Quality Assessment of Deep-Frying Palm Oil by Impedimetric Sensing with a Simple and Economic Electrochemical Cell
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents, Samples, and Electrodes
2.2. Deep-Frying Process
2.3. EIS Measurement
2.4. Viscosity
2.5. Acid Value
2.6. Total Polar Compounds
3. Results and Discussion
3.1. Effect of Electrochemical Cell Geometry on Impedimetric Sensing
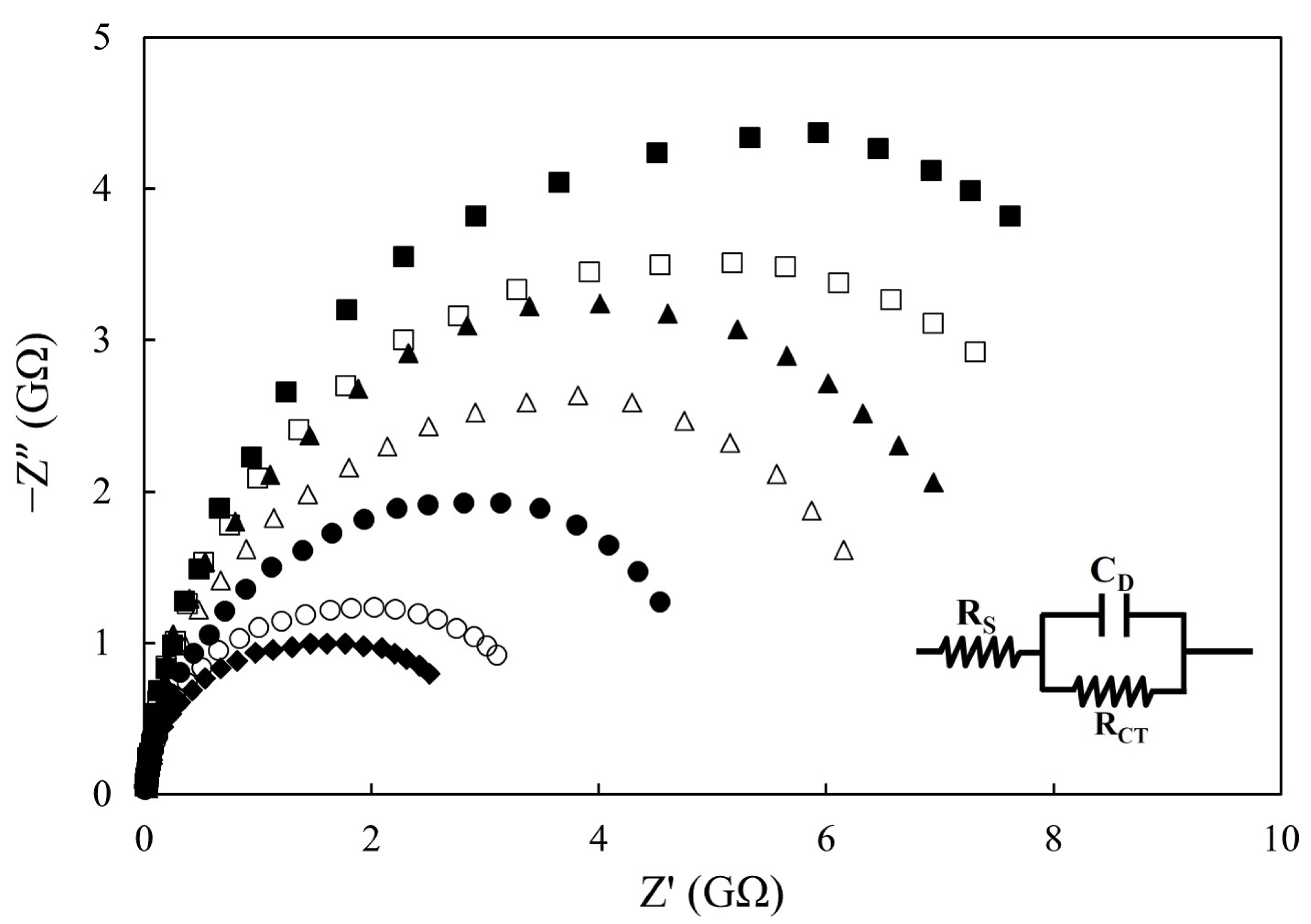
3.2. Effect of Deep-Frying Cycle on Impedimetric Sensing
3.3. Effect of Deep-Frying Cycle on Viscosity, AV, and TPC
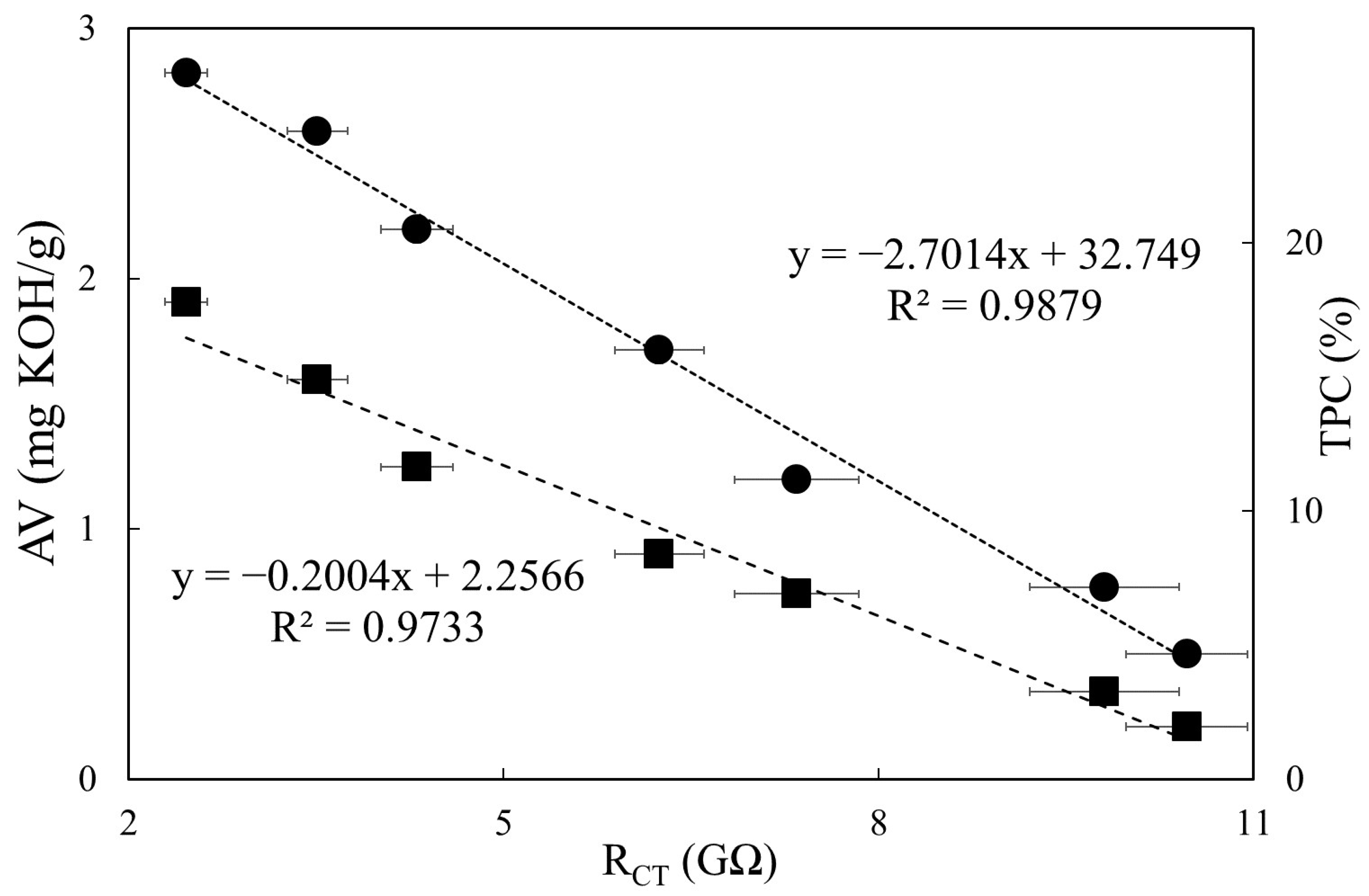
3.4. Relationships between Impedimetric Data and Regulatory Quality Indicators
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guillén, M.D.; Uriarte, P.S. Simultaneous control of the evolution of the percentage in weight of polar compounds, iodine value, acyl groups proportions and aldehydes concentrations in sunflower oil submitted to frying temperature in an industrial fryer. Food Control 2012, 24, 50–56. [Google Scholar] [CrossRef]
- Gertz, C. Chemical and physical parameters as quality indicators of used frying fats. Eur. J. Lipid Sci. Technol. 2000, 102, 566–572. [Google Scholar] [CrossRef]
- Stier, R.F. Tests to monitor quality of deep-frying fats and oils. Eur. J. Lipid Sci. Technol. 2004, 106, 766–771. [Google Scholar] [CrossRef]
- Stier, R.F. Ensuring the health and safety of fried foods. Eur. J. Lipid Sci. Technol. 2013, 115, 956–964. [Google Scholar] [CrossRef]
