Hybrid Sol-Gel Surface-Enhanced Raman Sensor for Xylene Detection in Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of Materials
2.2.1. Synthesis of Nanoparticles and Nanoshells
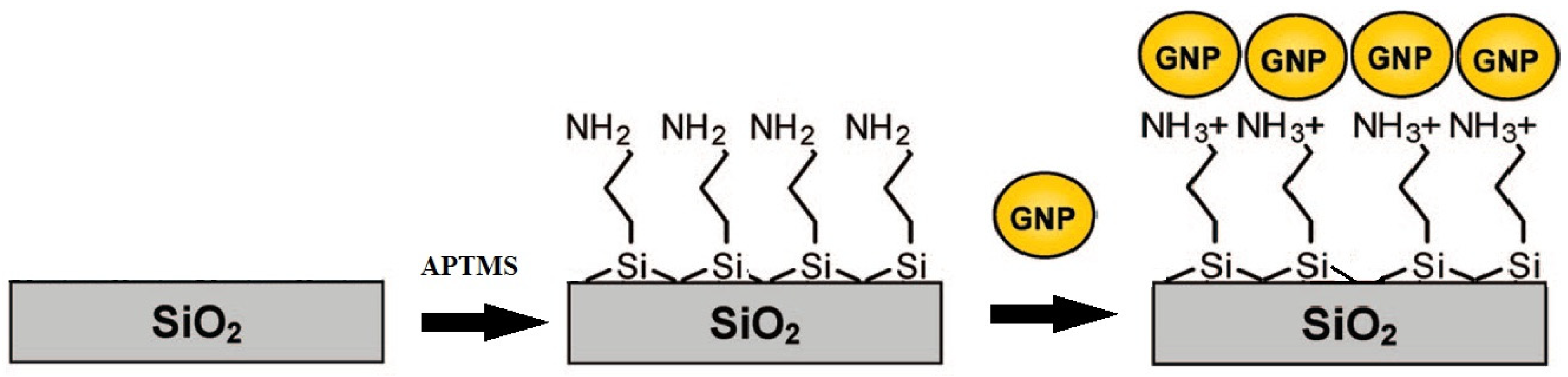
2.2.2. Fabrication of SERS Substrates
2.2.3. Synthesis of the Sol-Gel Matrix
2.3. Characterization Techniques
2.3.1. UV-Vis Absorption Spectroscopy, Dynamic Light Scattering and Z-Potential Measurements
2.3.2. Raman
2.3.3. Atomic Force Microscopy
2.3.4. Transmission Electron Microscopy
3. Results and Discussion
