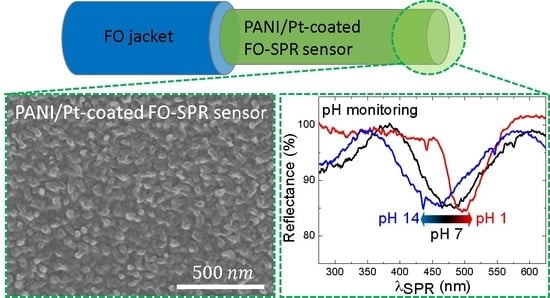
Sensitive pH Monitoring Using a Polyaniline-Functionalized Fiber Optic—Surface Plasmon Resonance Detector
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. FO-SPR Sensing Device
2.3. FO-SPR RI Measurements
2.4. pH Monitoring Using the PANI/Pt-Coated FO-SPR Sensors
2.5. Observations of the FO-SPR Surfaces
3. Results and Discussion
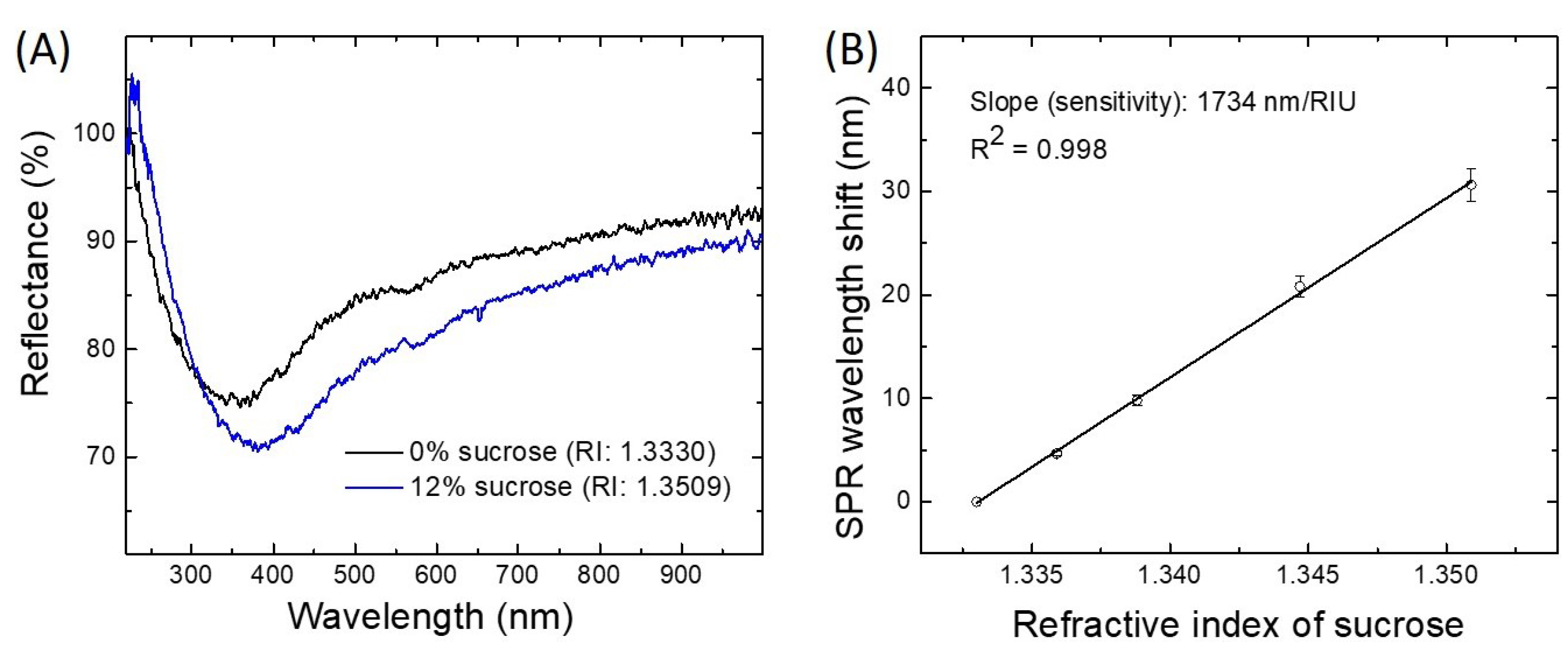
3.1. Platinum-Coated FO-SPR Sensor Bulk Sensitivity
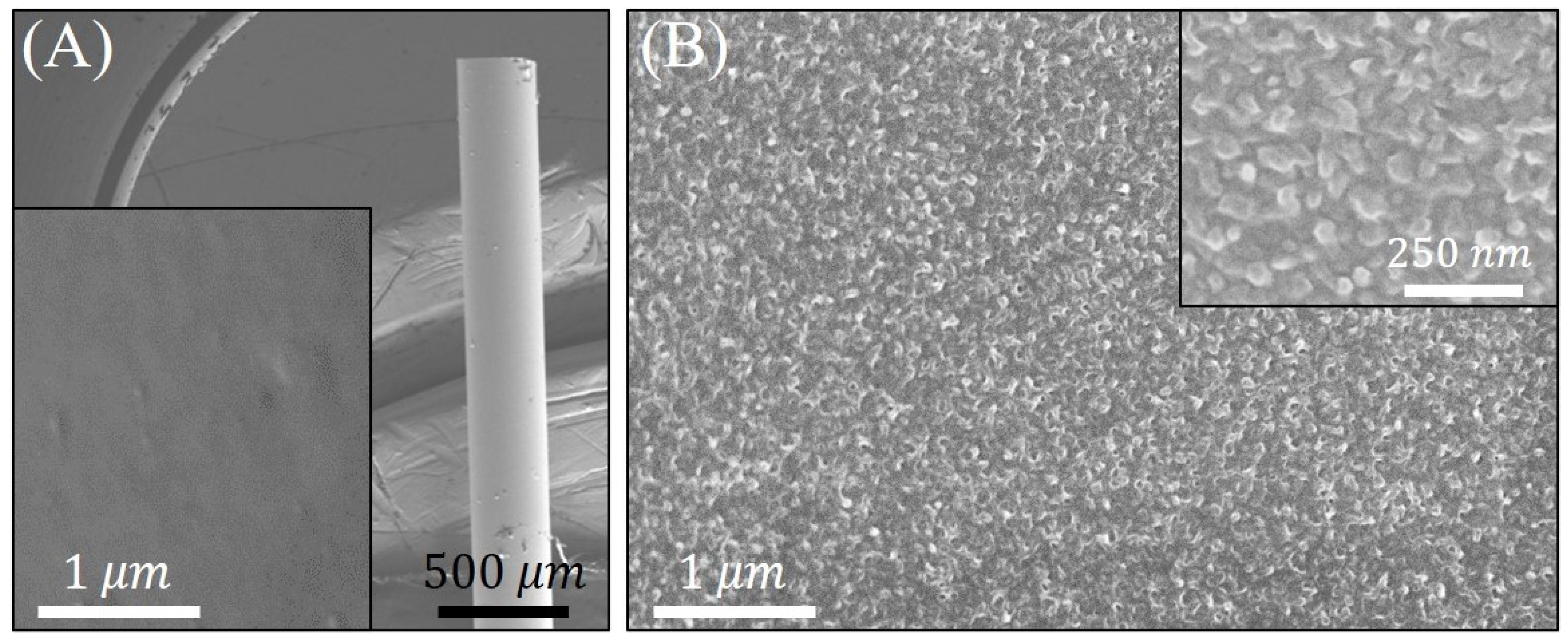
3.2. Morpho-Structural Characterization of PANI/Pt-Coated FO-SPR Sensor’ Surfaces
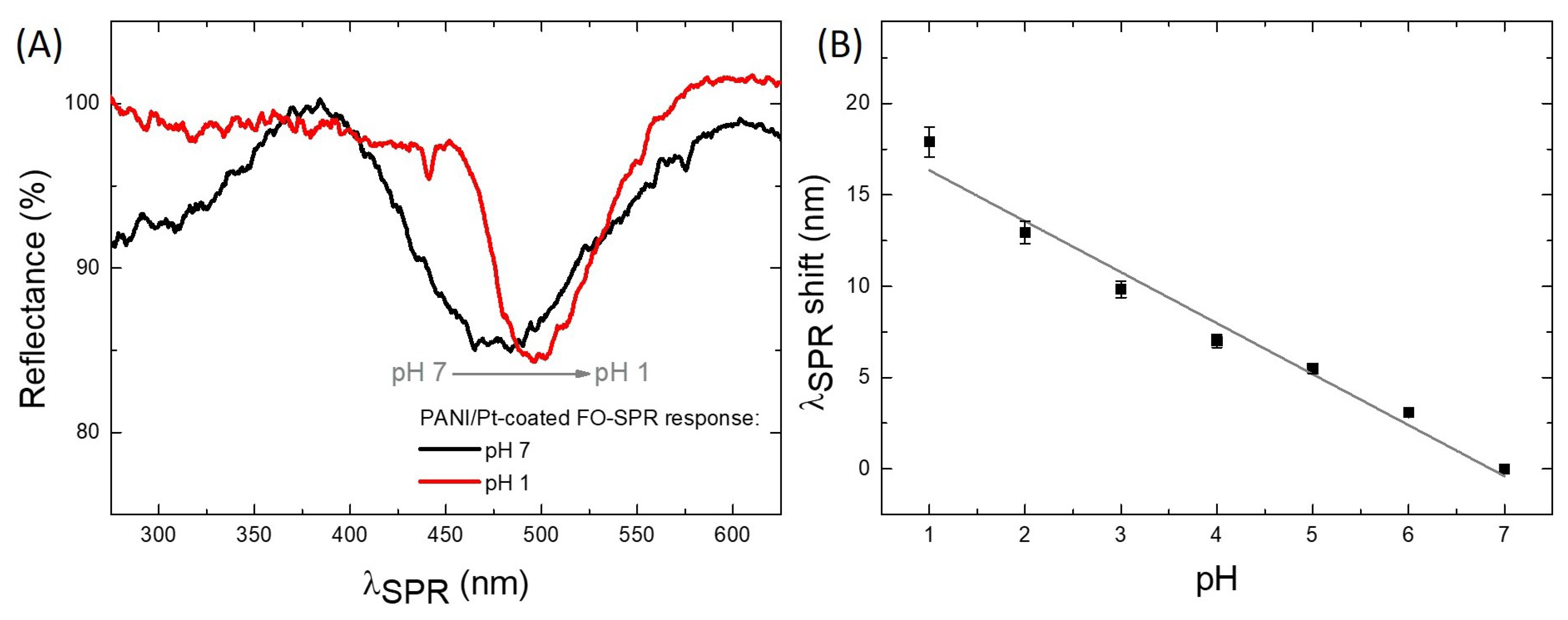
3.3. pH Measurements Using PANI/Pt-Coated FO-SPR Sensors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| R&D | Research & Development |
| MDEO | R&D Center for Materials and Electronic & Optoelectronic Devices |
| IMCN | Institute of Condensed Matter and Nanosciences |
| UCLouvain | Université Catholique de Louvain |
| INFLPR | National Institute for Laser, Plasma and Radiation Physics |
| FO-SPR | Fiber Optic—Surface Plasmon Resonance |
| Pt, Au, Ag, Al | Platinum, Gold, Silver, Aluminum |
| ITO | Indium-doped tin oxide |
| PANI | Polyaniline |
| XPS | X-ray Photoelectron Spectroscopy |
| FE-SEM | Field Emission—Scanning Electron Microscope |
| DIW | Deionized water |
| RI(U) | Refractive index (units) |
| S, FOM | Sensitivity, Figure of Merit |
| FWHM | Full width at half-maximum |
| UEFISCDI | Executive Unit for Financing Higher Education, Research, |
| Development and Innovation | |
| BSMA | Bio- and Soft Matter Research Division |
References
- Chaudhari, A.L.; Shaligram, A.D. Development of fiber optic pH meter based on colorimetric principle. Indian J. Pure Appl. Phys. 2002, 40, 132–136. [Google Scholar]
