Berry Cell Vitality Assessment and the Effect on Wine Sensory Traits Based on Chemical Fingerprinting, Canopy Architecture and Machine Learning Modelling
Abstract
:1. Introduction
2. Materials and Methods
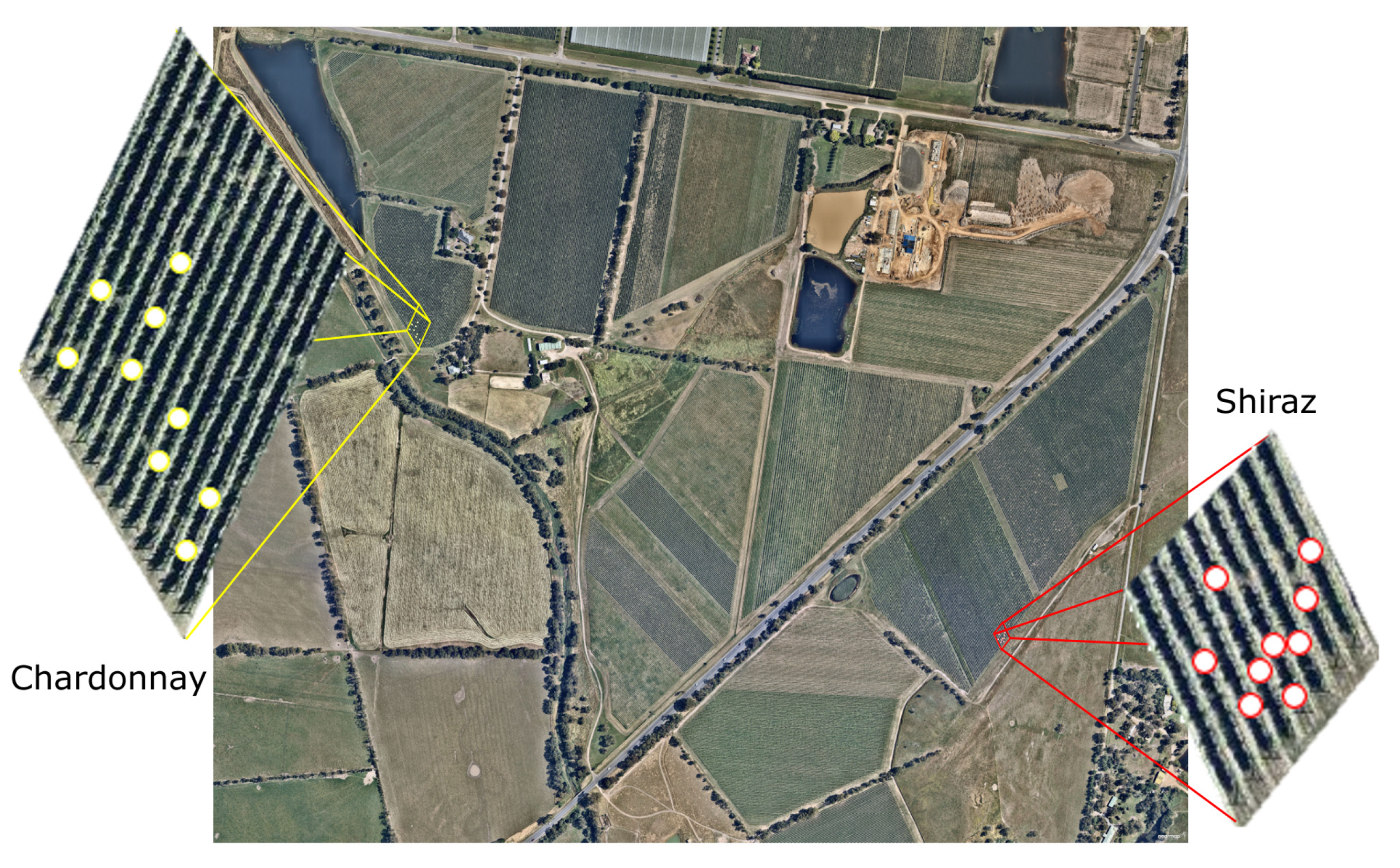
2.1. Site and Grapevine Cultivars Description
2.2. Canopy Imaging and Digital Measurements
2.3. Near-Infrared Spectroscopy
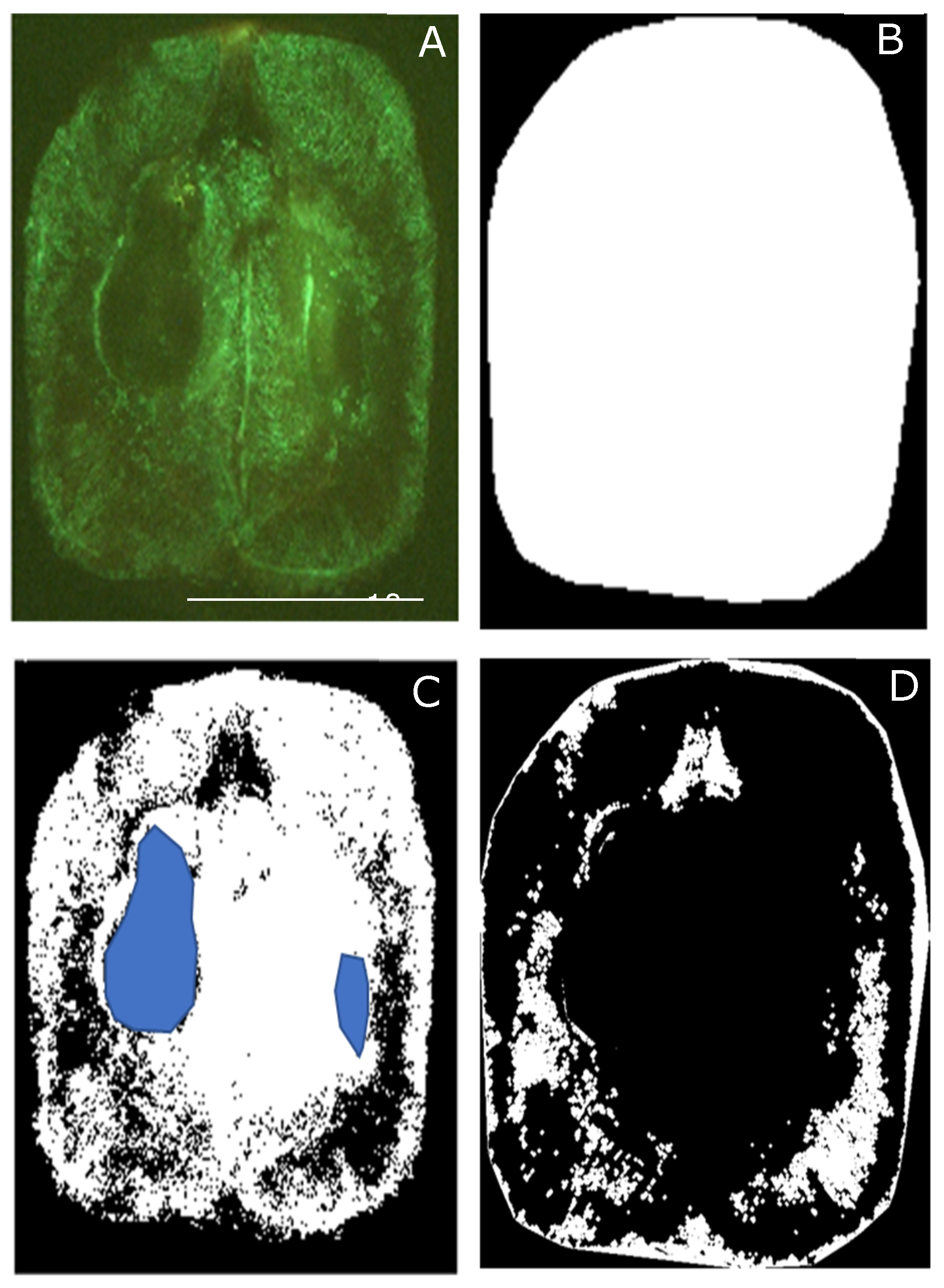
2.4. Living and Dead Tissue Analysis from Berries
2.5. Winemaking and Descriptive Sensory Analysis
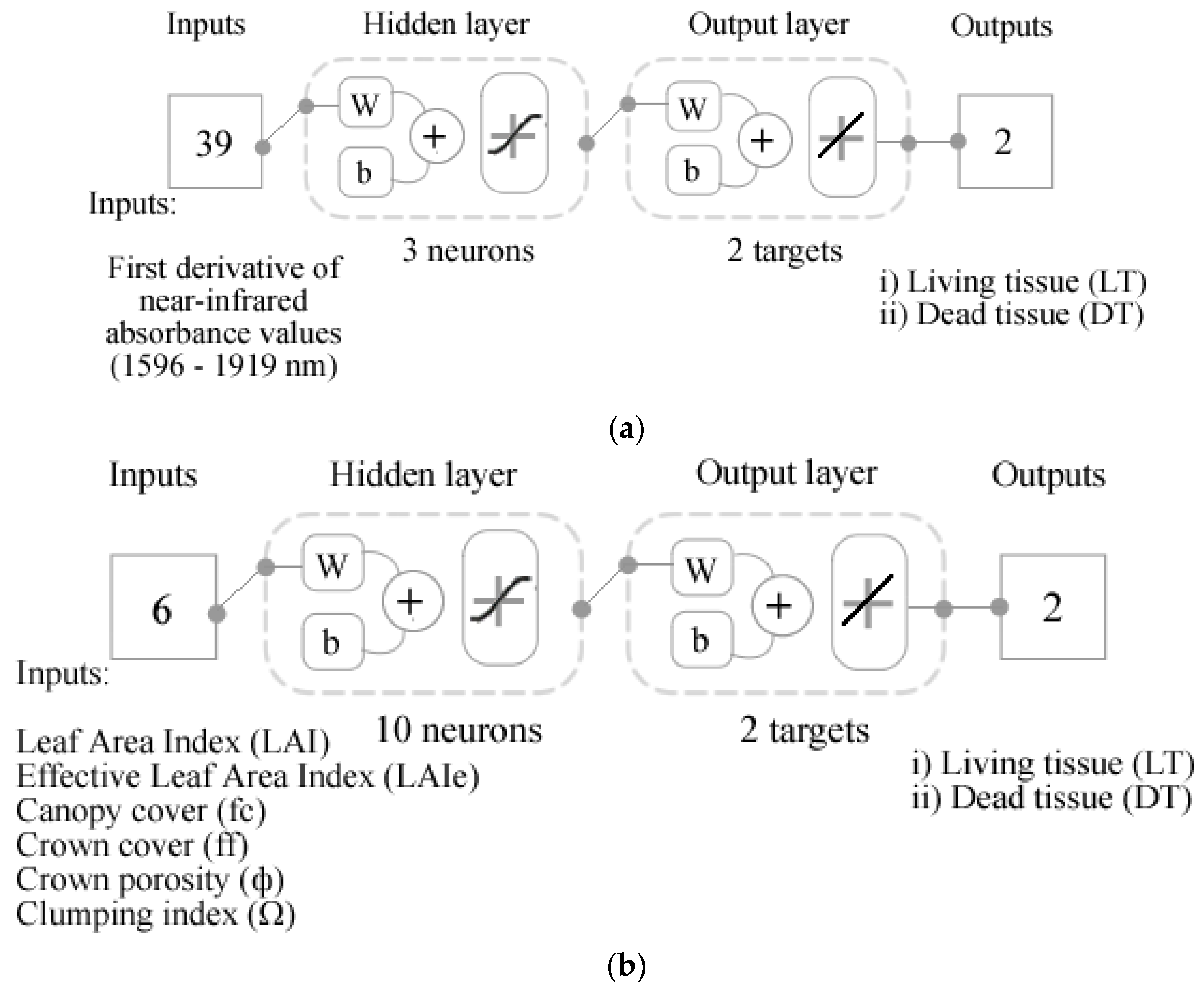
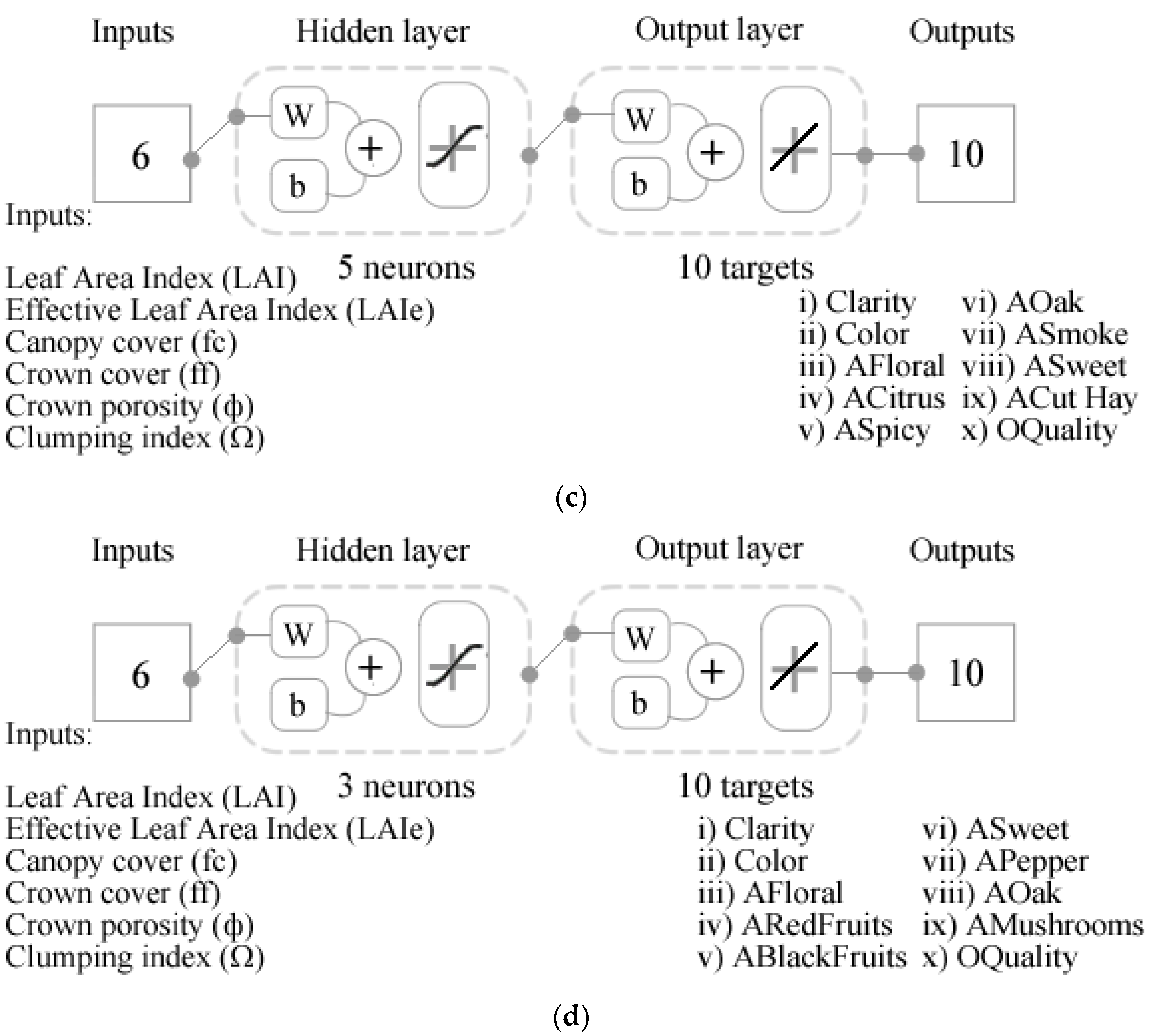
2.6. Statistical Analysis and Machine Learning Modeling
3. Results
4. Discussion
4.1. Dynamics of Berry Cell Death and Berry Composition
4.2. Proximal Near-Infrared Spectroscopy of Berries and Machine Learning Modeling
4.3. Canopy Architecture, Berry Cell Vitality and Machine Learning Modeling
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tilbrook, J.; Tyerman, S.D. Cell death in grape berries: Varietal differences linked to xylem pressure and berry weight loss. Funct. Plant Biol. 2008, 35, 173–184. [Google Scholar] [CrossRef] [Green Version]
- Hardie, W.J.; O’brien, T.; Jaudzems, V. Morphology, anatomy and development of the pericarp after anthesis in grape, Vitis vinifera L. Aust. J. Grape Wine Res. 1996, 2, 97–142. [Google Scholar] [CrossRef]
- Fuentes, S.; Tongson, E.; Chen, J.; Gonzalez Viejo, C. A Digital Approach to Evaluate the Effect of Berry Cell Death on Pinot Noir Wines’ Quality Traits and Sensory Profiles Using Non-Destructive Near-Infrared Spectroscopy. Beverages 2020, 6, 39. [Google Scholar] [CrossRef]
- Bondada, B.; Keller, M. Morphoanatomical symptomatology and osmotic behavior of grape berry shrivel. J. Am. Soc. Hortic. Sci. 2012, 137, 20–30. [Google Scholar] [CrossRef]
