The Role of 8-Amidoquinoline Derivatives as Fluorescent Probes for Zinc Ion Determination
Abstract
:1. Introduction
2. Significance, Development, and Challenges in the Detection of Zinc Ions with Various Analytical Methods
3. Zn2+ Fluorophore Based Sensors for the Analysis of Zn Ions
| Article/Year | Analyte | Binding Mode | Sensing Mechanism | Fluorescence Signals | Detection Limits |
|---|---|---|---|---|---|
| [44]/2015 | Zn2+ | Lower rim amide linked 8-amino quinoline acts as a receptor molecule, and 8-amino naphthalene moiety 1,3,5-triderivatives of calix[6]arene acts as a control molecule | Absorption and Electrospray Ionization (ESI) MS Spectra | 390 and 490 nm | - |
| [45]/2016 | CEA | Carcinoembryonic antigen aptamer (5′-nh2-ataccagcttattcaatt-3′) conjugated to hexanedioic acid (hda) modified ucps (hda-ucps) by edc-nhs coupling method. | Fluorescence Resonance Energy Transfer (FRET) | - | 0.8 pg/mL |
| [46]/2015 | Zn2+ and Cu2+ | Asymmetrical Diarylethene As Photoswitchable Core and Amidoquinoline As A Multi-Responsive Group Via A Piperazine Linkage | Fluorescence Chemosensor | 417 nm to 502 nm | - |
| [47]/2018 | Zn2+ | N’-(quinolin-8-ylmethylene)benzohydrazide group as the binding unit and perfluorodiarylethene as a photoswitching trigger | Fluorescence Sensor | 412 nm | 3.2 × 10−8 mol L−1 |
| [48]/2011 | Zn2+ and Cu2+ | Amide tautomerization | Fluorescence Sensor | 492 to 430 nm | 0.14 and 0.86 μm |
| [49]/2014 | Zn2+ | 4-amino-1,8-naphthalimide-pet, with iminodiacetic acid as a chelating metal group | PET-Fluorescent Sensor | 470 nm | - |
| [50]/2013 | Zn2+ and Cu2+ | 2-((benzylimino)- methyl)-naphthalen-1-ol | PET-Fluorescence Sensor | 300 nm, 370 nm | 0.35 and 0.82 µm |
| [51]/2017 | Al3+ | Coumarin-Derived Chemosensor with 2-Hydroxy-4-Methylbenzohydrazide and Acetylcoumarin | Fluorescence Chemosensor | 490 nm | 6.7 μm |
4. Quinoline and Derivatives for Zn2+ Fluorophores
Aminoquinoline as a Fluorophore for Zinc’s Recognition
5. 8-Amidoquinoline Derivatives as Zn Ions Recognition Probes and Their Properties
5.1. Binding Studies of 8-Amidoquinoline Probes via Solution Studies
5.2. Detection Limits for Zn2+ Ions and Possible Interferences
5.3. The Reliability of Zn Fluorophores for Zn Ion Determinations
5.4. Possible Mechanisms and Interactions Involved 8-Amidoquinoline Derivatives upon Binding with Zn2+
5.5. Another Recent Studies Related to 8-Amidoquinoline Derivatives as Fluorophores for Zinc’s Detection
5.6. Limitations of and the Future of Quinoline and Derivatives Fluorophore for Zn Ion Analysis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Basabe-Desmonts, L.; Müller, T.J.J.; Crego-Calama, M. Design of fluorescent materials for chemical sensing. Chem. Soc. Rev. 2007, 36, 993–1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janata, J. Introduction to Sensors. In Principles of Chemical Sensors; Springer: Boston, MA, USA, 2009; pp. 1–11. [Google Scholar]
- Compagnone, D.; Di Francia, G.; Di Natale, C.; Neri, G.; Seeber, R.; Tajani, A. Chemical sensors and biosensors in Italy: A review of the 2015 literature. Sensors 2017, 17, 868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, Z.; Le, X.; Zhou, P.; Dong, C.; Ma, J. An “off-on-off” fluorescent probe for the sequential detection of Zn2+ and hydrogen sulfide in aqueous solution. New J. Chem. 2014, 38, 1802–1808. [Google Scholar] [CrossRef]
- Kwon, N.; Hu, Y.; Yoon, J. Fluorescent chemosensors for various analytes including reactive oxygen species, biothiol, metal ions, and toxic gases. ACS Omega 2018, 3, 13731–13751. [Google Scholar] [CrossRef]
- Cuajungco, M.P.; Lees, G.J. Zinc metabolism in the brain: Relevance to human neurodegenerative disorders. Neurobiol. Dis. 1997, 4, 137–169. [Google Scholar] [CrossRef] [Green Version]
- Küry, S.; Dréno, B.; Bézieau, S.; Giraudet, S.; Kharfi, M.; Kamoun, R.; Moisan, J.P. Identification of SLC39A4, a gene involved in acrodermatitis enteropathica. Nat. Genet. 2002, 31, 239–240. [Google Scholar] [CrossRef]
- Koh, J.Y.; Suh, S.W.; Gwag, B.J.; He, Y.Y.; Hsu, C.Y.; Choi, D.W. The role of zinc in selective neuronal death after transient global cerebral ischemia. Science 1996, 272, 1013–1016. [Google Scholar] [CrossRef]
- Chowdhury, S.; Rooj, B.; Dutta, A.; Mandal, U. Review on Recent Advances in Metal Ions Sensing Using Different Fluorescent Probes. J. Fluoresc. 2018, 28, 999–1021. [Google Scholar] [CrossRef]
- Carpenter, M.C.; Lo, M.N.; Palmer, A.E. Techniques for measuring cellular zinc. Arch. Biochem. Biophys. 2016, 611, 20–29. [Google Scholar] [CrossRef] [Green Version]
- Moynier, F.; Le Borgne, M. High precision zinc isotopic measurements applied to mouse organs. J. Vis. Exp. 2015, e52479. [Google Scholar] [CrossRef] [Green Version]