- Wencel, D.; Abel, T.; McDonagh, C. Optical Chemical pH Sensors. Anal. Chem. 2014, 86, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Mukherjee, K.; Shoukat, R.; Dong, H. A review on pH sensitive materials for sensors and detection methods. Microsyst. Technol. 2017, 23, 4391–4404. [Google Scholar] [CrossRef]
- Gotor, R.; Ashokkumar, P.; Hecht, M.; Keil, K.; Rurack, K. Optical pH Sensor Covering the Range from pH 0–14 Compatible with Mobile-Device Readout and Based on a Set of Rationally Designed Indicator Dyes. Anal. Chem. 2017, 89, 8437–8444. [Google Scholar] [CrossRef] [Green Version]
- Manjakkal, L.; Szwagierczak, D.; Dahiya, R. Metal oxides based electrochemical pH sensors: Current progress and future perspectives. Prog. Mater. Sci. 2020, 109, 100635. [Google Scholar] [CrossRef]
- Podrazký, O.; Peterka, P.; Kaăík, I.; Vytykáčová, S.; Proboštová, J.; Mrázek, J.; Kuneš, M.; Závalová, V.; Radochová, V.; Lyutakov, O.; et al. In vivo testing of a bioresorbable phosphate-based optical fiber. J. Biophotonics 2019, 12, e201800397. [Google Scholar] [CrossRef]
- Aldaba, A.L.; González-Vila, A.; Debliquy, M.; Lopez-Amo, M.; Caucheteur, C.; Lahem, D. Polyaniline-coated tilted fiber Bragg gratings for pH sensing. Sens. Actuators B Chem. 2018, 254, 1087–1093. [Google Scholar] [CrossRef]
- Semwal, V.; Gupta, B.D. SPR based fiber optic pH sensor using polyaniline as a sensing layer. Front. Opt. Laser Sci. 2018. [Google Scholar] [CrossRef]
- Eddin, F.B.K.; Fen, Y.W. The Principle of Nanomaterials Based Surface Plasmon Resonance Biosensors and Its Potential for Dopamine Detection. Molecules 2020, 25, 2769. [Google Scholar] [CrossRef]