- Weisshaar, R. Quality control of used deep-frying oils. Eur. J. Lipid Sci. Technol. 2014, 116, 716–722. [Google Scholar] [CrossRef]
- Chen, W.-A.; Chiu, C.P.; Cheng, W.-C.; Hsu, C.-K.; Kuo, M.-I. Total polar compounds and acid values of repeatedly used frying oils measured by standard and rapid methods. J. Food Drug. Anal. 2013, 21, 58–65. [Google Scholar] [CrossRef]
- Mba, O.I.; Dumont, M.J.; Ngadi, M. Deterioration kinetics of crude palm oil, canola oil and blend during repeated deep-fat frying. J. Am. Oil. Chem. Soc. 2016, 93, 1243–1253. [Google Scholar] [CrossRef]
- Tarmizi, A.H.A.; Hishamuddin, E.; Razak, R.A.A. Impartial assessment of oil degradation through partitioning of polar compounds in vegetable oils under simulated frying practice of fast food restaurants. Food Control 2019, 96, 445–455. [Google Scholar] [CrossRef]
- Ibrahim, N.U.A.; Aziz, S.A.; Hashim, N.; Jamaludin, D.; Khaled, A.Y. Dielectric spectroscopy of palm olein during batch deep frying and their relation with degradation parameters. J. Food Sci. 2019, 84, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cai, W.; Sun, D.; Liu, Y. A quick method for determining total polar compounds of frying oils using electric conductivity. Food. Anal. Methods 2016, 9, 1444–1450. [Google Scholar] [CrossRef]
- Kalogianni, E.P.; Karastogiannidou, C. Development of a rapid method for the determination of frying oil quality based on capillary penetration. Int. J. Food Sci. Technol. 2015, 50, 1215–1223. [Google Scholar] [CrossRef]