- Frederickson, C.J.; Koh, J.Y.; Bush, A.I. The neurobiology of zinc in health and disease. Nat. Rev. Neurosci. 2005, 6, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Urgast, D.S.; Hill, S.; Kwun, I.S.; Beattie, J.H.; Goenaga-Infante, H.; Feldmann, J. Zinc isotope ratio imaging of rat brain thin sections from stable isotope tracer studies by LA-MC-ICP-MS. Metallomics 2012, 4, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Hare, D.; Burger, F.; Austin, C.; Fryer, F.; Grimm, R.; Reedy, B.; Scolyer, R.A.; Thompson, J.F.; Doble, P. Elemental bio-imaging of melanoma in lymph node biopsies. Analyst 2009, 134, 450–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pornwilard, M.M.; Ralf, W.; Nikolaus, G.; Anja, K.B.; Sabine Becker, J. Novel bioimaging techniques of metals by laser ablation inductively coupled plasma mass spectrometry for diagnosis of fibrotic and cirrhotic liver disorders. PLoS ONE 2013, 8, e58702. [Google Scholar] [CrossRef]
- Ondrasek, G.; Clode, P.L.; Kilburn, M.R.; Guagliardo, P.; Romić, D.; Rengel, Z. Zinc and cadmium mapping in the apical shoot and hypocotyl tissues of radish by high-resolution secondary ion mass spectrometry (NanoSIMS) after short-term exposure to metal contamination. Int. J. Environ. Res. Public Health 2019, 16, 373. [Google Scholar] [CrossRef] [Green Version]
- Migeon, A.; Audinot, J.; Eybe, T.; Richaud, P.; Damien, B.; Migeon, H.; Chalot, M. Cadmium and zinc localization by SIMS in leaves of Populus deltoides (cv. Lena) grown in a metal polluted soil. Surf. Interface Anal. 2011, 367–369. [Google Scholar] [CrossRef]
- McRae, R.; Lai, B.; Fahrni, C.J. Subcellular redistribution and mitotic inheritance of transition metals in proliferating mouse fibroblast cells. Metallomics 2013, 5, 52–61. [Google Scholar] [CrossRef] [Green Version]
- Que, E.L.; Bleher, R.; Duncan, F.E.; Kong, B.Y.; Gleber, S.C.; Vogt, S.; Chen, S.; Garwin, S.A.; Bayer, A.R.; Dravid, V.P.; et al. Quantitative mapping of zinc fluxes in the mammalian egg reveals the origin of fertilization-induced zinc sparks. Nat. Chem. 2014, 7, 130–139. [Google Scholar] [CrossRef]
- Kim, A.M.; Bernhardt, M.L.; Kong, B.Y.; Ahn, R.W.; Vogt, S.; Woodru, T.K.; Halloran, T.V.O. Zinc sparks are triggered by fertilization and facilitate cell cycle resumption in mammalian eggs. ACS Chem. Biol. 2011, 6, 716–723. [Google Scholar] [CrossRef]
- Bozym, R.A.; Thompson, R.B.; Fierke, C.A. Importance of measuring free zinc in cells. Rev. Fluoresc. 2006, 399–419. [Google Scholar] [CrossRef]
- Sivaraman, G.; Iniya, M.; Anand, T.; Kotla, N.G.; Sunnapu, O.; Singaravadivel, S.; Gulyani, A.; Chellappa, D. Chemically diverse small molecule fluorescent chemosensors for copper ion. Coord. Chem. Rev. 2018, 357, 50–104. [Google Scholar] [CrossRef]
- Jung, J.M.; Yun, D.; Lee, H.; Kim, K.T.; Kim, C. Selective chemosensor capable of sensing both CN− and Zn2+: Its application to zebrafish. Sens. Actuators B-Chem. 2019, 297, 126814. [Google Scholar] [CrossRef]
- Chen, L.; Wu, D.; Yoon, J. Recent Advances in the Development of Chromophore-Based Chemosensors for Nerve Agents and Phosgene. ACS Sens. 2018, 3, 27–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mako, T.L.; Racicot, J.M.; Levine, M. Supramolecular Luminescent Sensors. Chem. Rev. 2018, 119, 322–477. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Zhu, W.H.; Xie, Y. Development of Ion Chemosensors Based on Porphyrin Analogues. Chem. Rev. 2017, 117, 2203–2256. [Google Scholar] [CrossRef]
- Li, Z.; Yi, Y.; Luo, X.; Xiong, N.; Liu, Y.; Li, S.; Sun, R.; Wang, Y.; Hu, B.; Chen, W.; et al. Development and Clinical Application of A Rapid IgM-IgG Combined Antibody Test for SARS-CoV-2 Infection Diagnosis. J. Med. Virol. 2020. [Google Scholar] [CrossRef]
- Wu, D.; Sedgwick, A.C.; Gunnlaugsson, T.; Akkaya, E.U.; Yoon, J.; James, T.D. Fluorescent chemosensors: The past, present, and future. Chem. Soc. Rev. 2017, 46, 7105–7123. [Google Scholar] [CrossRef] [Green Version]
- Ganesabaskaran, S.; Kandasamy, K. Chemosensors for Hg Ions: A Review of Literature. Biosens. J. 2015. [Google Scholar] [CrossRef]
- Okutan, E.; Tümay, S.O.; Yeşilot, S. Colorimetric Fluorescent Sensors for Hemoglobin Based on BODIPY Dyes. J. Fluoresc. 2016, 26, 2333–2343. [Google Scholar] [CrossRef]
- Yang, J.; Yuan, Z.; Yu, G.; He, S.; Hu, Q.; Wu, Q.; Jiang, B.; Wei, G. Single Chemosensor for Double Analytes: Spectrophotometric Sensing of Cu2+ and Fluorogenic Sensing of Al3+ Under Aqueous Conditions. J. Fluoresc. 2016, 26, 43–51. [Google Scholar] [CrossRef]
- Obare, S.O.; De, C.; Guo, W.; Haywood, T.L.; Samuels, T.A.; Adams, C.P.; Masika, N.O.; Murray, D.H.; Anderson, G.A.; Campbell, K.; et al. Fluorescent Chemosensors for Toxic Organophosphorus Pesticides: A Review. Sensors 2010, 10, 7018–7043. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, X.; Zhang, C.; Wu, Y.; Liu, Z. Coumarin-hydrazone based high selective fluorescence sensor for copper (II) detection in aqueous solution. Inorg. Chem. Commun. 2013, 34, 8–11. [Google Scholar] [CrossRef]