- Aray, A.; Chiavaioli, F.; Arjmand, M.; Trono, C.; Tombelli, S.; Giannetti, A.; Cennamo, N.; Soltanolkotabi, M.; Zeni, L.; Baldini, F. SPR-based plastic optical fibre biosensor for the detection of C-reactive protein in serum. J. Biophotonics 2016, 9, 1077–1084. [Google Scholar] [CrossRef]
- Antohe, I.; Schouteden, K.; Goos, P.; Delport, F.; Spasic, D.; Lammertyn, J. Thermal annealing of gold coated fiber optic surfaces for improved plasmonic biosensing. Sens. Actuators B Chem. 2016, 229, 678–685. [Google Scholar] [CrossRef]
- Antohe, I.; Spasic, D.; Delport, F.; Li, J.; Lammertyn, J. Nanoscale patterning of gold-coated optical fibers for improved plasmonic sensing. Nanotechnology 2017, 28, 215301. [Google Scholar] [CrossRef]
- Arghir, I.; Delport, F.; Spasic, D.; Lammertyn, J. Smart design of fiber optic surfaces for improved plasmonic biosensing. New Biotechnol. 2015, 32, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Arghir, I.; Spasic, D.; Verlinden, B.E.; Delport, F.; Lammertyn, J. Improved surface plasmon resonance biosensing using silanized optical fibers. Sens. Actuators B Chem. 2015, 216, 518–526. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Gupta, B.D. Fabrication and characterization of a highly sensitive surface plasmon resonance based fiber optic pH sensor utilizing high index layer and smart hydrogel. Sens. Actuators B Chem. 2012, 173, 268–273. [Google Scholar] [CrossRef]
- Mishra, S.K.; Gupta, B.D. Surface plasmon resonance based fiber optic pH sensor utilizing Ag/ITO/Al/hydrogel layers. Analyst 2013, 138, 2640–2646. [Google Scholar] [CrossRef]
- Semwal, V.; Gupta, B.D. Highly sensitive surface plasmon resonance based fiber optic pH sensor utilizing rGO-Pani nanocomposite prepared by in situ method. Sens. Actuators B Chem. 2019, 283, 632–642. [Google Scholar] [CrossRef]