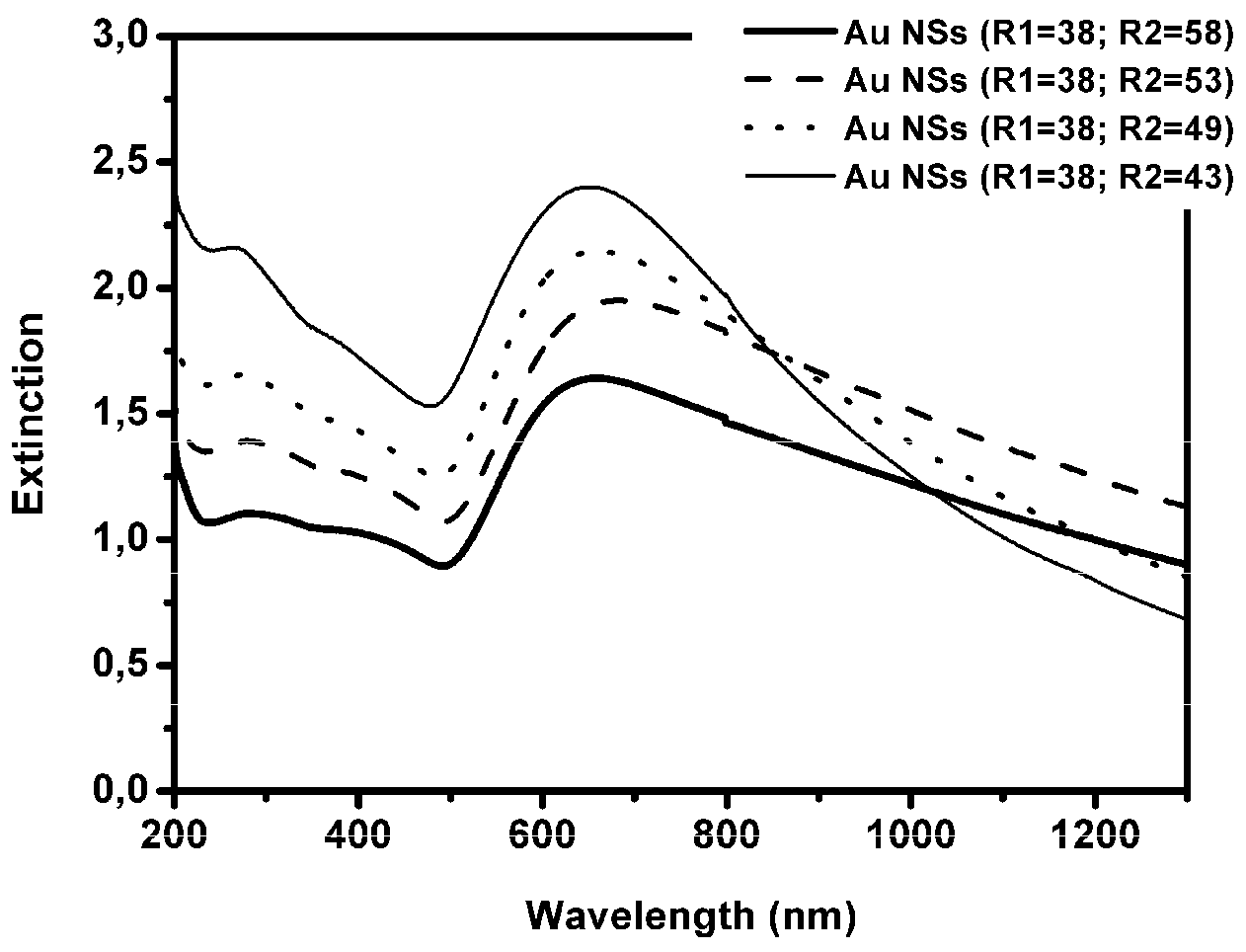
3.1. Optical and Morphological Characterization of Nanoparticles, Nanoshells, and Substrates
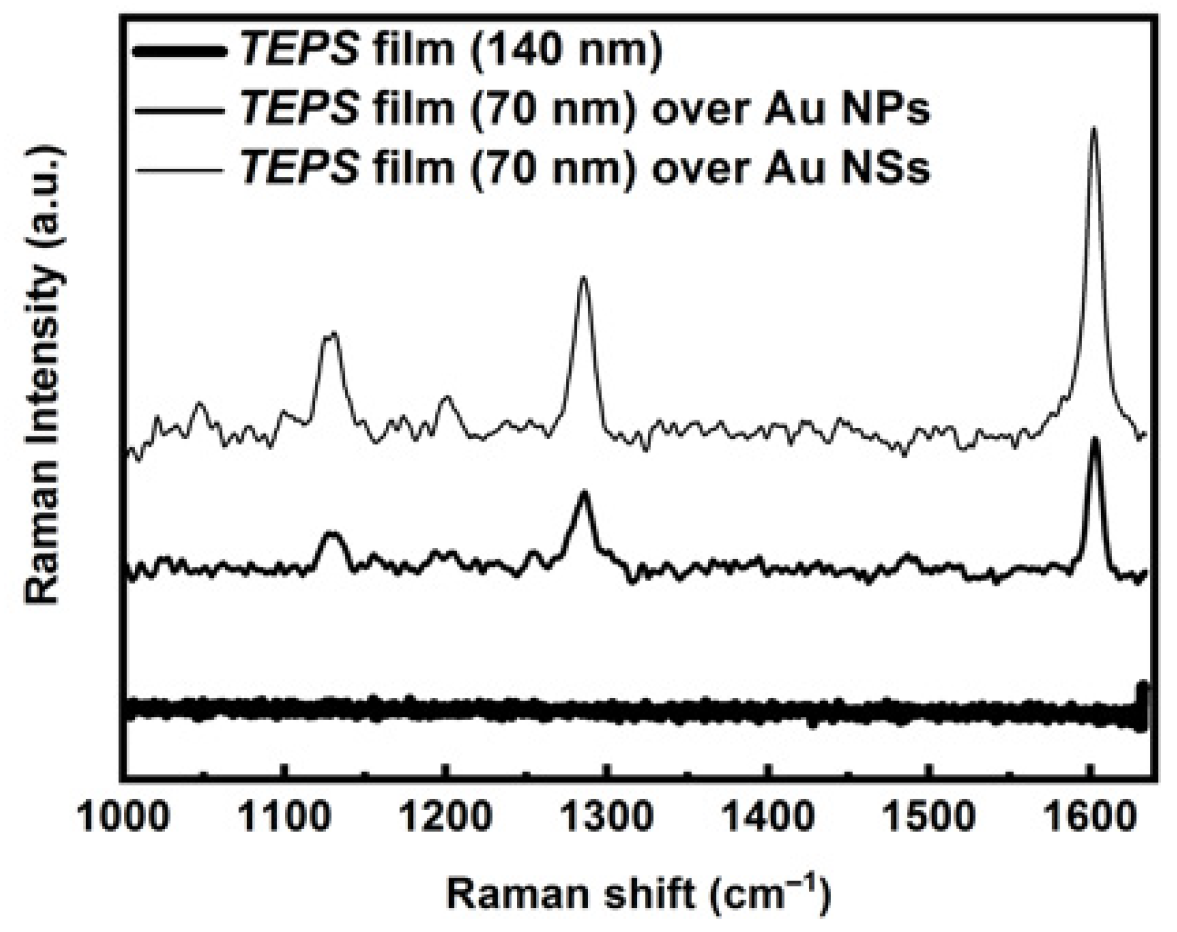
3.2. Raman Characterization of Materials and Substrates
3.3. Hybrid Sol Gel Matrix Spin Coated on Plasmonic Substrate
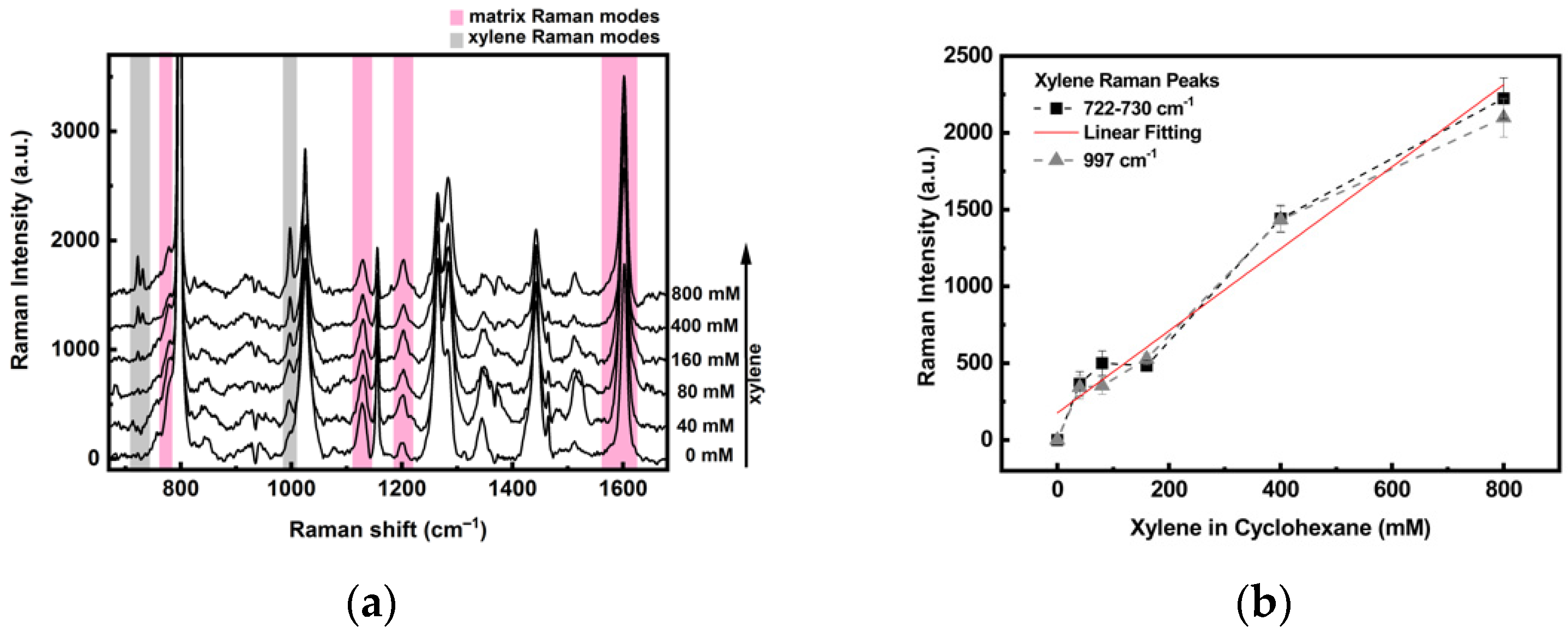
3.4. Detection of Xylene
- (a)
- The laser heats the sample, causing desorption of the xylene molecules from the matrix. Measurement of the temperature reached by the substrate upon illumination was carried out by looking at the Stokes / anti-Stokes ratio (see Supplementary Materials), revealing that T can vary from 60 °C (laser power 8 µW) to 110 °C (laser power 800 µW).
- (b)
- The xylene molecules absorbed in the TEPS layer lie not so close to the hot spots in the plasmonic substrate, not allowing an efficient exploitation of the SERS enhancement of the substrate.
- (c)
- The porosity of the TEPS layer could prevent the migration of the xylene molecules toward the plasmonic amplified region at the interface with the NSs.