- El-Naggar, E.A. The evaluation of deep frying oil quality with the spectrophotometric method for the rapid assessment of total polar compounds. Zagazig J. Agric. Res. 2019, 46, 1489–1502. [Google Scholar] [CrossRef]
- Khaled, A.Y.; Aziz, S.A.; Rokhani, F.Z. Capacitive sensor probe to assess frying oil degradation. Inf. Process. Agric. 2015, 2, 142–148. [Google Scholar] [CrossRef] [Green Version]
- Khaled, A.; Aziz, S.; Rokhani, F. Development and evaluation of an impedance spectroscopy sensor to assess cooking oil quality. Int. J. Environ. Sci. Dev. 2014, 5, 299–302. [Google Scholar] [CrossRef]
- Vasilescu, A.; Vezeanu, A.; Badea, M. Electrochemical impedance spectroscopy investigations focused on food allergens. Sens. Electroanal. 2014, 8, 59–83. [Google Scholar]
- Zhao, X.; Zhuang, H.; Yoon, S.C.; Dong, Y.; Wang, W.; Zhao, W. Electrical impedance spectroscopy for quality assessment of meat and fish: A review on basic principles, measurement methods, and recent advances. J. Food Qual. 2017, 2017, 6370739. [Google Scholar] [CrossRef] [Green Version]
- van Grinsven, B.; Vandenryt, T.; Duchateau, S.; Gaulke, A.; Grieten, L.; Thoelen, R.; Ingebrandt, S.; De Ceuninck, W.; Wagner, P. Customized impedance spectroscopy device as possible sensor platform for biosensor applications. Phys. Status Solidi A 2010, 207, 919–923. [Google Scholar] [CrossRef]
- Broeders, L.; Duchateau, S.; Van Grinsven, B.; Vanaken, W.; Peeters, M.; Cleij, T.; Thoelen, R.; Wagner, P.; De Ceuninck, W. Miniaturised eight-channel impedance spectroscopy unit as sensor platform for biosensor applications. Phys. Status Solidi A 2011, 208, 1357–1363. [Google Scholar] [CrossRef]