- Hariharan, P.S.; Anthony, S.P. Substitutional group dependent colori/fluorimetric sensing of Mn2+, Fe3+ and Zn2+ ions by simple Schiff base chemosensor. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 136, 1658–1665. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.H.; Sun, Y.; Qi, J.; Pei, P.X.; Lin, Q.; Zhang, Y.M. A colorimetric and “turn-on” fluorimetric chemosensor for the selective detection of cyanide and its application in food samples. RSC Adv. 2016, 6, 100401–100406. [Google Scholar] [CrossRef]
- Kundu, A.; Hariharan, P.S.; Prabakaran, K.; Anthony, S.P. Synthesis of new colori/fluorimetric chemosensor for selective sensing of biologically important Fe3+, Cu2+, and Zn2+ metal ions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 151, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Shu, Q.; Zhu, J.; Jin, S.; Li, N.; Chen, X.; Chen, S. A new multifunctional Schiff-based chemosensor for mask-free fluorimetric and colorimetric sensing of F− and CN−. Talanta 2016, 152, 39–44. [Google Scholar] [CrossRef]
- Yin, P.; Niu, Q.; Wei, T.; Li, T.; Li, Y.; Yang, Q. A new thiophene-based dual functional chemosensor for ultrasensitive colorimetric detection of Cu2+ in aqueous solution and highly selective fluorimetric detection of Al3+ in living cells. J. Photochem. Photobiol. A Chem. 2020, 389, 112249. [Google Scholar] [CrossRef]
- Haldar, U.; Lee, H.-L. BODIPY-derived multi-channel polymeric chemosensor with pH-tunable sensitivity: Selective colorimetric and fluorimetric detection of Hg2+ and HSO4− in aqueous media. Polym. Chem. 2018, 9, 4882–4890. [Google Scholar] [CrossRef]
- Xu, Z.; Yoon, J.; Spring, D.R. Fluorescent chemosensors for Zn2+. Chem. Soc. Rev. 2010, 39, 1996–2006. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Fan, R.; Chen, W.; Wang, P.; Yang, Y. A simple quinolone Schiff-base containing CHEF based fluorescence “turn-on” chemosensor for distinguishing Zn2+ and Hg2+ with high sensitivity, selectivity, and reversibility. Dalt. Trans. 2017, 46, 6769–6775. [Google Scholar] [CrossRef]
- Boonkitpatarakul, K.; Smata, A.; Kongnukool, K.; Srisurichan, S.; Chainok, K.; Sukwattanasinitt, M. An 8-aminoquinoline derivative as a molecular platform for fluorescent sensors for Zn(II) and Cd(II) ions. J. Lumin. 2018, 198, 59–67. [Google Scholar] [CrossRef]
- Czaplinska, B.; Spaczynska, E.; Musiol, R. Quinoline fluorescent probes for zinc—From diagnostic to therapeutic molecules in treating neurodegenerative diseases. Med. Chem. 2018, 14, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Mummidivarapu, V.V.S.; Bandaru, S.; Yarramala, D.S.; Samanta, K.; Mhatre, D.S.; Rao, C.P. Binding and ratiometric dual ion recognition of Zn2+ and Cu2+ by 1,3,5-tris-amidoquinoline conjugate of calix[6]arene by spectroscopy and its supramolecular features by microscopy. Anal. Chem. 2015, 87, 4988–4995. [Google Scholar] [CrossRef] [PubMed]
- Vongnam, K.; Muangnoi, C.; Rojsitthisak, P.; Sukwattanasinitt, M.; Rashatasakhon, P. A highly selective turn-on fluorescent sensor for glucosamine from amidoquinoline-napthalimide dyads. Biosens. Bioelectron. 2016, 86, 472–476. [Google Scholar] [CrossRef]
- Xia, S.; Liu, G.; Pu, S. A highly selective fluorescence sensor for Zn2+ and Cu2+ based on diarylethene with a piperazine-linked amidoquinoline unit. J. Mater. Chem. C 2015, 3, 4023–4029. [Google Scholar] [CrossRef]
- Wang, R.; Wang, N.; Tu, Y.; Liu, G.; Pu, S. A new fluorescence sensor based on diarylethene with a N’-(quinolin-8-ylmethylene)benzohydrazide group for Zn2+ detection. J. Photochem. Photobiol. A Chem. 2018, 364, 32–39. [Google Scholar] [CrossRef]
- Jiang, J.; Jiang, H.; Tang, X.; Yang, L.; Dou, W.; Liu, W.; Fang, R.; Liu, W. An efficient sensor for Zn2+ and Cu2+ based on different binding modes. Dalt. Trans. 2011, 40, 6367–6370. [Google Scholar] [CrossRef]
- Liu, D.Y.; Qi, J.; Liu, X.Y.; He, H.R.; Chen, J.T.; Yang, G.M. 4-Amino-1,8-naphthalimide-based fluorescent sensor with high selectivity and sensitivity for Zn2+ imaging in living cells. Inorg. Chem. Commun. 2014, 43, 173–178. [Google Scholar] [CrossRef]
- Ganguly, A.; Ghosh, S.; Kar, S.; Guchhait, N. Selective fluorescence sensing of Cu(II) and Zn(II) using a simple Schiff base ligand: Naked eye detection and elucidation of photoinduced electron transfer (PET) mechanism. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 143, 72–80. [Google Scholar] [CrossRef]
- Hossain, S.M.; Singh, K.; Lakma, A.; Pradhan, R.N.; Singh, A.K. A Schiff base ligand of coumarin derivative as an ICT-Based fluorescence chemosensor for Al3+. Sens. Actuators B Chem. 2017, 239, 1109–1117. [Google Scholar] [CrossRef]
- Sutariya, P.G.; Modi, N.R.; Pandya, A.; Joshi, B.K.; Joshi, K.V.; Menon, S.K. An ICT based “turn on/off” quinoline armed calix[4]arene fluoroionophore: Its sensing efficiency towards fluoride from wastewater and Zn2+ from blood serum. Analyst 2012, 137, 5491–5494. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Duan, D.; Cui, X.; Sun, J.; Fang, J. A selective and sensitive fluorescence probe for imaging endogenous zinc in living cells. Tetrahedron Lett. 2012, 69, 15–21. [Google Scholar] [CrossRef]