- (d)
- The heating induced desorption of xylene can be overcome by using a cooling system [31] and choosing a precursor of the sol-gel film characterized by a stronger affinity for xylenes.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Langer, J.; de Aberasturi, D.J.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.J.; Boisen, A.; Brolo, A.G.; et al. Present and future of surface-enhanced Raman scattering. ACS Nano 2020, 14, 28–117. [Google Scholar] [CrossRef] [Green Version]
- Pilot, R.; Signorini, R.; Durante, C.; Orian, L.; Bhamidipati, M.; Fabris, L. A review on surface-enhanced raman scattering. Biosensors 2019, 9, 57. [Google Scholar] [CrossRef] [Green Version]
- Hakonen, A.; Andersson, P.O.; Stenbæk Schmidt, M.; Rindzevicius, T.; Käll, M. Explosive and chemical threat detection by surface-enhanced Raman scattering: A review. Anal. Chim. Acta 2015, 893, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gillibert, R.; Huang, J.Q.; Zhang, Y.; Fu, W.L.; Lamy de la Chapelle, M. Explosive detection by Surface Enhanced Raman Scattering. TrAC-Trends Anal. Chem. 2018, 105, 166–172. [Google Scholar] [CrossRef]
- Ong, T.T.X.; Blanch, E.W.; Jones, O.A.H. Surface enhanced raman spectroscopy in environmental analysis, monitoring and assessment. Sci. Total Environ. 2020, 720, 137601. [Google Scholar] [CrossRef]
- Ellis, D.I.; Muhamadali, H.; Haughey, S.A.; Elliott, C.T.; Goodacre, R. Point-and-shoot: Rapid quantitative detection methods for on-site food fraud analysis—Moving out of the laboratory and into the food supply chain. Anal. Methods 2015, 7, 9401–9414. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; He, L. Surface-enhanced raman spectroscopy for the chemical analysis of food. Compr. Rev. Food Sci. Food Saf. 2014, 13, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Cialla-May, D.; Zheng, X.-S.; Weber, K.; Popp, J. Recent progress in surface-enhanced Raman spectroscopy for biological and biomedical applications: From cells to clinics. Chem. Soc. Rev. 2017, 46, 3857–4112. [Google Scholar] [CrossRef]
- Baumberg, J.; Bell, S.; Bonifacio, A.; Chikkaraddy, R.; Chisanga, M.; Corsetti, S.; Delfino, I.; Eremina, O.; Fasolato, C.; Faulds, K.; et al. SERS in biology/biomedical SERS: General discussion. Faraday Discuss. 2017, 205, 429–456. [Google Scholar] [CrossRef] [PubMed]
- Casadio, F.; Leona, M.; Lombardi, J.R.; Van Duyne, R.P. Identification of organic colorants in fibers, paints, and glazes by surface enhanced raman spectroscopy. Acc. Chem. Res. 2010, 43, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Li, A.J.; Pal, V.K.; Kannan, K. A review of environmental occurrence, toxicity, biotransformation and biomonitoring of volatile organic compounds. Environ. Chem. Ecotoxicol. 2021, 3, 91–116. [Google Scholar] [CrossRef]