- Hoja, J.; Lentka, G. Interface circuit for impedance sensors using two specialized single-chip microsystems. Sens. Actuator A Phys. 2010, 63, 191–197. [Google Scholar] [CrossRef]
- Critcher, S.; Freeborn, T.J. Localized bioimpedance measurements with the max3000x integrated circuit: Characterization and demonstration. Sensors 2021, 21, 3013. [Google Scholar] [CrossRef]
- Kung, Y.; Hsieh, B.-C.; Cheng, T.-J.; Huang, C.-K.; Chen, R.L.C. Impedimetric sensing of the biodiesel contents in diesel fuels with a carbon paste electrode pair. Fuel 2012, 102, 724–728. [Google Scholar] [CrossRef]
- Pliquett, U. Bioimpedance: A review for food processing. Food Eng. Rev. 2010, 2, 74–94. [Google Scholar] [CrossRef]
- ISO Method 660. Animal and Vegetable Fats and Oils: Determination of Acid Value and Acidity; ISO: Geneva, Switzerland, 2009. [Google Scholar]
- Prevc, T.; Cigić, B.; Vidrih, R.; Poklar, U.N.; Šegatin, N. Correlation of basic oil quality indices and electrical properties of model vegetable oil systems. J. Agric. Food Chem. 2013, 61, 11355–11362. [Google Scholar] [CrossRef] [PubMed]
- Timmer, B.; Sparreboom, W.; Olthuis, W.; Bergveld, P.; van den Berg, A. Optimization of an electrolyte conductivity detector for measuring low ion concentrations. Lab. Chip. 2002, 2, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Pignitter, M.; Somoza, V. Critical evaluation of methods for the measurement of oxidative rancidity in vegetable oils. J. Food Drug Anal. 2012, 20, 772–777. [Google Scholar] [CrossRef]