- Xu, Z.; Baek, K.H.; Kim, H.N.; Cui, J.; Qian, X.; Spring, D.R.; Shin, I.; Yoon, J. Zn2+-triggered amide tautomerization produces a highly Zn2+-selective, cell-permeable, and ratiometric fluorescent sensor. J. Am. Chem. Soc. 2010, 132, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.Y.; Zhao, K.; Song, J.; Wang, C.K. Responsive mechanism and molecular design of di-2-picolylamine-based two-photon fluorescent probes for zinc ions. Chin. Phys. B 2018, 27, 023302. [Google Scholar] [CrossRef]
- Alreja, P.; Saini, D.; Gautam, S.S.; Navneet; Kaur, N. Novel 1,10-phenanthroline—Di-2-picolylamine scaffold as a selective chemosensor for copper and cyanide ions. Inorg. Chem. Commun. 2016, 70, 125–128. [Google Scholar] [CrossRef]
- Shao, J. Quinoline-based “on-off” fluorescent sensor for acetate: Effect of link mode between binding sites and fluorophore on fluorescence changes. Spectrosc. Lett. 2012, 45, 262–268. [Google Scholar] [CrossRef]
- Kanagaraj, K.; Xiao, C.; Rao, M.; Fan, C.; Borovkov, V.; Cheng, G.; Zhou, D.; Zhong, Z.; Su, D.; Yu, X.; et al. A Quinoline-Appended Cyclodextrin Derivative as a Highly Selective Receptor and Colorimetric Probe for Nucleotides. IScience 2020, 23, 100927. [Google Scholar] [CrossRef] [Green Version]
- Dorazco-González, A.; Alamo, M.F.; Godoy-Alcántar, C.; Höpfl, H.; Yatsimirsky, A.K. Fluorescent anion sensing by bisquinolinium pyridine-2,6-dicarboxamide receptors in water. RSC Adv. 2014, 4, 455–466. [Google Scholar] [CrossRef]
- Mehta, P.K.; Oh, E.T.; Park, H.J.; Lee, K.H. Ratiometric fluorescent probe based on symmetric peptidyl receptor with picomolar affinity for Zn2+ in aqueous solution. Sens. Actuators B Chem. 2017, 245, 996–1003. [Google Scholar] [CrossRef]
- Shabunina, O.V.; Kapustina, D.Y.; Krinochkin, A.P.; Kim, G.A.; Kopchuk, D.S.; Zyryanov, G.V.; Li, F.; Chupakhin, O.N. π-Extended fluorophores based on 5-aryl-2,2’-bipyridines: Synthesis and photophysical studies. Mendeleev Commun. 2017, 27, 602–604. [Google Scholar] [CrossRef]
- Moseev, T.D.; Varaksin, M.V.; Gorlov, D.A.; Nikiforov, E.A.; Kopchuk, D.S.; Starnovskaya, E.S.; Khasanov, A.F.; Zyryanov, G.V.; Charushin, V.N.; Chupakhin, O.N. Direct C[sbnd]H/C[sbnd]Li coupling of 1,2,4-triazines with C6F5Li followed by aza-Diels-Alder reaction as a pot, atom, and step economy (PASE) approach towards novel fluorinated 2,2′-bipyridine fluorophores. J. Fluor. Chem. 2019, 224, 89–99. [Google Scholar] [CrossRef]
- Bencini, A.; Lippolis, V. Probing biologically and environmentally important metal ions with fluorescent chemosensors: Thermodynamic versus optical response selectivity in some study cases. Coord. Chem. Rev. 2012, 256, 149–169. [Google Scholar] [CrossRef]
- Volkova, Y.A.; Brizet, B.; Harvey, P.D.; Averin, A.D.; Goze, C.; Denat, F. BODIPY Dyes Functionalized with Pendant Cyclic and Acyclic Polyamines. Eur. J. Org. Chem. 2013, 2013, 4270–4279. [Google Scholar] [CrossRef]
- Seto, D.; Maki, T.; Soh, N.; Nakano, K.; Ishimatsu, R.; Imato, T. A simple and selective fluorometric assay for dopamine using a calcein blue-Fe2+ complex fluorophore. Talanta 2012, 94, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Takahira, I.; Fuchida, H.; Tabata, S.; Shindo, N.; Uchinomiya, S.; Hamachi, I.; Ojida, A. Design of a binuclear Ni(II)-iminodiacetic acid (IDA) complex for selective recognition and covalent labeling of His-tag fused proteins. Bioorg. Med. Chem. Lett. 2014, 24, 2855–2858. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ding, H.; Zhu, L.; Wang, Y.; Chen, Z.; Tian, Z. A indole-trizole-rhodamine triad as ratiometric fluorescent probe for nanomolar-concentration level Hg2+ sensing with high selectivity. J. Fluoresc. 2015, 25, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Cui, W.; Zhou, S.; Ma, F. The binding behavior of itraconazole with hemoglobin: Studies from multi-spectroscopic techniques. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2014, 131, 407–412. [Google Scholar] [CrossRef]
- Gumus, A.; Gumus, S.; Yuzuncu, V. Synthesis of Novel Triazole-Linked Schiff Base Derivative. Technol. Eng. Math. 2019, 7, 87–92. [Google Scholar]
- Kang, L.; Liu, Y.T.; Li, N.N.; Dang, Q.X.; Xing, Z.Y.; Li, J.L.; Zhang, Y. A Schiff-base receptor based naphthalimide derivative: Highly selective and colorimetric fluorescent turn-on sensor for Al3+. J. Lumin. 2017, 186, 48–52. [Google Scholar] [CrossRef]
- Gan, W. Synthesis and Design of Fluorescence Ligands to Act as Sensor for Zinc. Master’s Thesis, University of North Carolina Wilmington, Wilmington, NC, USA, 2009. [Google Scholar]
- Tharmaraj, V.; Pitchumani, K. A highly selective ratiometric fluorescent chemosensor for Cu(II) based on dansyl-functionalized thiol stabilized silver nanoparticles. J. Mater. Chem. B 2013, 1, 1962–1967. [Google Scholar] [CrossRef]