- Sweeney, W.A.; Bryan, P.F. BTX processing. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2000. [Google Scholar] [CrossRef]
- Tursi, A.; Chidichimo, F.; Bagetta, R.; Beneduci, A. BTX removal from open Aqueous systems by modified cellulose fibers and evaluation of competitive evaporation kinetics. Water 2020, 12, 3154. [Google Scholar] [CrossRef]
- Masih, A.; Lall, A.S.; Taneja, A.; Singhvi, R.; Team, R.; States, U.; Protection, E. Exposure levels and health risk assessment of ambient BTX at urban and rural environments of a terai region of northern India. Env. Pollut. 2018, 242, 1678–1683. [Google Scholar] [CrossRef]
- Singh, A.P.; Mukherji, S.; Tewari, A.K.; Kalsi, W.R.; Sarpal, A.S. Determination of benzene and total aromatics in commercial gasolines using packed column GC and NMR techniques. Fuel 2003, 82, 23–33. [Google Scholar] [CrossRef]
- Sieg, K.; Fries, E.; Puttmann, W. Analysis of benzene, toluene, ethylbenzene, xylenes and n-aldehydes in melted snow water via solid-phase dynamic extraction combined with gas chromatography/mass spectrometry. J. Chromatogr. A 2008, 1178, 178–186. [Google Scholar] [CrossRef]
- Bianchin, J.N.; Nardini, G.; Merib, J.; Dias, A.N.; Martendal, E.; Carasek, E. Simultaneous determination of polycyclic aromatic hydrocarbons and benzene, toluene, ethylbenzene and xylene in water samples using a new sampling strategy combining different extraction modes and temperatures in a single extraction solid-phase microextraction-gas chromatography–mass spectrometry procedure. J. Chromatogr. A 2012, 1233, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Tan, J.; Ding, H.; Tan, X.; Xing, J.; Xing, L.; Zhai, Y.; Li, Z. Detection of polycyclic aromatic hydrocarbons in water samples by annular platform-supported ionic liquid-based headspace liquid-phase microextraction. J. Anal. Methods Chem. 2018, 2018, 3765682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wittkamp, B.L.; Hawthorne, S.B.; Tilotta, D.C. Determination of Aromatic compounds in water by solid phase microextraction and ultraviolet absorption spectroscopy. Anal. Chem. 1997, 69, 1197–1203. [Google Scholar] [CrossRef]
- Chatzimichail, S.; Rahimi, F.; Saifuddin, A.; Surman, A.J.; Taylor-Robinson, S.D.; Salehi-Reyhani, A. Hand-portable HPLC with broadband spectral detection enables analysis of complex polycyclic aromatic hydrocarbon mixtures. Comm. Chem. 2021, 4, 17. [Google Scholar] [CrossRef]
- Cheng, C.S.; Chen, Y.Q.; Lu, C.J. Organic vapour sensing using localized surface plasmon resonance spectrum of metallic nanoparticles self assemble monolayer. Talanta 2007, 73, 358–365. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Lu, C.J. Surface modification on silver nanoparticles for enhancing vapor selectivity of localized surface plasmon resonance sensors. Sens. Actuators B Chem. 2009, 135, 492–498. [Google Scholar] [CrossRef]
