; Jing Wang, H.; Qing Zhao, Y.; Di Fan, S. Simultaneous electrochemical detection of dopamine and ascorbic acid at a poly (p-toluene sulfonic acid) modified electrode. Microchim. Acta 2007, 157, 41–47. [Google Scholar] [CrossRef]
- Al-Graiti, W.; Foroughi, J.; Liu, Y.; Chen, J. Hybrid Graphene/Conducting Polymer Strip Sensors for Sensitive and Selective Electrochemical Detection of Serotonin. ACS Omega 2019, 4, 22169. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Ma, Y.; Liu, Y.; Xin, G.; Wang, M.; Zhang, Z.; Liu, Z. Electrochemical sensor based on a three dimensional nanostructured MoS2 nanosphere-PANI/reduced graphene oxide composite for simultaneous detection of ascorbic acid, dopamine, and uric acid. RSC Adv. 2019, 9, 2997–3003. [Google Scholar] [CrossRef] [Green Version]
- Blili, S.; Zaaboub, Z.; Maaref, H.; Said, A.H. Synthesis of a new p-conjugated redox oligomer: Electrochemical and optical investigation. J. Mol. Struct. 2017, 1128, 111–116. [Google Scholar] [CrossRef]
- Bizid, S.; Blili, S.; Mlika, R.; Said, A.H.; Youssoufi, H.K. Direct Electrochemical DNA Sensor based on a new redox oligomer modified with ferrocene and carboxylic acid: Application to the detection of Mycobacterium Tuberculosis mutant strain. Anal. Chim. Acta 2017, 994, 10–18. [Google Scholar] [CrossRef]
- Kühnel, M.; Overgaard, M.H.; Hels, M.C.; Cui, A.; Vosch, T.; Nygård, T.; Li, T.; Laursen, B.W.; Nørgaard, K. High-Quality Reduced Graphene Oxide Electrodes for Sub-Kelvin Studies of Molecular Monolayer Junctions. J. Phys. Chem. C 2018, 122, 25102–25109. [Google Scholar] [CrossRef]
- Bizid, S.; Blili, S.; Mlika, R.; Saida, A.H.; Youssouf, H.K. Direct E-DNA sensor of Mycobacterium tuberculosis mutant strain based on new nanocomposite transducer (Fc-ac-OMPA/MWCNTs). Talanta 2016, 184, 475–483. [Google Scholar] [CrossRef]
- McCreery, R.L. Advanced Carbon Electrode Materials for Molecular Electrochemistry. Chem. Rev. 2008, 108, 2646–2687. [Google Scholar] [CrossRef]
- Randviir, E.P. A cross examination of electron transfer rate constants for carbon screen-printed electrodes using Electrochemical Impedance Spectroscopy and cyclic voltammetry. Electrochim. Acta 2018, 286, 179–186. [Google Scholar] [CrossRef]
- Taleb, M.; Ivanov, R.; Berezne, S.; Kazemi, S.H.; Hussainova, I. Ultra-sensitive voltammetric simultaneous determination of dopamine, uric acid and ascorbic acid based on a graphene-coated alumina electrode. Microchim. Acta 2017, 184, 4603–4610. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, N.; Zhang, L.; Wang, H.; Shi, H.; Liu, Q. Simultaneous voltammetric detection of dopamine, ascorbic acid and uric acid using a poly(2-(N -morpholine)ethane sulfonic acid)/RGO modified electrode. RSC Adv. 2018, 8, 5280–5285. [Google Scholar] [CrossRef] [Green Version]
- Begum, K.; Ahmed, M.S.; Jeon, S. New Approach for Porous Chitosan–Graphene Matrix Preparation through Enhanced Amidation for Synergic Detection of Dopamine and Uric Acid. ACS Omega 2017, 2, 3043–3054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, L.; Zheng, Y.; Wang, A.; Cai, W.; Deng, B.; Zhang, Z. An Electrochemical Sensor Based on Reduced Graphene Oxide and ZnO Nanorods-Modified Glassy Carbon Electrode for Uric Acid Detection. Arab. J. Sci. Eng. 2016, 41, 135–141. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, H.; Liu, Q.; Li, L.; Kong, J. Electrodeposition of Three-Dimensional Network Nanostructure PEDOT/PANI for Simultaneous Voltammetric Detection of Ascorbic Acid, Dopamine and Uric Acid. Anal. Chem. 2020, 5, 1288–1293. [Google Scholar] [CrossRef]