- You, Q.H.; Chan, P.S.; Chan, W.H.; Hau, S.C.K.; Lee, A.W.M.; Mak, N.K.; Mak, T.C.W.; Wong, R.N.S. A quinolinyl antipyrine based fluorescence sensor for Zn2+ and its application in bioimaging. RSC Adv. 2012, 2, 11078–11083. [Google Scholar] [CrossRef]
- Urano, Y.; Kamiya, M.; Kanda, K.; Ueno, T.; Hirose, K.; Nagano, T. Evolution of fluorescein as a platform for finely tunable fluorescence probes. J. Am. Chem. Soc. 2005, 127, 4888–4894. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Jain, A.K.; Maheshwari, G. A new Zn2+-selective potentiometric sensor based on dithizone—PVC membrane. Chem. Anal. 2006, 51, 889–898. [Google Scholar]
- Shamsipur, M.; Yousefi, M.; Hosseini, M.; Ganjali, M.R.; Sharghi, H.; Naeimi, H. A Schiff base complex of Zn(II) as a neutral carrier for highly selective PVC membrane sensors for the sulfate ion. Anal. Chem. 2001, 73, 2869–2874. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.W.; Jensen, K.B.; Jensen, M.S.; Silva, D.S.; Kesslak, P.J.; Danscher, G.; Frederickson, C.J. Histochemically-reactive zinc in amyloid plaques, angiopathy, and degenerating neurons of Alzheimer’s diseased brains. Brain Res. 2000, 852, 274–278. [Google Scholar] [CrossRef]
- Meeusen, J.W.; Tomasiewicz, H.; Nowakowski, A.; Petering, D.H. TSQ (6-methoxy-8-p-toluenesulfonamido-quinoline), a common fluorescent sensor for cellular zinc, images zinc proteins. Inorg. Chem. 2011, 50, 7563–7573. [Google Scholar] [CrossRef] [Green Version]
- Kimura, E.; Aoki, S. Chemistry of zinc(II) fluorophore sensors. Zinc Biochem. Physiol. Homeost. 2001, 2, 5–18. [Google Scholar] [CrossRef]
- Li, J.; Yin, C.; Huo, F. Development of fluorescent zinc chemosensors based on various fluorophores and their applications in zinc recognition. Dye. Pigment. 2016. [Google Scholar] [CrossRef]
- Mikata, Y.; Wakamatsu, M.; Kawamura, A.; Yamanaka, N.; Yano, S.; Odani, A.; Morihiro, K.; Tamotsu, S. Methoxy-Substituted TQEN Family of Fluorescent Zinc Sensors. Inorg. Chem. 2006, 45, 3889–3895. [Google Scholar] [CrossRef]
- Dai, Z. Steric and Stereochemical Modulation in Pyridyl- and Quinolyl-Containing Ligands. Molecules 2016, 21, 1647. [Google Scholar] [CrossRef] [Green Version]
- Mikata, Y.; Yamanaka, A.; Yamashita, A.; Yano, S. Isoquinoline-Based TQEN Family as TPEN-Derived Fluorescent Zinc Sensors. Inorg. Chem. 2008, 47, 7295–7301. [Google Scholar] [CrossRef] [PubMed]
- Mikata, Y.; Takeuchi, S.; Konno, H.; Iwatsuki, S.; Akaji, S.; Hamagami, I.; Aoyama, M.; Yasuda, K.; Tamotsu, S.; Burdette, S.C. Bis(2-quinolylmethyl)ethylenediaminediacetic acids (BQENDAs), TQEN-EDTA hybrid molecules as fluorescent zinc sensors. Dalt. Trans. 2014, 43, 10013–10022. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gan, Q.; Wang, X.; Xue, L.; Liu, S. A Water-Soluble, Small Molecular Fluorescent Sensor with Femtomolar Sensitivity for Zinc Ion. Org. Lett. 2007, 9, 4995–4998. [Google Scholar] [CrossRef] [PubMed]
- Burdette, S.C.; Walkup, G.K.; Spingler, B.; Tsien, R.Y.; Lippard, S.J. Fluorescent Sensors for Zn2+ Based on a Fluorescein Platform: Synthesis, Properties, and Intracellular Distribution. J. Am. Chem. Soc. 2001, 123, 7831–7841. [Google Scholar] [CrossRef] [Green Version]
- Burdette, S.C.; Frederickson, C.J.; Bu, W.; Lippard, S.J. ZP4, an Improved Neuronal Zn2+ Sensor of the Zinpyr Family. J. Am. Chem. Soc. 2003, 195, 1778–1787. [Google Scholar] [CrossRef]
- Royzen, M.; Durandin, A.; Young, V.G.; Geacintov, N.E.; Canary, J.W. A Sensitive Probe for the Detection of Zn(II) by Time-Resolved Fluorescence. J. Am. Chem. Soc. 2006, 128, 3854–3855. [Google Scholar] [CrossRef]
- Royzen, M.; Canary, J.W. Structural parameters of Zn(II) complexes of 8-hydroxyquinoline-based tripodal ligands affect fluorescence quantum yield. Polyhedron 2013, 58, 85–91. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, N.; Chen, Y.; Wang, L. Fluorescence Sensing and Binding Behavior of Aminobenzenesulfonamido- quinolino-βcyclodextrin to Zn2+. Org. Lett. 2007, 9, 315–318. [Google Scholar] [CrossRef]
- Yang, R.; Wang, K.; Xiao, D.; Yang, X. Fluorometric study of the inclusion interaction of b-cyclodextrin derivatives with tetraphenylporphyrin and its analytical application. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2001, 57, 1595–1602. [Google Scholar] [CrossRef]
- Yu, M.R.; Gao, T.; Sun, H. Synthesis and Characterization of Pyrene/Quinoline Based Zn2+ Selective Fluorescent Sensor. J. Toxicol. Environ. 2010, 2, 158–161. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, X.; Jia, L.; Xu, S.; Xu, Z.; Zheng, L.; Qian, X. Substituent-dependent fluorescent sensors for zinc ions based on carboxamidoquinoline. Dalton Trans. 2012, 41, 11776–11782. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Chen, L.; Lin, J.; Liu, Q.; Ding, J.; Gao, X.; Guo, Z. Novel zinc fluorescent probe bearing dansyl and aminoquinoline groups. Chem. Commun. 2002, 13, 1424–1425. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guo, X.; Si, W.; Jia, L.; Qian, X. Ratiometric and water-soluble fluorescent zinc sensor of carboxamidoquinoline with an alkoxyethylamino chain as receptor. Org. Lett. 2008, 10, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Shi, Y.; Hou, F.; Liu, H.; Huang, L.; Xi, P.; Chen, F.; Zeng, Z. A highly selective fluorescent and colorimetric chemosensor for Zn II and its application in cell imaging. Eur. J. Inorg. Chem. 2012, 327–332. [Google Scholar] [CrossRef]