- Bonada, M.; Sadras, V.; Fuentes, S. Effect of elevated temperature on the onset and rate of mesocarp cell death in berries of S hiraz and C hardonnay and its relationship with berry shrivel. Aust. J. Grape Wine Res. 2013, 19, 87–94. [Google Scholar] [CrossRef]
- Fuentes, S.; Sullivan, W.; Tilbrook, J.; Tyerman, S. A novel analysis of grapevine berry tissue demonstrates a variety-dependent correlation between tissue vitality and berry shrivel. Aust. J. Grape Wine Res. 2010, 16, 327–336. [Google Scholar] [CrossRef]
- Bonada, M.; Sadras, V.; Moran, M.; Fuentes, S. Elevated temperature and water stress accelerate mesocarp cell death and shrivelling, and decouple sensory traits in Shiraz berries. Irrig. Sci. 2013, 31, 1317–1331. [Google Scholar] [CrossRef]
- Sutton, R.T.; Hawkins, E. ESD Ideas: Global climate response scenarios for IPCC AR6. Earth Syst. Dyn. Discuss. 2020, 2020, 1–4. [Google Scholar]
- Caravia Bayer, L. Heat Wave Mitigation Strategies for Wine Grape Production and Measures of the Impact of Heat on Berry Ripening and Wine Composition. Ph.D. Thesis, University of Adelaide, Adelaide, SA, Australia, 2016. [Google Scholar]
- Caravia, L.; Collins, C.; Tyerman, S. Electrical impedance of S hiraz berries correlates with decreasing cell vitality during ripening. Aust. J. Grape Wine Res. 2015, 21, 430–438. [Google Scholar] [CrossRef]
- Pimenta, A.M.; Scafi, S.H.F.; Pasquini, C.; Raimundo, I.M., Jr.; Rohwedder, J.J.; Montenegro, M.d.C.B.; Araújo, A.N. Determination of hydrogen peroxide by near infrared spectroscopy. J. Near Infrared Spectrosc. 2003, 11, 49–53. [Google Scholar] [CrossRef]