- Chen, K.J.; Lu, C.J. A vapor sensor array using multiple localized surface plasmon resonance bands in a single UV-vis spectrum. Talanta 2010, 81, 1670–1675. [Google Scholar] [CrossRef] [PubMed]
- Carron, K.; Peltersen, L.; Lewis, M. Octadecylthiol-modified surface-enhanced raman spectroscopy substrates: A new method for the detection of aromatic compounds. Environ. Sci. Technol. 1992, 26, 1950–1954. [Google Scholar] [CrossRef]
- Mosier-Boss, P.A.; Lieberman, S.H. Detection of volatile organic compounds using surface enhanced Raman spectroscopy substrates mounted on a thermoelectric cooler. Anal. Chim. Acta 2003, 488, 15–23. [Google Scholar] [CrossRef]
- Wittkamp, B.L.L.; Tilotta, D.C.C. Determination of BTEX compounds in water by solid-phase microextraction and Raman spectroscopy. Anal. Chem. 1995, 67, 600–605. [Google Scholar] [CrossRef]
- Qian, C.; Guo, Q.; Xu, M.; Yuan, Y.; Yao, J. Improving the SERS detection sensitivity of aromatic molecules by a PDMS-coated Au nanoparticle monolayer film. RSC Adv. 2015, 5, 53306–53312. [Google Scholar] [CrossRef]
- Jung, H.S.; Koh, E.H.; Mun, C.W.; Min, J.; Sohng, W.; Chung, H.; Yang, J.Y.; Lee, S.; Kim, H.J.; Park, S.G.; et al. Hydrophobic hBN-coated surface-enhanced Raman scattering sponge sensor for simultaneous separation and detection of organic pollutants. J. Mater. Chem. C 2019, 7, 13059–13069. [Google Scholar] [CrossRef]
- Xia, D.; Guo, Q.; Ge, M.; Yuan, Y.; Xu, M.; Yao, J. On-line sensitive detection of aromatic vapor through PDMS/C3H7S-assisted SERS amplification. RSC Adv. 2016, 6, 53289–53295. [Google Scholar] [CrossRef]
- Men, D.; Feng, S.; Liu, G.; Hang, L.; Zhang, T. A sensitive “optical nose” for detection of volatile organic molecules based on Au@MOFs nanoparticle arrays through surface-enhanced raman scattering. Part. Part. Syst. Charact. 2020, 37, 1–8. [Google Scholar] [CrossRef]
- Oh, M.K.; De, R.; Yim, S.Y. Highly sensitive VOC gas sensor employing deep cooling of SERS film. J. Raman Spectrosc. 2018, 49, 800–809. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, S.; Zheng, J.; He, L. Surface-enhanced Raman spectroscopy (SERS) combined techniques for high-performance detection and characterization. Trends Anal. Chem. 2017, 90, 1–13. [Google Scholar] [CrossRef]
- Tijunelyte, I.; Betelu, S.; Moreau, J.; Ignatiadis, I.; Berho, C.; Lidgi-Guigui, N.; Guénin, E.; David, C.; Vergnole, S.; Rinnert, E.; et al. Diazonium salt-based surface-enhanced raman spectroscopy nanosensor: Detection and quantitation of aromatic hydrocarbons in water samples. Sensors 2017, 17, 1198. [Google Scholar] [CrossRef]