- Lee, H.G.; Lee, J.H.; Jang, S.P.; Hwang, I.H.; Kim, S.J.; Kim, Y.; Kim, C.; Harrison, R.G. Zinc selective chemosensors based on the flexible dipicolylamine and quinoline. Inorganica Chim. Acta 2013, 394, 542–551. [Google Scholar] [CrossRef]
- Xue, L.; Liu, C.; Jiang, H. Highly sensitive and selective fluorescent sensor for distinguishing cadmium from zinc ions in aqueous media. Org. Lett. 2009, 11, 1655–1658. [Google Scholar] [CrossRef]
- Du, J.; Fan, J.; Peng, X.; Li, H.; Sun, S. The quinoline derivative of ratiometric and sensitive fluorescent zinc probe based on deprotonation. Sens. Actuators B Chem. 2010, 144, 337–341. [Google Scholar] [CrossRef]
- Ma, Q.J.; Zhang, X.B.; Han, Z.X.; Huang, B.; Jiang, Q.; Shen, G.L.; Yu, R.Q. A ratiometric fluorescent probe for zinc ions based on the quinoline fluorophore. Int. J. Environ. Anal. Chem. 2011, 91, 74–86. [Google Scholar] [CrossRef]
- Xie, G.; Xi, P.; Wang, X.; Zhao, X.; Huang, L.; Chen, F.; Wu, Y.; Yao, X.; Zeng, Z. A highly zinc(II) -selective fluorescent sensor based on 8-aminoquinoline and its application in biological imaging. Eur. J. Inorg. Chem. 2011, 19, 2927–2931. [Google Scholar] [CrossRef]
- Zhu, J.; Yuan, H.; Chan, W.; Lee, A.W.M. A colorimetric and fluorescent turn-on chemosensor operative in aqueous media for Zn2+ based on a multi functionalized spirobenzopyran derivative. Org. Biomol. Chem. 2010, 17, 3957–3964. [Google Scholar] [CrossRef] [Green Version]
- Goswami, S.; Sen, D.; Das, K.; Fun, H. A new rhodamine based colorimetric ‘off—on’ fluorescence sensor selective for Pd2+ along with the first bound X-ray crystal structure. Chem. Comm. 2011, 47, 9101–9103. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Dong, Z.; Ren, J.; Jin, J.; Wang, P.; Jiang, J.; Li, R.; Ma, J. A quinoline group modified SBA-15 inhibit logic gate with [Cu2+ and Zn2+] or [Cu2+ and Cd2+] as inputs. Micropor Mesopor Mat. 2010, 135, 170–177. [Google Scholar] [CrossRef]
- He, C.; Zhu, W.; Xu, Y.; Zhong, Y.; Zhou, J.; Qian, X. Ratiometric and reusable fluorescent nanoparticles for Zn2+ and H2PO4. A detection in aqueous solution and living cells. J. Mater. Chem. 2010, 20, 10755–10764. [Google Scholar] [CrossRef]
- Pal, P.; Rastogi, S.K.; Gibson, C.M.; Aston, D.E.; Branen, A.L.; Bitterwolf, T.E. Fluorescence Sensing of Zinc (II) Using Ordered Mesoporous Silica Material (MCM-41) Functionalized with N—(Quinolin-8-yl)-2-[3-(triethoxysilyl) propylamino] acetamide. ACS Appl. Mater. Interfaces 2011, 3, 279–286. [Google Scholar] [CrossRef]
- Zhang, L.; Cui, X.; Sun, J.; Wang, Y.; Li, W.; Fang, J. 8-Aminoquinoline-based ratiometric zinc probe: Unexpected binding mode and its application in living cells. Bioorg. Med. Chem. Lett. 2013, 23, 3511–3514. [Google Scholar] [CrossRef]
- Fu, H.; Liu, H.; Zhao, L.; Xiao, B.; Fan, T.; Jiang, Y. A quinoline-based selective ‘turn on’ chemosensor for zinc(II) via quad-core complex, and its application in live cell imaging. Tetrahedron 2019, 75, 130710. [Google Scholar] [CrossRef]
- Chen, X.; Ma, Y.; Chen, H.; Wang, F.; Kambam, S.; Wang, Y.; Mao, C. A highly sensitive and selective ratiometric fluorescent sensor for Zn2+ ion based on ICT and FRET. Dye. Pigment. 2014, 102, 301–307. [Google Scholar] [CrossRef]
- Song, H.; Zhang, Z. A quinoline-based ratiometric fluorescent probe for discriminative detection of Zn2+ and Cd2+ with different binding modes, and its Zn2+ complex for relay sensing of pyrophosphate and adenosine triphosphate. Dye. Pigment. 2019, 165, 172–181. [Google Scholar] [CrossRef]
- Li, Q.F.; Wang, J.T.; Wu, S.; Ge, G.W.; Huang, J.; Wang, Z.; Yang, P.; Lin, J. A water-soluble fluorescent chemosensor having a high affinity and sensitivity for Zn2+ and its biological application. Sens. Actuators B Chem. 2018, 259, 484–491. [Google Scholar] [CrossRef]
- Yue, Y.; Dong, Q.; Zhang, Y.; Sun, Y.; Gong, Y. A highly selective “turn-on” fluorescent chemosensor based on 8-aminoquinoline for detection of Zn2+. Anal. Methods 2015, 7, 5661–5666. [Google Scholar] [CrossRef]
- Liu, Z.; Li, G.; Ma, Q.; Liu, L.; Su, X. A near-infrared turn-on fluorescent nanosensor for zinc(II) based on CuInS2 quantum dots modified with 8-aminoquinoline. Microchim. Acta 2014, 181, 1385–1391. [Google Scholar] [CrossRef]
- Rastogi, S.K.; Pal, P.; Aston, D.E.; Bitterwolf, T.E.; Branen, A.L. 8-aminoquinoline functionalized silica nanoparticles: A fluorescent nanosensor for detection of divalent zinc in aqueous and in yeast cell suspension. ACS Appl. Mater. Interfaces 2011, 3, 1731–1739. [Google Scholar] [CrossRef] [PubMed]