- Xiao, Z.; Rogiers, S.Y.; Sadras, V.O.; Tyerman, S.D. Hypoxia in grape berries: The role of seed respiration and lenticels on the berry pedicel and the possible link to cell death. J. Exp. Bot. 2018, 69, 2071–2083. [Google Scholar] [CrossRef] [Green Version]
- De Bei, R.; Fuentes, S.; Gilliham, M.; Tyerman, S.; Edwards, E.; Bianchini, N.; Smith, J.; Collins, C. VitiCanopy: A free computer App to estimate canopy vigor and porosity for grapevine. Sensors 2016, 16, 585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuentes, S.; Chacon, G.; Torrico, D.D.; Zarate, A.; Gonzalez Viejo, C. Spatial Variability of Aroma Profiles of Cocoa Trees Obtained through Computer Vision and Machine Learning Modelling: A Cover Photography and High Spatial Remote Sensing Application. Sensors 2019, 19, 3054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuentes, S.; Palmer, A.R.; Taylor, D.; Zeppel, M.; Whitley, R.; Eamus, D. An automated procedure for estimating the leaf area index (LAI) of woodland ecosystems using digital imagery, MATLAB programming and its application to an examination of the relationship between remotely sensed and field measurements of LAI. Funct. Plant Biol. 2008, 35, 1070–1079. [Google Scholar] [CrossRef]
- Fuentes, S.; Poblete-Echeverría, C.; Ortega-Farias, S.; Tyerman, S.; De Bei, R. Automated estimation of leaf area index from grapevine canopies using cover photography, video and computational analysis methods. Aust. J. Grape Wine Res. 2014, 20, 465–473. [Google Scholar] [CrossRef]
- Carrasco-Benavides, M.; Mora, M.; Maldonado, G.; Olguín-Cáceres, J.; von Bennewitz, E.; Ortega-Farías, S.; Gajardo, J.; Fuentes, S. Assessment of an automated digital method to estimate leaf area index (LAI) in cherry trees. N. Z. J. Crop Hortic. Sci. 2016, 44, 247–261. [Google Scholar] [CrossRef]