- Attout, A.; Yunus, S.; Bertrand, P. Electroless deposition of polyaniline: Synthesis and characterization. Surf. Interface Anal. 2008, 40, 657–660. [Google Scholar] [CrossRef]
- Ahmad, K.; Mobin, S.M. Construction of polyanilne/ITO electrode for electrochemical sensor applications. Mater. Res. Express 2019, 6, 085508. [Google Scholar] [CrossRef]
- Zujovic, Z.; Kilmartin, P.A.; Travas-Sejdic, J. The Applications of Solid-State NMR to Conducting Polymers. The Special Case on Polyaniline. Molecules 2020, 25, 444. [Google Scholar] [CrossRef] [Green Version]
- Omale, J.O.; Rupp, R.; Velthem, P.V.; Kerckhoven, V.V.; Antohe, V.A.; Vlad, A.; Piraux, L. Three-dimensional microsupercapacitors based on interdigitated patterns of interconnected nanowire networks. Energy Storage Mater. 2019, 21, 77–84. [Google Scholar] [CrossRef]
- Antohe, I.; Iordache, I.; Antohe, V.A.; Socol, G. A polyaniline/platinum coated fiber optic surface plasmon resonance sensor for picomolar detection of 4-nitrophenol. Sci. Rep. 2021, 11, 10086. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Gao, L.; Zhou, F.; Duan, G. Facile Synthesis of Polyaniline Nanotubes Using Self-Assembly Method Based on the Hydrogen Bonding: Mechanism and Application in Gas Sensing. Polymers 2017, 9, 544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antohe, V.A.; Radu, A.; Mátéfi-Tempfli, M.; Attout, A.; Yunus, S.; Bertrand, P.; Duţu, C.A.; Vlad, A.; Melinte, S.; Mátéfi-Tempfli, S.; et al. Nanowire-templated microelectrodes for high-sensitivity pH detection. Appl. Phys. Lett. 2009, 94, 073118. [Google Scholar] [CrossRef]