- Kreno, L.E.; Greeneltch, N.G.; Farha, O.K.; Hupp, J.T.; Van Duyne, R.P. SERS of molecules that do not adsorb on Ag surfaces: A metal-organic framework-based functionalization strategy. Analyst 2014, 139, 4073–4080. [Google Scholar] [CrossRef]
- Guizard, C.; Bac, A.; Barboiu, M.; Hovnanian, N. Hybrid organic-inorganic membranes with specific transport properties: Application in separation and sensors technologies. Sep. Purif. Technol. 2001, 25, 167–180. [Google Scholar] [CrossRef]
- Shea, K.J.; Loy, D.A. Bridged polysilsesquioxanes. molecular-engineered hybrid organic—Inorganic materials. Chem. Mater. 2001, 13, 3306–3319. [Google Scholar] [CrossRef]
- Dabrowski, A.; Barczak, M.; Robens, E.; Stolyarchuk, N.V.; Yurchenko, G.R.; Matkovskii, O.K.; Zub, Y.L. Ethylene and phenylene bridged polysilsesquioxanes functionalized by amine and thiol groups as adsorbents of volatile organic compounds. Appl. Surf. Sci. 2007, 253, 5747–5751. [Google Scholar] [CrossRef]
- Brigo, L.; Cittadini, M.; Artiglia, L.; Rizzi, G.A.; Granozzi, G.; Guglielmi, M.; Martucci, A.; Brusatin, G. Xylene sensing properties of aryl-bridged polysilsesquioxane thin films coupled to gold nanoparticles. J. Mater. Chem. C 2013, 1, 4252–4260. [Google Scholar] [CrossRef]
- Ishizaki, K.; Komarneni, S.; Nanko, M. Sol-gel processing, designing porosity, pore size and polarity, and shaping processes. In Porous Materials. Materials Technology Series; Springer: Boston, MA, USA, 1998; Volume 4. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. The formation of colloidal gold. J. Phys. Chem. 1953, 57, 670–673. [Google Scholar] [CrossRef]
- Oldenburg, S.J.; Averitt, R.; Westcott, S.L.; Halas, N.J. Nanoengineering of optical resonances. Chem. Phys. Lett. 1998, 288, 243–247. [Google Scholar] [CrossRef]
- Stober, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1967, 26, 62–69. [Google Scholar] [CrossRef]
- Freeman, R.G.; Grabar, K.C.; Allison, K.J.; Bright, R.M.; Davis, J.A.; Guthrie, A.P.; Hommer, M.B.; Jackson, M.A.; Smith, P.C.; Walter, D.G.; et al. Self-assembled metal colloid monolayers: An approach to sers substrates. Science 1995, 267, 1629–1632. [Google Scholar] [CrossRef]
- Grabar, K.C.; Freeman, R.G.; Hommer, M.B.; Natan, M.J. Preparation and characterization of Au colloid monolayers. Anal. Chem. 1995, 67, 735–743. [Google Scholar] [CrossRef]
- Brigo, L.; Gazzola, E.; Cittadini, M.; Zilio, P.; Zacco, G.; Romanato, F.; Martucci, A.; Guglielmi, M.; Brusatin, G. Short and long range surface plasmon polariton waveguides for xylene sensing. Nanotechnology 2013, 24, 155502. [Google Scholar] [CrossRef]
- Burleigh, M.C.; Markowitz, M.A.; Spector, M.S.; Gaber, B.P. Porous polysilsesquioxanes for the adsorption of phenols. Environ. Sci. Technol. 2002, 36, 2515–2518. [Google Scholar] [CrossRef]