- Mozell, M.R.; Thach, L. The impact of climate change on the global wine industry: Challenges & solutions. Wine Econ. Policy 2014, 3, 81–89. [Google Scholar]
- Anderson, K.; Findlay, C.; Fuentes, S.; Tyerman, S. Viticulture, wine and climate change. Garnaut Clim. Chang. Rev. 2008, 560, 1–16. [Google Scholar]
- Van Leeuwen, C.; Darriet, P. The impact of climate change on viticulture and wine quality. J. Wine Econ. 2016, 11, 150–167. [Google Scholar] [CrossRef] [Green Version]
- Lammertyn, J.; Peirs, A.; De Baerdemaeker, J.; Nicolaı, B. Light penetration properties of NIR radiation in fruit with respect to non-destructive quality assessment. Postharvest Biol. Technol. 2000, 18, 121–132. [Google Scholar] [CrossRef]
- Larraín, M.; Guesalaga, A.R.; Agosín, E. A multipurpose portable instrument for determining ripeness in wine grapes using NIR spectroscopy. IEEE Trans. Instrum. Meas. 2008, 57, 294–302. [Google Scholar] [CrossRef]
- Martelo-Vidal, M.J.; Vazquez, M. Evaluation of ultraviolet, visible, and near infrared spectroscopy for the analysis of wine compounds. Czech J. Food Sci. 2014, 32, 37–47. [Google Scholar] [CrossRef] [Green Version]
- Macfarlane, C.; Arndt, S.K.; Livesley, S.J.; Edgar, A.C.; White, D.A.; Adams, M.A.; Eamus, D. Estimation of leaf area index in eucalypt forest with vertical foliage, using cover and fullframe fisheye photography. For. Ecol. Manag. 2007, 242, 756–763. [Google Scholar] [CrossRef] [Green Version]
- Chianucci, F.; Cutini, A. Estimation of canopy properties in deciduous forests with digital hemispherical and cover photography. Agric. For. Meteorol. 2013, 168, 130–139. [Google Scholar] [CrossRef]