- Tanwar, S.; Ho, J.A. Green Synthesis of Novel Polyaniline Nanofibers: Application in pH Sensing. Molecules 2015, 20, 18585–18596. [Google Scholar] [CrossRef]
- Sharma, N.K.; Shukla, S.; Sajal, V. Surface plasmon resonance based fiber optic sensor using an additional layer of platinum: A theoretical study. Optik 2017, 133, 43–50. [Google Scholar] [CrossRef]
- Shukla, S.; Rani, M.; Sharma, N.K.; Sajal, V. Sensitivity enhancement of a surface plasmon resonance based fiber optic sensor utilizing platinum layer. Optik 2015, 126, 4636–4639. [Google Scholar] [CrossRef]
- Yunus, S.; Attout, A.; Bertrand, P. Controlled Aniline Polymerization Strategies for Polyaniline Micro- and Nano Self-Assembling into Practical Electronic Devices. Langmuir 2009, 25, 1851–1854. [Google Scholar] [CrossRef] [PubMed]
- Ran, Y.; Xiao, P.; Zhang, Y.; Hu, D.; Xu, Z.; Liang, L.; Guan, B. A Miniature pH Probe Using Functional Microfiber Bragg Grating. Optics 2020, 1, 202–212. [Google Scholar] [CrossRef]
- Moulder, J.; Stickle, W.; Sobol, P.; Bomben, K. A Reference Book of Standard Spectra for Identification and Interpretation of XPS Data. In Handbook of X-ray Photoelectron Spectroscopy; Chastain, J., Ed.; Physical Electronics Division; Perkin-Elmer Corporation: Waltham, MA, USA, 1992. [Google Scholar]
- Wu, C.G.; Hsiao, H.T.; Yeh, Y.R. Electroless surface polymerization of polyaniline films on aniline primed ITO electrodes: A simple method to fabricate good modified anodes for polymeric light emitting diodes. J. Mater. Chem. 2001, 11, 2287–2292. [Google Scholar] [CrossRef]
- Chandra, S.; Mukherji, S. Polyaniline modified u-bent fiber optic pH sensor for physiological use. In Proceedings of the 2018 3rd International Conference on Microwave and Photonics (ICMAP), Dhanbad, India, 9–11 February 2018; pp. 1–2. [Google Scholar] [CrossRef]