- Yamada, K.; Terao, J.; Matsushita, S. Electrochemical detection of phospholipid hydroperoxides in reverse-phase high performance liquid chromatography. Lipids 1987, 22, 125–128. [Google Scholar] [CrossRef]
- Korytowski, W.; Bachowski, G.J.; Girotti, A.W. Chromatographic Separation and electrochemical determination of hydroperoxides. Anal. Biochem. 1991, 197, 149–156. [Google Scholar] [CrossRef]
- Perini, N.; Prado, A.R.; Sad, C.M.S.; Castro, E.V.R.; Freitas, M.B.J.G. Electrochemical impedance spectroscopy for in situ petroleum analysis and water-in-oil emulsion characterization. Fuel 2012, 91, 224–228. [Google Scholar] [CrossRef] [Green Version]

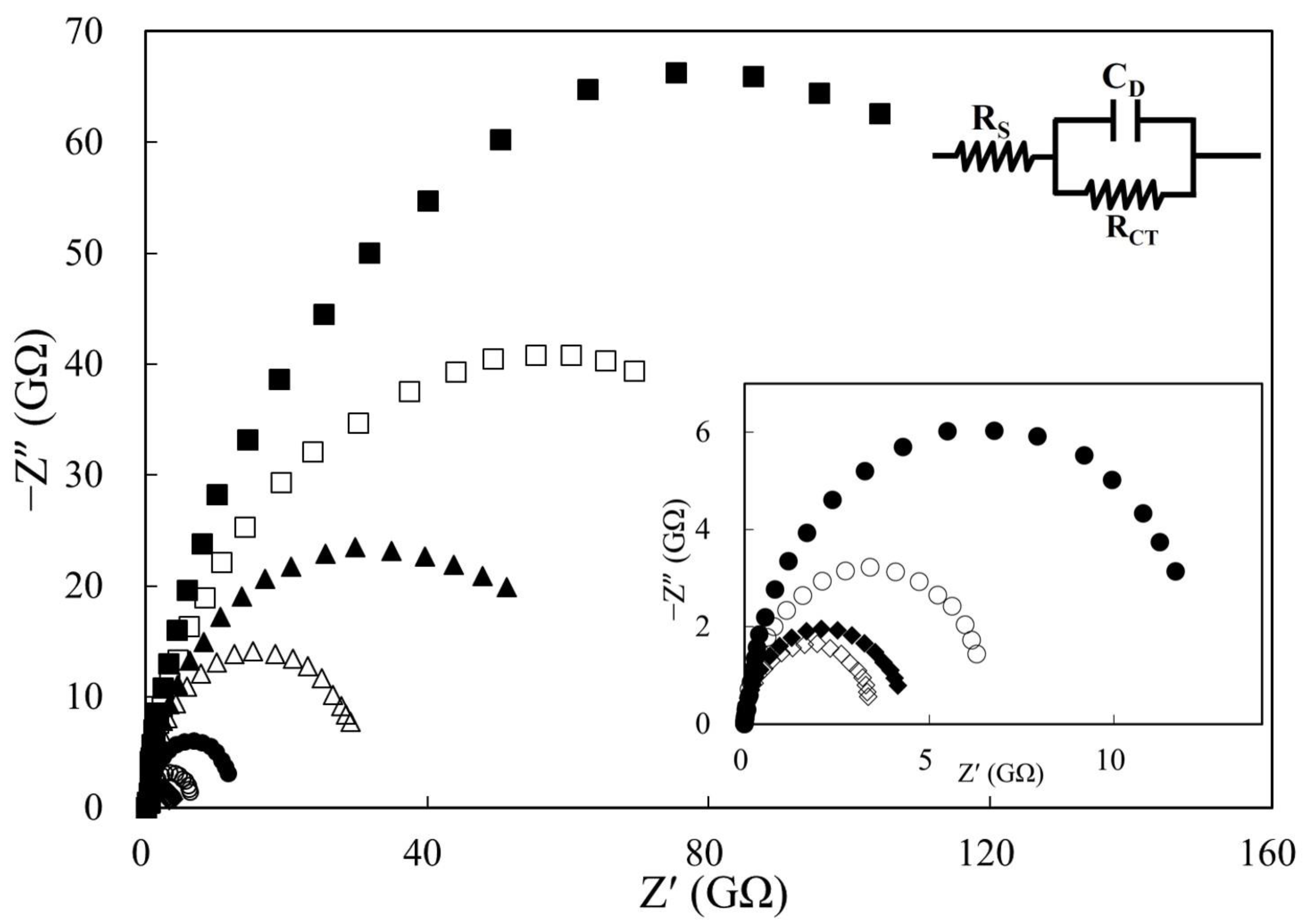


| Geometry | Distance (L, cm) | Area (A, cm2) | Cell Constant (cm−1) | RS (MΩ) | CD (pF) | RCT (GΩ) |
|---|---|---|---|---|---|---|
| #1 | 0.0385 | 19.63 × 102 | 19.61 × 10−2 | 860 ± 303 | 19.83 ± 2.89 | 165.33 ± 30.14 |
| #2 | 0.0275 | 19.63 × 102 | 14.01 × 10−2 | 628 ± 177 | 22.47 ± 2.03 | 118.50 ± 17.97 |
| #3 | 0.0165 | 19.63 × 102 | 8.40 × 10−2 | 687 ± 338 | 28.50 ± 1.41 | 71.83 ± 7.77 |
| #4 | 0.0055 | 19.63 × 102 | 2.80 × 10−2 | 238 ± 179 | 48.87 ± 3.21 | 34.97 ± 1.43 |
| #5 | 0.0055 | 78.54 × 102 | 0.70 × 10−2 | 139 ± 80 | 61.27 ± 2.64 | 10.67 ± 0.61 |
| #6 | 0.0055 | 176.71 × 102 | 0.31 × 10−2 | 110 ± 46 | 79.50 ± 2.85 | 6.17 ± 0.93 |
| #7 | 0.0055 | 314.16 × 102 | 0.18 × 10−2 | 55 ± 17 | 101.57 ± 5.44 | 3.95 ± 0.79 |
| #8 | 0.0055 | 490.87 × 102 | 0.11 × 10−2 | 28 ± 16 | 126.67 ± 2.08 | 3.21 ± 0.87 |