- Rurack, K. Flipping the light switch ‘ON’—the design of sensor molecules that show cation-induced fluorescence enhancement with heavy and transition metal ions. Spectrochim. Acta Part A 2001, 57, 2161–2195. [Google Scholar] [CrossRef]
- Atalay, Y.B.; Di Toro, D.M.; Carbonaro, R.F. Estimation of stability constants for metal-ligand complexes containing neutral nitrogen donor atoms with applications to natural organic matter. Geochim. Cosmochim. Acta 2013, 122, 464–477. [Google Scholar] [CrossRef]
- Ghorai, P.; Pal, K.; Karmakar, P.; Saha, A. The development of two fluorescent chemosensors for the selective detection of Zn2+ and Al3+ ions in a quinoline platform by tuning the substituents in the receptor part: Elucidation of the structures of the metal-bound chemosensors and biological studies. Dalton Trans. 2020, 49, 4758–4773. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Nguyen, M.; Liu, Y.; Robert, A.; Meunier, B. Synthesis and characterization of 8-aminoquinolines, substituted by electron-donating groups, as high-affinity copper chelators for the treatment of Alzheimer’s disease. Comptes Rendus Chim. 2019, 22, 419–427. [Google Scholar] [CrossRef]
- Haroon, I.; Abdelrahman, A.; Elbadawi, M.A.S. Optical and Electrical Properties of Eggshell. In Proceedings of the 6th Annual Conference of the Graduate College, Alneelain University, Khartuam, Sudan, 21 December 2014; pp. 1–16. [Google Scholar]
- Hirose, K. Determination of Binding Constants. Anal. Methods Supramol. Chem. 2007, 2. [Google Scholar] [CrossRef]
- Yingchen, B.A.I.; Fengchang, W.U.; Guojiang, W.A.N.; Congqiang, L.I.U.; Pingqing, F.U.; Wen, L.I. Ultraviolet absorbance titration for the determination of conditional stability constants of Hg (II) and dissolved organic matter. Chin. J. Geochem. 2008, 27, 46–52. [Google Scholar] [CrossRef]
- Hang, L.Z.; Na, L.I.; Hao, F.Z.; Ke, L.I. Spectroscopic Study on the Interaction between Methylene Blue and Chondroitin 4-Sulfate and Its Analytical Application. Anal. Sci. 2004, 20, 445–450. [Google Scholar]
- Haq, I.; Lincoln, P.; Suh, D.; Norden, B.; Chowdhry, B.Z.; Chaires, J.B. Interaction of. delta.-and. lambda.-[Ru (phen) 2DPPZ] 2+ with DNA: A calorimetric and equilibrium binding study. J. Am. Chem. Soc. 1995, 17, 4788–4796. [Google Scholar] [CrossRef]
- Tamayo, A.; Lodeiro, C.; Escriche, L.; Casabo, J.; Covelo, B.; Gonza, P. New Fluorescence PET Systems Based on N 2 S 2 Pyridine-Anthracene-Containing Macrocyclic Ligands. Spectrophotometric, Spectrofluorimetric, and Metal Ion Binding Studies. Inorg. Chem. 2005, 44, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, C.; Otting, G. NMR studies of ligand binding. Curr. Opin. Struct. Biol. 2018, 48, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Ba´nyai, I. Dynamic NMR for coordination chemistry. New J. Chem. 2018, 42, 7569–7581. [Google Scholar] [CrossRef]
- Loock, H.P.; Wentzell, P.D. Detection limits of chemical sensors: Applications and misapplications. Sens. Actuators B Chem. 2012, 173, 157–163. [Google Scholar] [CrossRef] [Green Version]
- Massarini, E.; Wästerby, P.; Landström, L.; Lejon, C.; Beck, O.; Andersson, P.O. Methodologies for assessment of limit of detection and limit of identification using surface-enhanced Raman spectroscopy. Sens Actuators B. Chem. 2015, 207, 437–446. [Google Scholar] [CrossRef]
- Allegrini, F.; Olivieri, A.C. IUPAC-consistent approach to the limit of detection in partial least-squares calibration. Anal. Chem. 2014, 86, 7858–7866. [Google Scholar] [CrossRef]
- Thanh, N.M.; Van Hop, N.; Luyen, N.D.; Phong, N.H.; Toan, T.T.T.; Mai, H.D. Simultaneous Determination of Zn(II), Cd(II), Pb(II), and Cu(II) Using Differential Pulse Anodic Stripping Voltammetry at a Bismuth Film-Modified Electrode. Adv. Mater. Sci. Eng. 2019, 2019. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.L.; Li, S.P.; Wang, Y.; Wu, W.N.; Zhao, X.L.; Li, H.J.; Xu, Z.H. Quinoline-based hydrazone for colorimetric detection of Co2+ and fluorescence turn-on response of Zn2+. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 230, 118025. [Google Scholar] [CrossRef]
- Chang, L.L.; Gao, Q.; Liu, S.; Hu, C.C.; Zhou, W.J.; Zheng, M.M. Selective and differential detection of Hg2+ and Cu2+ with use of a single rhodamine hydrazone-type probe in the absence and presence of UV irradiation. Dye. Pigment. 2018, 153, 117–124. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Br. Med. J. 2009, 339, 332–336. [Google Scholar] [CrossRef] [Green Version]
- Breaux, J.; Jones, K.; Boulas, P. Analytical methods development and validation. Pharm. Technol. 2003, 1, 6–13. [Google Scholar]
- Peris-Vicente, J.; Esteve-Romero, J.; Carda-Broch, S. Validation of Analytical Methods Based on Chromatographic Techniques: An Overview. Anal. Sep. Sci. 2015, 1757–1808. [Google Scholar] [CrossRef]
- Ogundele, O.M.; Adekeye, A.O.; Adeniyi, P.A.; Ogedengbe, O.O.; Enye, L.A.; Saheed, S.; Omotosho, D.R.; Anglais, A.E. Basic Principles of Fluorescence Microscopy. World J. Pharm. Med. Res. 2013, 2, 17–22. [Google Scholar]