- Yulianti, I.; Supa’at, A.S.M.; Idrus, S.M.; Kurdi, O.; Anwar, M.R.S. Sensitivity improvement of a fibre Bragg grating pH sensor with elastomeric coating. Meas. Sci. Technol. 2011, 23, 015104. [Google Scholar] [CrossRef]
- Zhao, Q.; Yin, M.; Zhang, A.P.; Prescher, S.; Antonietti, M.; Yuan, J. Hierarchically Structured Nanoporous Poly(Ionic Liquid) Membranes: Facile Preparation and Application in Fiber-Optic pH Sensing. J. Am. Chem. Soc. 2013, 135, 5549–5552. [Google Scholar] [CrossRef] [PubMed]




| FO-SPR pH Sensor Configuration | pH Operational Range | Sensitivity (nm/pH) |
|---|---|---|
| FO-SPR based on | 3–11 | 19.5 |
| Ag/ITO/Al/hydrogel layers [16] | ||
| Nanoporous Poly(Ionic Liquid) | 7–10 | 2.48 |
| membrane on FO-SPR sensor [34] | ||
| Fibre Bragg grating FO-SPR [33] | 3–8 | 0.166 |
| Reflection-type PANI/Pt-coated | 1–7 | 2.77 |
| FO-SPR sensor (present study) | 7–14 | 3.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antohe, I.; Jinga, L.-I.; Antohe, V.-A.; Socol, G. Sensitive pH Monitoring Using a Polyaniline-Functionalized Fiber Optic—Surface Plasmon Resonance Detector. Sensors 2021, 21, 4218. https://doi.org/10.3390/s21124218
Antohe I, Jinga L-I, Antohe V-A, Socol G. Sensitive pH Monitoring Using a Polyaniline-Functionalized Fiber Optic—Surface Plasmon Resonance Detector. Sensors. 2021; 21(12):4218. https://doi.org/10.3390/s21124218
Chicago/Turabian StyleAntohe, Iulia, Luiza-Izabela Jinga, Vlad-Andrei Antohe, and Gabriel Socol. 2021. "Sensitive pH Monitoring Using a Polyaniline-Functionalized Fiber Optic—Surface Plasmon Resonance Detector" Sensors 21, no. 12: 4218. https://doi.org/10.3390/s21124218