- Pekin, B.; Macfarlane, C. Measurement of crown cover and leaf area index using digital cover photography and its application to remote sensing. Remote Sens. 2009, 1, 1298–1320. [Google Scholar] [CrossRef] [Green Version]
- Poblete-Echeverría, C.; Fuentes, S.; Ortega-Farias, S.; Gonzalez-Talice, J.; Yuri, A.J. Digital Cover Photography for Estimating Leaf Area Index (LAI) in Apple Trees Using a Variable Light Extinction Coefficient. Sensors 2015, 15, 2860–2872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mora, M.; Avila, F.; Carrasco-Benavides, M.; Maldonado, G.; Olguín-Cáceres, J.; Fuentes, S. Automated computation of leaf area index from fruit trees using improved image processing algorithms applied to canopy cover digital photograpies. Comput. Electron. Agric. 2016, 123, 195–202. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; De Bei, R.; Fuentes, S.; Collins, C. Influence of Canopy Management Practices on Canopy Architecture and Reproductive Performance of Semillon and Shiraz Grapevines in a Hot Climate. Am. J. Enol. Vitic. 2019, 70, 360–372. [Google Scholar] [CrossRef]
- De Bei, R.; Wang, X.; Papagiannis, L.; Cocco, M.; O’Brien, P.; Zito, M.; Ouyang, J.; Fuentes, S.; Gilliham, M.; Tyerman, S. Postveraison Leaf Removal Does Not Consistently Delay Ripening in Semillon and Shiraz in a Hot Australian Climate. Am. J. Enol. Vitic. 2019, 70, 398–410. [Google Scholar] [CrossRef]
- De Bei, R.; Fuentes, S.; Collins, C. Vineyard variability: Can we assess it using smart technologies? IVES Tech. Rev. Vine Wine 2019. [Google Scholar] [CrossRef]
- De Bei, R.; Kidman, C.; Wotton, C.; Shepherd, J.; Fuentes, S.; Gilliham, M.; Tyerman, S.; Collins, C. Canopy Architecture Is Linked to Grape and Wine Quality in Australian Shiraz. Wine Australia. 2018. Available online: https://www.wineaustralia.com/news/articles/canopy-management-to-optimise-performance (accessed on 20 August 2021).