- Croneya, J.C.; Jamesona, D.M.; Learmonthb, R.P. Fluorescence spectroscopy in biochemistry: Teaching basic principles with visual demonstrations. Biochem. Mol. Biol. Educ. 2001, 29, 60–65. [Google Scholar] [CrossRef]
- Sun, Z.; Li, H.; Guo, D.; Sun, J.; Cui, G.; Liu, Y.; Tian, Y.S.Y. Multifunctional magnetic core-shell fibrous silica sensing probe for highly sensitive detection and removal of Zn2+ in aqueous solution. J. Mater. Chem. 2015, 18, 4713–4722. [Google Scholar] [CrossRef]
- Fu, Y.; Fan, C.; Liu, G.; Cui, S.; Pu, S. A highly selective and sensitive ratiometric fluorescent chemosensor for Zn2+ based on diarylethene with a benzyl-linked 8-aminoquinoline-2-aminomethylpyridine unit. Dye. Pigment. 2016, 126, 121–130. [Google Scholar] [CrossRef]
- Zhang, X.; Li, H.; Liu, G.; Pu, S. A novel diarylethene-based fluorescent switch with a carboxamidoquinoline unit for sensing of Zn(II) ion. J. Photochem. Photobiol. A 2016, 330, 22–29. [Google Scholar] [CrossRef]
- Brahma, R.; Baruah, J.B. Self-assemblies of zinc complexes for aggregation-induced emission luminogen precursors. ACS Omega 2020, 5, 3774–3785. [Google Scholar] [CrossRef] [Green Version]
- Park, G.J.; Kim, H.; Lee, J.J.; Kim, Y.S.; Lee, S.Y.; Lee, S.; Noh, I.; Kim, C. A highly selective turn-on chemosensor capable of monitoring Zn2+ concentrations in living cells and aqueous solution. Sens. Actuators B Chem. 2015, 215, 568–576. [Google Scholar] [CrossRef]
- Du, K.; Niu, S.; Chen, X.; Zhang, P. A novel highly selective ratiometric fluorescent sensor for relay recognition of Zn2+ and H2PO4−. Tetrahedron Lett. 2017, 59, 356–360. [Google Scholar] [CrossRef]
- Kumar, S.S.; Kumar, R.S.; Kumar, S.A. An “Off—On—Off” type fluorescent chemosensor for the relay detection of Zn2+ and H2PO4− in aqueous environment. Inorg. Chim. Acta 2020, 502, 119348. [Google Scholar] [CrossRef]
- Ojida, A.; Mito-oka, Y.; Sada, K.; Hamachi, I. Molecular recognition and fluorescence sensing of monophosphorylated peptides in aqueous solution by bis (zinc(II)—Dipicolylamine) -based artificial receptors. J. Am. Chem. Soc. 2004, 126, 2454–2463. [Google Scholar] [CrossRef] [PubMed]
- Gale, P.A.; Gale, P.A. Amidopyrroles: From anion receptors to membrane transport agents. Chem. Commun. 2005, 30, 3761–3772. [Google Scholar] [CrossRef] [PubMed]
- Vongnam, K.; Aree, T.; Sukwattanasinitt, M. Aminoquinoline-salicylaldimine dyads as highly selective turn-on fluorescent sensors for zinc(II) ions. ChemistrySelect 2018, 3, 3495–3499. [Google Scholar] [CrossRef]

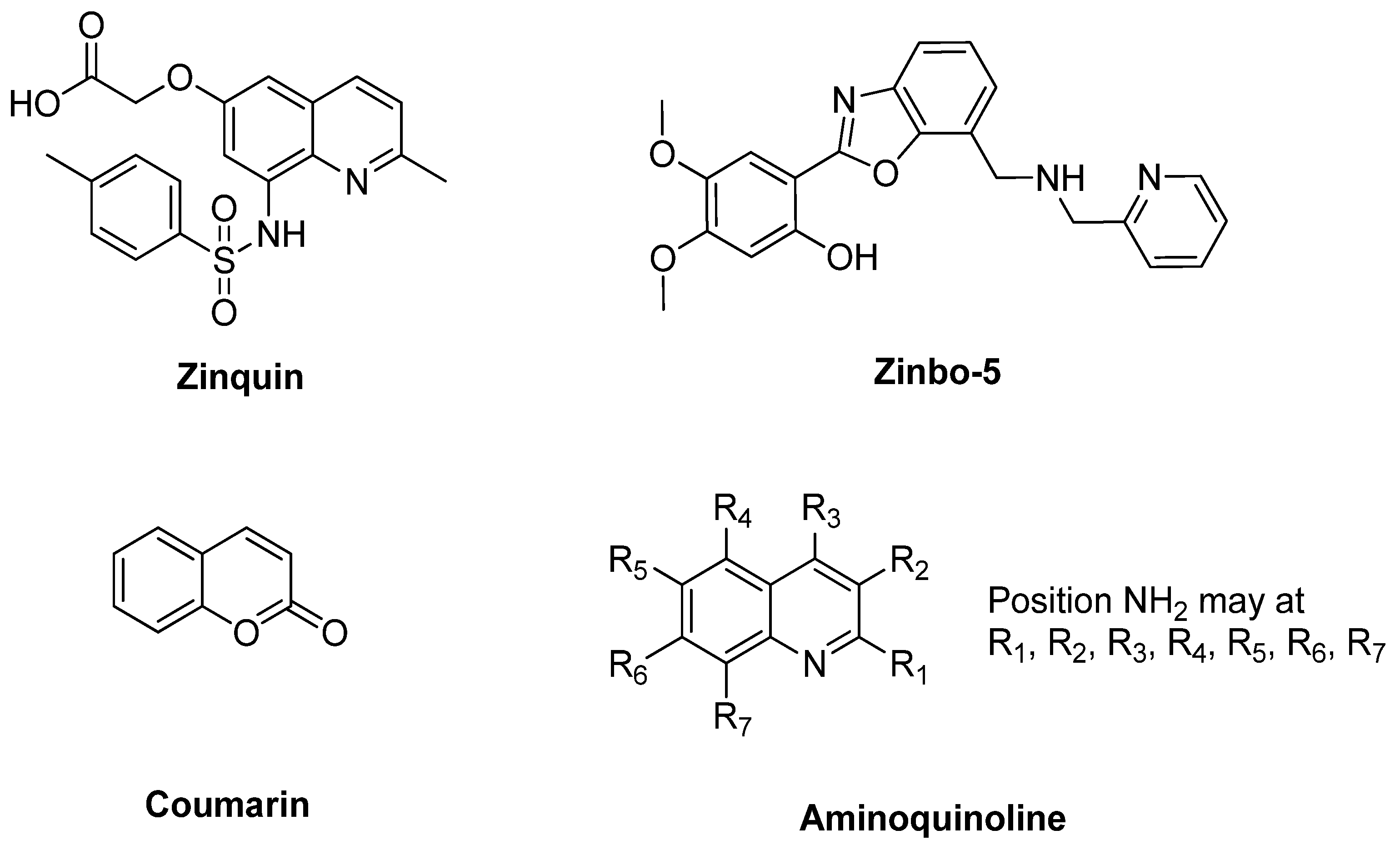




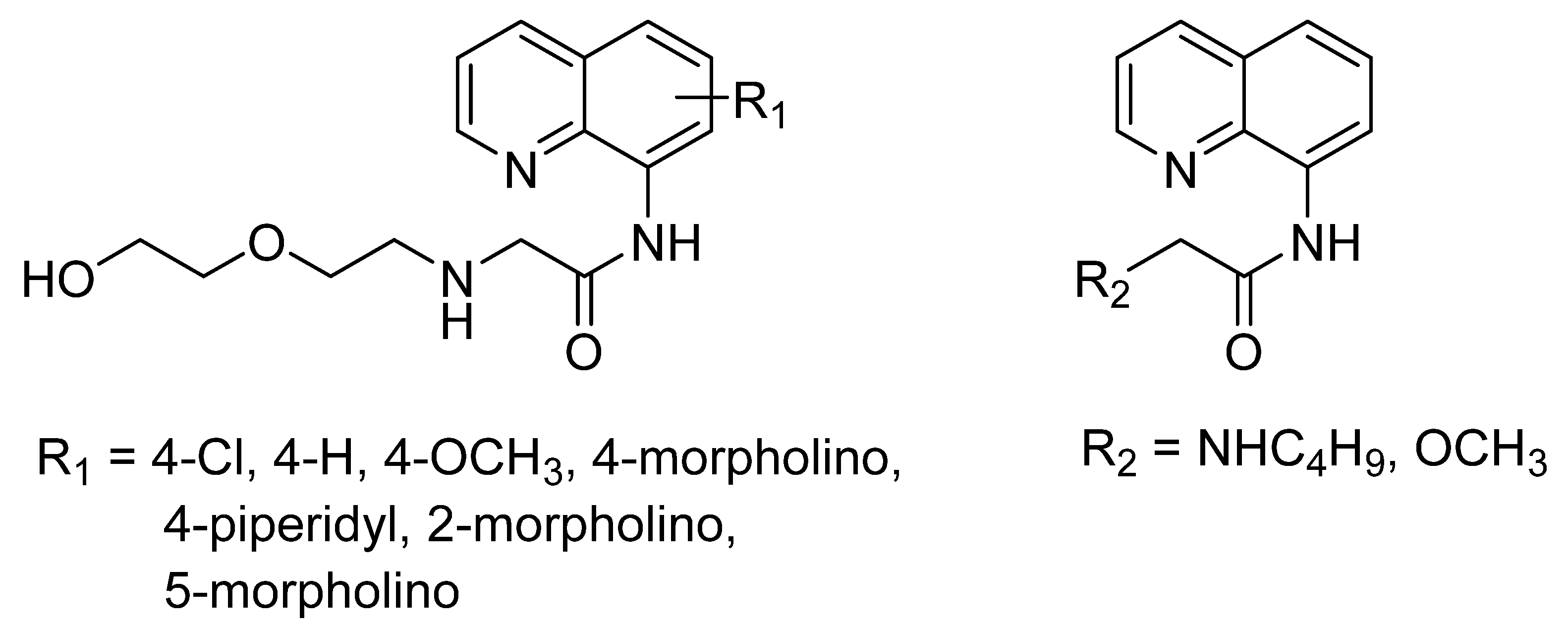





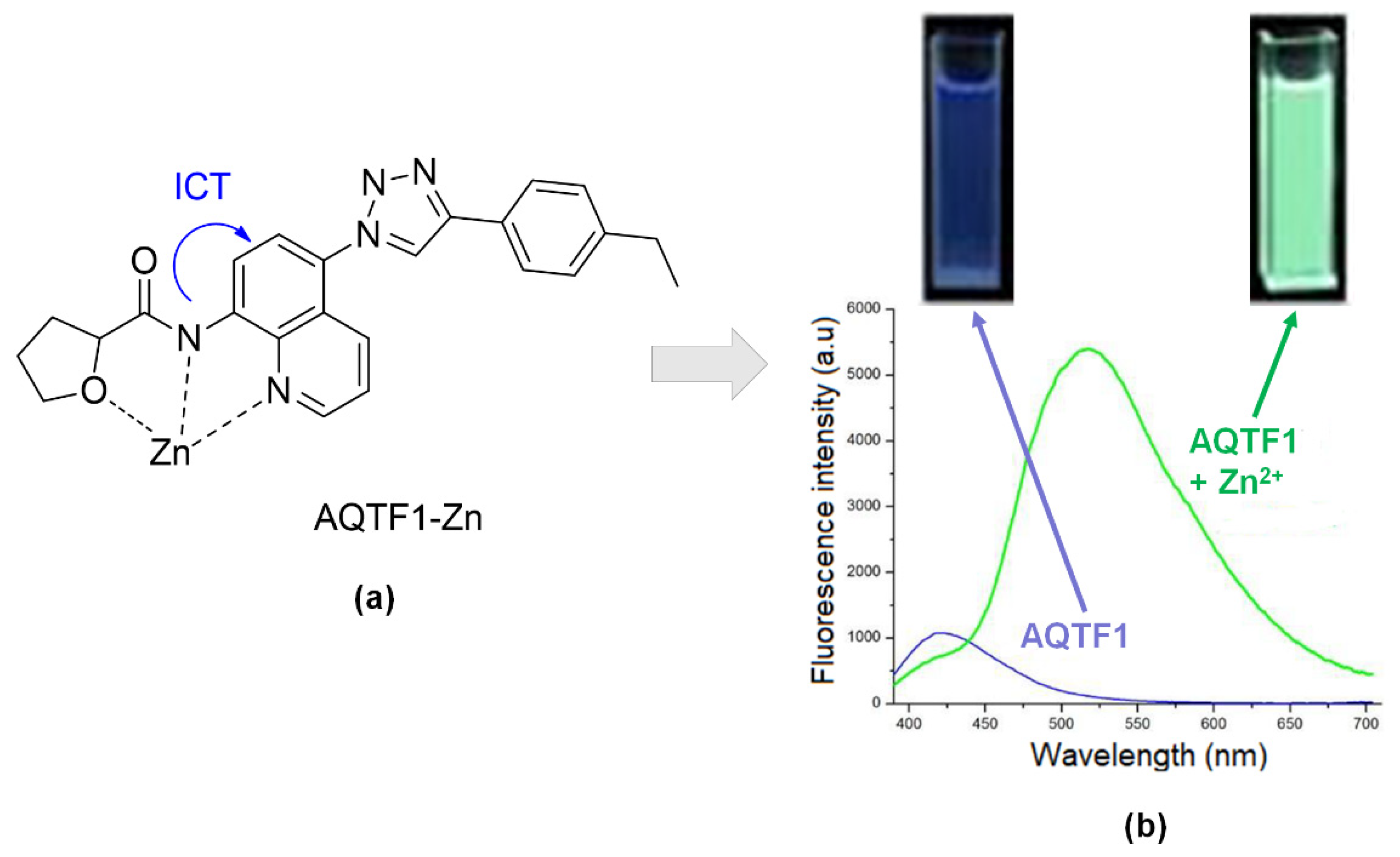


| Articles | Year | University/Countries | Probe | Binding Mode (Probe: Zn) | Interferences | Detection Limits | Real Sample |
|---|---|---|---|---|---|---|---|
| [42] | 2018 | Thailand | 1 | 1:1 | Ni2+, Cu2+, Co2+ | 160 × 10−8 M | Cabbage |
| [73] | 2012 | The Chinese University of Hong Kong, China | QPA | 1:1 | Cd2+, Fe2+, Cu2+ | 13 × 10−8 M | HK-1 cells |
| [96] | 2012 | Lanzhou University, China | L1 | - | Cd2+, Ni2+, Cu2+, Hg2+ | 100 × 10−8 M | Human bladder cancer |
| [97] | 2013 | Korea | QP | - | Cu2+, Fe2+, and Co2+ | - | - |
| [53] | 2012 | Lanzhou University, China | Probe 1 | 1:1 | Hg2+, Cu2+, Cr3+, Ag2+ | 8.14 × 10−8 M | HELA cells |
| [107] | 2013 | Lanzhou University, China | PMQA | 2:1 | Cd2+, Co2+, Cu2+ | 8.85 × 10−8 M | HELA cells |
| [108] | 2019 | Shenzhen University, China | QLNPY | - | Cu2+ | 3.8 × 10−8 M | HepG2 cells |
| [109] | 2014 | Nanjing Normal University, China | QA | 1: 1 | Hg2+, Cu2+ | 3.36 × 10−8 M | Intracellular cells |
| [110] | 2019 | Xi’an Jiaotong University, China | Probe 1 | 1:1 | Cr3+ | 6.3 × 10−8 M | Tapwater |
| [111] | 2018 | Zhoukou University, China | AQZ-2COOH | - | Cu2+ | 10.2 × 10−8 M | HeLa cells |
| [112] | 2013 | Xinxiang University, China | HAQT | 1: 1 | Ni2+, Cu2+ | 25.6 × 10−8 M | River water |
| [113] | 2014 | Jilin University, China | CuInS2 QDs/8-aminoquinoline | 1:1 | Pb2+, Hg2+ | 445 × 10−8 M | Tap water |
| [114] | 2011 | United States | QTEPA-SiNPs | - | Fe2+, Cu2+, | 10 × 10−8 M | Yeast cells |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamad, N.S.; Zakaria, N.H.; Daud, N.; Tan, L.L.; Ta, G.C.; Heng, L.Y.; Hassan, N.I. The Role of 8-Amidoquinoline Derivatives as Fluorescent Probes for Zinc Ion Determination. Sensors 2021, 21, 311. https://doi.org/10.3390/s21010311
Mohamad NS, Zakaria NH, Daud N, Tan LL, Ta GC, Heng LY, Hassan NI. The Role of 8-Amidoquinoline Derivatives as Fluorescent Probes for Zinc Ion Determination. Sensors. 2021; 21(1):311. https://doi.org/10.3390/s21010311
Chicago/Turabian StyleMohamad, Nur Syamimi, Nur Hanis Zakaria, Nurulhaidah Daud, Ling Ling Tan, Goh Choo Ta, Lee Yook Heng, and Nurul Izzaty Hassan. 2021. "The Role of 8-Amidoquinoline Derivatives as Fluorescent Probes for Zinc Ion Determination" Sensors 21, no. 1: 311. https://doi.org/10.3390/s21010311