| Deep-Frying Time (h) | RS (MΩ) | CD (pF) | RCT (GΩ) |
|---|---|---|---|
| 0 | 291 ± 72 | 40.47 ± 1.80 | 10.47 ± 0.49 |
| 4 | 318 ± 92 | 40.33 ± 1.71 | 9.81 ± 0.60 |
| 8 | 327 ± 60 | 41.03 ± 3.40 | 7.35 ± 0.50 |
| 12 | 249 ± 72 | 46.03 ± 2.31 | 6.24 ± 0.36 |
| 16 | 320 ± 92 | 49.20 ± 7.81 | 4.31 ± 0.29 |
| 20 | 259 ± 45 | 47.73 ± 3.60 | 3.51 ± 0.24 |
| 24 | 353 ± 52 | 48.70 ± 7.04 | 2.46 ± 0.17 |
| Deep-Frying Time (h) | Viscosity (cP) | Acid Value (mg KOH/g Oil) | TPC (%) |
|---|---|---|---|
| 0 | 55.23 ± 0.21 | 0.21 ± 0.01 | 4.67 ± 0.29 |
| 4 | 57.90 ± 0.36 | 0.35 ± 0.01 | 7.17 ± 0.29 |
| 8 | 60.57 ± 0.15 | 0.74 ± 0.02 | 11.17 ± 0.29 |
| 12 | 64.43 ± 0.35 | 0.90 ± 0.01 | 16.00 ± 0.50 |
| 16 | 68.47 ± 0.25 | 1.25 ± 0.03 | 20.50 ± 0.50 |
| 20 | 73.23 ± 0.25 | 1.60 ± 0.03 | 24.17 ± 0.29 |
| 24 | 75.80 ± 0.20 | 1.91 ± 0.03 | 26.33 ± 0.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kung, Y.; Hsieh, B.-C. Quality Assessment of Deep-Frying Palm Oil by Impedimetric Sensing with a Simple and Economic Electrochemical Cell. Sensors 2021, 21, 7093. https://doi.org/10.3390/s21217093
Kung Y, Hsieh B-C. Quality Assessment of Deep-Frying Palm Oil by Impedimetric Sensing with a Simple and Economic Electrochemical Cell. Sensors. 2021; 21(21):7093. https://doi.org/10.3390/s21217093
Chicago/Turabian StyleKung, Yi, and Bo-Chuan Hsieh. 2021. "Quality Assessment of Deep-Frying Palm Oil by Impedimetric Sensing with a Simple and Economic Electrochemical Cell" Sensors 21, no. 21: 7093. https://doi.org/10.3390/s21217093