- Sebastian, B.; Baeza, P.; Santesteban, L.G.; de Miguel, P.S.; De La Fuente, M.; Lissarrague, J.R. Response of grapevine cv. Syrah to irrigation frequency and water distribution pattern in a clay soil. Agric. Water Manag. 2015, 148, 269–279. [Google Scholar] [CrossRef]
- Keller, M.; Romero, P.; Gohil, H.; Smithyman, R.P.; Riley, W.R.; Casassa, L.F.; Harbertson, J.F. Deficit irrigation alters grapevine growth, physiology, and fruit microclimate. Am. J. Enol. Vitic. 2016, 67, 426–435. [Google Scholar] [CrossRef]
- Peterson, J.C.D.; Walker, M.A. Grapevine Rootstock Influence on Scion Development and Initiation of Senescence. Catal. Discov. Pract. 2017, 1, 48–54. [Google Scholar] [CrossRef]
- Mendes, J.P.; Pinho, T.M.; Neves dos Santos, F.; Sousa, J.J.; Peres, E.; Boaventura-Cunha, J.; Cunha, M.; Morais, R. Smartphone Applications Targeting Precision Agriculture Practices—A Systematic Review. Agronomy 2020, 10, 855. [Google Scholar] [CrossRef]
- Fuentes, S.; Gonzalez Viejo, C.; Wang, X.; Torrico, D.D. Aroma and quality assessment for vertical vintages using machine learning modelling based on weather and management information. In Proceedings of the 21st GiESCO International Meeting, Thessaloniki, Greece, 23–28 June 2021; pp. 23–28. [Google Scholar]


| Descriptor | Label | Anchors |
|---|---|---|
| Chardonnay | ||
| Clarity | Clarity | Turbid-Brilliant |
| Color | Color | Colorless-Green-yellow-Yellow-Golden-brown |
| Aroma Floral | AFloral | Absent-Intense |
| Aroma Citrus | ACitrus | Absent-Intense |
| Aroma Spicy | ASpicy | Absent-Intense |
| Aroma Oak | AOak | Absent-Intense |