- Li, D.; Liu, M.; Zhan, Y.; Su, Q.; Zhang, Y.; Zhang, D. Electrodeposited poly(3,4-ethylenedioxythiophene) doped with graphene oxide for the simultaneous voltammetric determination of ascorbic acid, dopamine and uric acid. Microchim. Acta 2020, 187, 94–104. [Google Scholar] [CrossRef]
- Krishnan, S.; Tong, L.; Liu, S.; Xing, R. A mesoporous silver-doped TiO2-SnO2 nanocomposite on g-C3N4 nanosheets and decorated with a hierarchical core−shell metal-organic framework for simultaneous voltammetric determination of ascorbic acid, dopamine and uric acid. Microchim. Acta 2020, 187, 82–91. [Google Scholar] [CrossRef]
- Raj, M.; Gupta, P.; Goyal, R.N.; Shim, Y.B. Graphene/conducting polymer nano-composite loaded screen printed carbon sensor for simultaneous determination of dopamine and5-hydroxytryptamine. Sens. Actuators B 2017, 239, 993–1002. [Google Scholar] [CrossRef]






| Modified Electrode | Dynamic Range (µM) | LOD (µM) | EP (mV) | Ref | |||||
|---|---|---|---|---|---|---|---|---|---|
| Analyte | AA | DA | UA | AA | DA | UA | AA-DA | DA-UA | |
| PMES/RGO/GCE | 30−100 | 0.05−100 | 0.1−100 | 0.43 | 0.0062 | 0.056 | - | - | [33] |
| GCE/GS/CS | - | 1−700 | 1−800 | - | 0.14 | 0.17 | - | - | [34] |
| GCE/RGO/ZnO | 50−2350 | 1−70 | 3−330 | 3.77 | 0.33 | 1.08 | 236 | 132 | [35] |
| GCE/PEDOT/PANI | 10−4−102 | 30−1000 | 0.7−100 | 24.2 | 4.58 | 2.23 | 216 | 320 | [36] |
| GO/PEDOT/GCE | 100−1000 | 6.0−200 | 40−240 | 20 | 2 | 10 | 97 | 129 | [37] |
| ITO/g-C3N4/NC@GC/h-ATS | 0.1−200 | 2.5−100 | 2.5−500 | 0.02 | 0.01 | 0.06 | 236 | 204 | [38] |
| GONRs/PETDOT/PSS | 250−1500* | 0.5−800 | 0.5−1200 | 250 | 0.5 | 0.5 | 220.5 | 115.1 | [21] |
| *0.05−16.55 | 0.05−16.55 | 0.05−1655 | 0.041 | 0.030 | 0.011 | ||||
| Au/CRGO/Fc-ac-PPP | 0.1−104 | 10−4−103 | 0.1−104 | 0.046 | 2.8×10−5 | 0.013 | 445.7 | 359.30 | This work |
| *0.1−104 | 10−4−103 | 0.1-103 | 0.022 | 1.2×10−5 | 0.012 | This work | |||
| Sample | Analyte | Added (µM) | Found (µM) | Recovery % | RSD % |
|---|---|---|---|---|---|
| Urine1 | AA | 1000 | 1010 | 101 | 0.4 |
| DA | 10 | 11.1 | 111 | 2.1 | |
| UA | 1000 | 1020 | 102 | 0.5 | |
| Urine2 | AA | 100 | 85 | 85 | 0.9 |
| DA | 0.1 | 0.1 | 100 | 1.5 | |
| UA | 100 | 100 | 100 | 0.9 | |
| Urine3 | AA | 10 | 10.5 | 105 | 0.4 |
| DA | 0.01 | 0.0097 | 97 | 3.4 | |
| UA | 10 | 10.5 | 105 | 0.2 | |
| Urine4 | AA | 0.1 | 0.095 | 95 | 2.4 |
| DA | 0.001 | 0.001 | 100 | 2.7 | |
| UA | 0.1 | 0.11 | 110 | 0.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsine, Z.; Bizid, S.; Mlika, R.; Sauriat-Dorizon, H.; Haj Said, A.; Korri-Youssoufi, H. Nanocomposite Based on Poly (para-phenylene)/Chemical Reduced Graphene Oxide as a Platform for Simultaneous Detection of Ascorbic Acid, Dopamine and Uric Acid. Sensors 2020, 20, 1256. https://doi.org/10.3390/s20051256
Hsine Z, Bizid S, Mlika R, Sauriat-Dorizon H, Haj Said A, Korri-Youssoufi H. Nanocomposite Based on Poly (para-phenylene)/Chemical Reduced Graphene Oxide as a Platform for Simultaneous Detection of Ascorbic Acid, Dopamine and Uric Acid. Sensors. 2020; 20(5):1256. https://doi.org/10.3390/s20051256
Chicago/Turabian StyleHsine, Zouhour, Salma Bizid, Rym Mlika, Hélène Sauriat-Dorizon, Ayoub Haj Said, and Hafsa Korri-Youssoufi. 2020. "Nanocomposite Based on Poly (para-phenylene)/Chemical Reduced Graphene Oxide as a Platform for Simultaneous Detection of Ascorbic Acid, Dopamine and Uric Acid" Sensors 20, no. 5: 1256. https://doi.org/10.3390/s20051256