| Aroma Smoke | ASmoke | Absent-Intense |
| Aroma Sweet | ASweet | Absent-Intense |
| Aroma Cut Hay | ACut Hay | Absent-Intense |
| Overall Quality | OQuality | Unacceptable-Extraordinary |
| Shiraz | ||
| Clarity | Clarity | Turbid-Brilliant |
| Color | Color | Purple-Ruby-Garnet-Tawny |
| Aroma Floral | AFloral | Absent-Intense |
| Aroma Red Fruits | ARedFruits | Absent-Intense |
| Aroma Black Fruits | ABlackFruits | Absent-Intense |
| Aroma Sweet | ASweet | Absent-Intense |
| Aroma Pepper | APepper | Absent-Intense |
| Aroma Oak | AOak | Absent-Intense |
| Aroma Mushrooms | AMushrooms | Absent-Intense |
| Overall Quality | OQuality | Unacceptable-Extraordinary |
| LAI | Canopy Cover (fc) | Crown Cover (ff) | Crown Porosity (ϕ) | Clumping Index (Ω) | |
|---|---|---|---|---|---|
| Chardonnay NS | |||||
| Group 1 | 2.13 | 0.67 | 0.80 | 0.16 | 0.75 |
| ±0.06 | ±0.01 | ±0.02 | ±0.01 | ±0.02 | |
| Group 2 | 1.99 | 0.64 | 0.78 | 0.18 | 0.76 |
| ±0.24 | ±0.04 | ±0.05 | ±0.03 | ±0.05 | |
| Group 3 | 1.93 | 0.66 | 0.81 | 0.19 | 0.80 |
| ±0.12 | ±0.03 | ±0.04 | ±0.01 | ±0.03 | |
| Shiraz | |||||
| Group 1 | 1.27 b | 0.49 b | 0.67 b | 0.28 a | 0.78 NS |
| ±0.17 | ±0.05 | ±0.06 | ±0.02 | ±0.02 | |
| Group 2 | 1.61 ab | 0.57 ab | 0.72 ab | 0.21 a | 0.75 |
| ±0.07 | ±0.02 | ±0.02 | ±0.01 | ±0.02 | |
| Group 3 | 2.00 a | 0.68 a | 0.84 a | 0.19 b | 0.82 |
| ±0.07 | ±0.02 | ±0.02 | ±0.01 | ±0.02 | |
| Stage | Samples | Observations | R | Slope | Performance (MSE) |
|---|---|---|---|---|---|
| Model 1: inputs: near-infrared absorbance; targets: living and dead tissue | |||||
| Training | 324 | 648 | 0.91 | 0.82 | 35.2 |
| Testing | 108 | 216 | 0.77 | 0.81 | 106.5 |
| Overall | 432 | 864 | 0.87 | 0.82 | - |
| Model 2: inputs: canopy architecture; targets: living and dead tissue | |||||
| Training | 260 | 520 | 0.98 | 0.95 | 8.9 |
| Validation | 86 | 172 | 0.98 | 0.91 | 11.5 |
| Testing | 86 | 172 | 0.98 | 0.91 | 11.4 |
| Overall | 432 | 864 | 0.98 | 0.93 | - |
| Stage | Samples | Observations | R | Slope | Performance (MSE) |
|---|---|---|---|---|---|
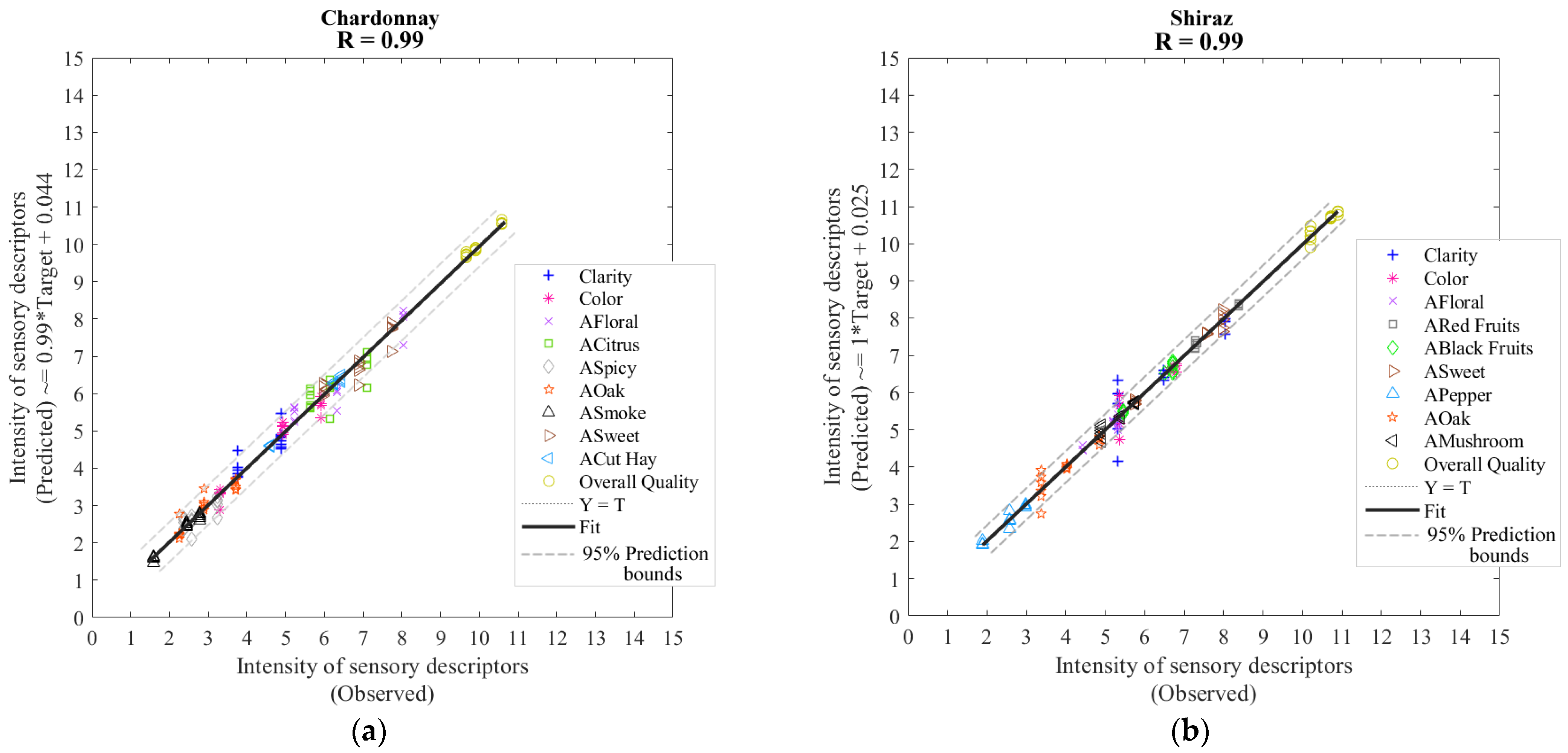
| Model 3: Chardonnay | |||||
| Training | 130 | 1300 | 0.99 | 0.99 | 0.04 |
| Validation | 43 | 430 | 0.99 | 0.99 | 0.06 |
| Testing | 43 | 430 | 0.99 | 0.99 | 0.06 |
| Overall | 216 | 2160 | 0.99 | 0.99 | - |
| Model 4: Shiraz | |||||
| Training | 130 | 1300 | 0.99 | 0.99 | 0.03 |
| Validation | 43 | 430 | 0.99 | 1.00 | 0.05 |
| Testing | 43 | 430 | 0.99 | 1.00 | 0.05 |
| Overall | 216 | 2160 | 0.99 | 1.00 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuentes, S.; Gonzalez Viejo, C.; Hall, C.; Tang, Y.; Tongson, E. Berry Cell Vitality Assessment and the Effect on Wine Sensory Traits Based on Chemical Fingerprinting, Canopy Architecture and Machine Learning Modelling. Sensors 2021, 21, 7312. https://doi.org/10.3390/s21217312
Fuentes S, Gonzalez Viejo C, Hall C, Tang Y, Tongson E. Berry Cell Vitality Assessment and the Effect on Wine Sensory Traits Based on Chemical Fingerprinting, Canopy Architecture and Machine Learning Modelling. Sensors. 2021; 21(21):7312. https://doi.org/10.3390/s21217312
Chicago/Turabian StyleFuentes, Sigfredo, Claudia Gonzalez Viejo, Chelsea Hall, Yidan Tang, and Eden Tongson. 2021. "Berry Cell Vitality Assessment and the Effect on Wine Sensory Traits Based on Chemical Fingerprinting, Canopy Architecture and Machine Learning Modelling" Sensors 21, no. 21: 7312. https://doi.org/10.3390/s21217312