- Brigo, L.; Faustini, M.; Pistore, A.; Kang, H.K.; Ferraris, C.; Schutzmann, S.; Brusatin, G. Porous inorganic thin films from bridged silsesquioxane sol–gel precursors. J. Non. Cryst. Solids 2016, 432, 399–405. [Google Scholar] [CrossRef]
- Amendola, V.; Meneghetti, M. Size evaluation of gold nanoparticles by UV-vis spectroscopy. J. Phys. Chem. C 2009, 113, 4277–4285. [Google Scholar] [CrossRef]
- Preston, T.C.; Signorell, R. Growth and optical properties of gold nanoshells prior to the formation of a continuous metallic layer. ACS Nano 2009, 3, 3696–3706. [Google Scholar] [CrossRef] [PubMed]
- Socrates, G. Infrared & Raman Characteristic Group Frequencies: Tables & Charts; John Wiley and Sons: Chichester, UK, 2001. [Google Scholar]
- Varsanyi, G. Vibrational Spectra of Benzene Derivatives; Academic Press: New York, NY, USA; London, UK, 1969. [Google Scholar]
- Wilmshurst, J.K.; Bernstein, H.J. The infrared and Raman spectra of toluene, toluene-a-d3, m-xylene, and m-xylene-aa’-d6. Can. J. Chem. 1957, 35, 911–925. [Google Scholar] [CrossRef]
- McFarland, A.D.; Young, M.A.; Dieringer, J.A.; Van Duyne, R.P. Wavelength-scanned surface-enhanced Raman excitation spectroscopy. J. Phys. Chem. B 2005, 109, 11279–11285. [Google Scholar] [CrossRef] [PubMed]
- Weber, V.; Feis, A.; Gellini, C.; Pilot, R.; Salvi, P.R.; Signorini, R. Far- and near-field properties of gold nanoshells studied by photoacoustic and surface-enhanced Raman spectroscopies. Phys. Chem. Chem. Phys. 2015, 17, 21190–21197. [Google Scholar] [CrossRef]
- Hunter, C.A.; Sanders, J.K.M. The Nature of π-π Interactions. J. Am. Chem. Soc. 1990, 112, 5525–5534. [Google Scholar] [CrossRef]
- Péron, O.; Rinnert, E.; Toury, T.; Lamy de la Chapellec, M.; Compèrea, C. Quantitative SERS sensors for environmental analysis of naphthalene. Analyst 2011, 136, 1018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xuegang Gong, X.; Liao, X.; Li, Y.; Cao, H.; Zhao, Y.; Lia, H.; Cassidyc, D.P. Sensitive detection of polycyclic aromatic hydrocarbons with gold colloid coupled chloride ion SERS sensor. Analyst 2019, 144, 6698. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhong, P.; Jiang, A.; Shen, X.; Li, X.; Xu, Z.; Shen, Y.; Sun, Y.; Lei, H. Raman spectroscopy coupled with chemometrics for food authentication: A review. Trends Anal. Chem. 2020, 131, 116017. [Google Scholar] [CrossRef]
- Zhou, H.; Li, X.; Wang, L.; Liang, Y.; Jialading, A.; Wang, Z.; Zhang, J. Application of SERS quantitative analysis method in food safety detection. Rev. Anal. Chem. 2021, 40, 173–186. [Google Scholar] [CrossRef]



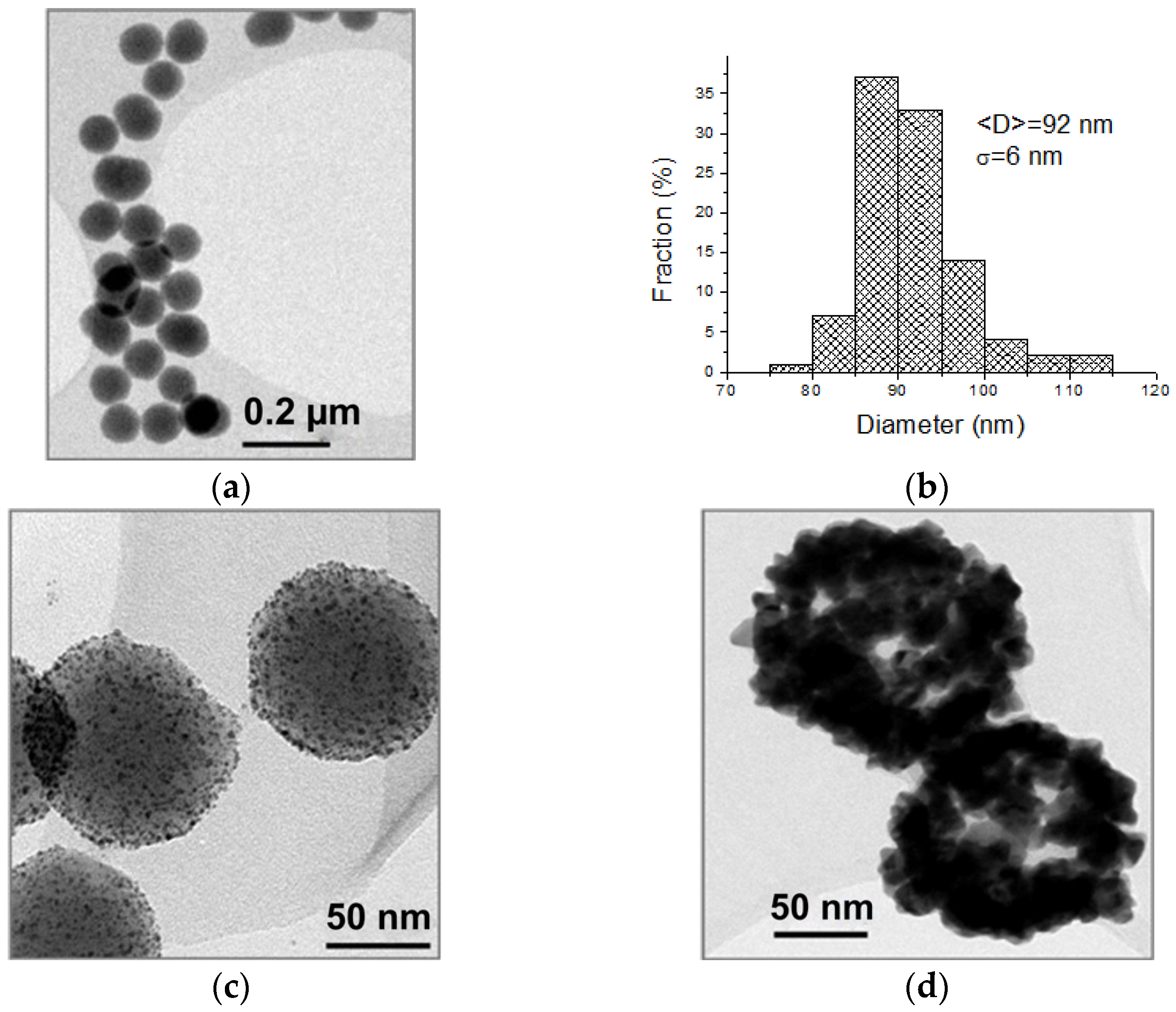
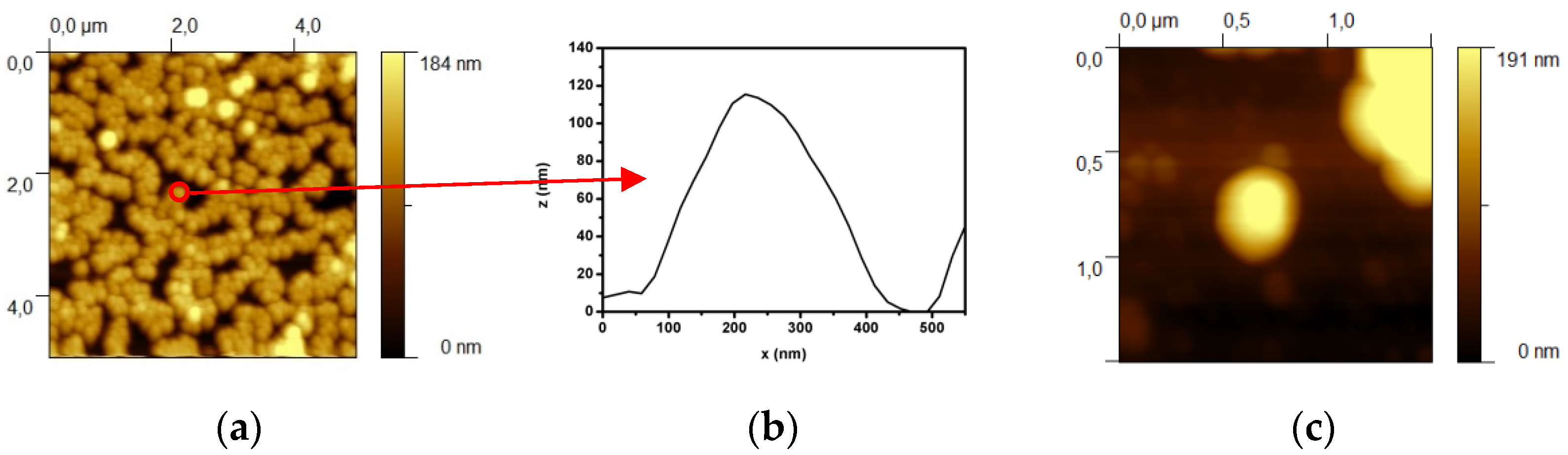
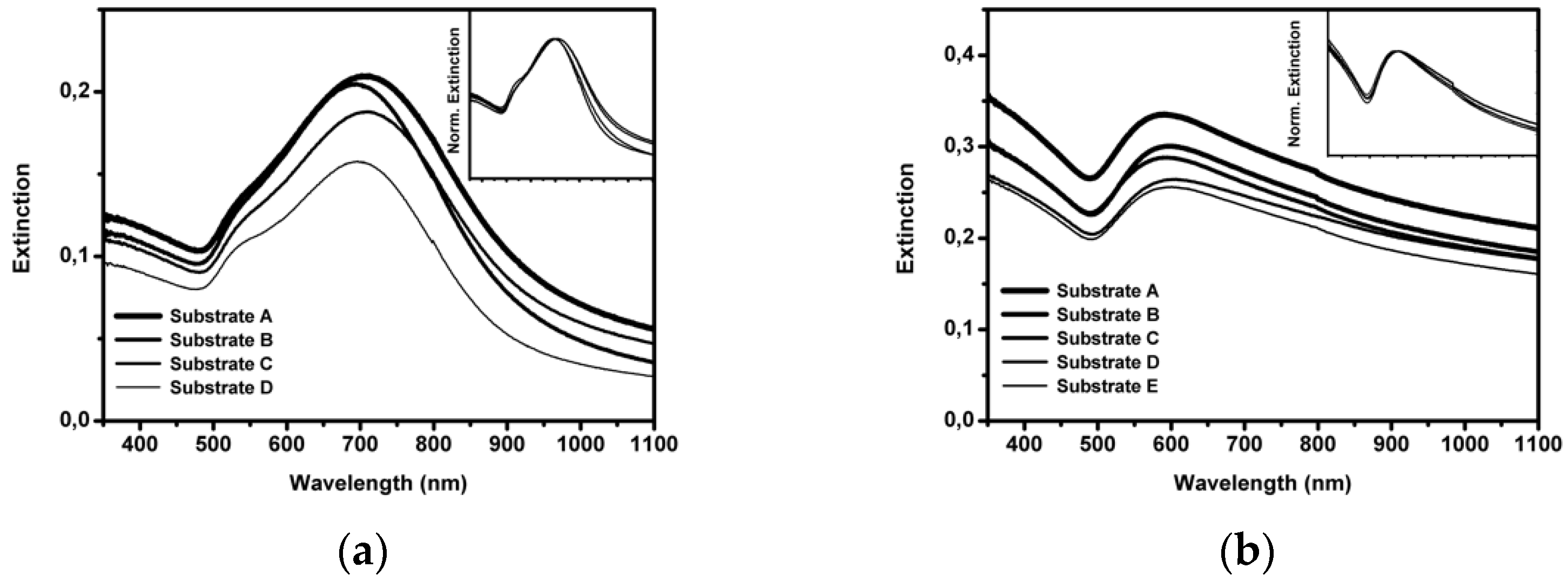

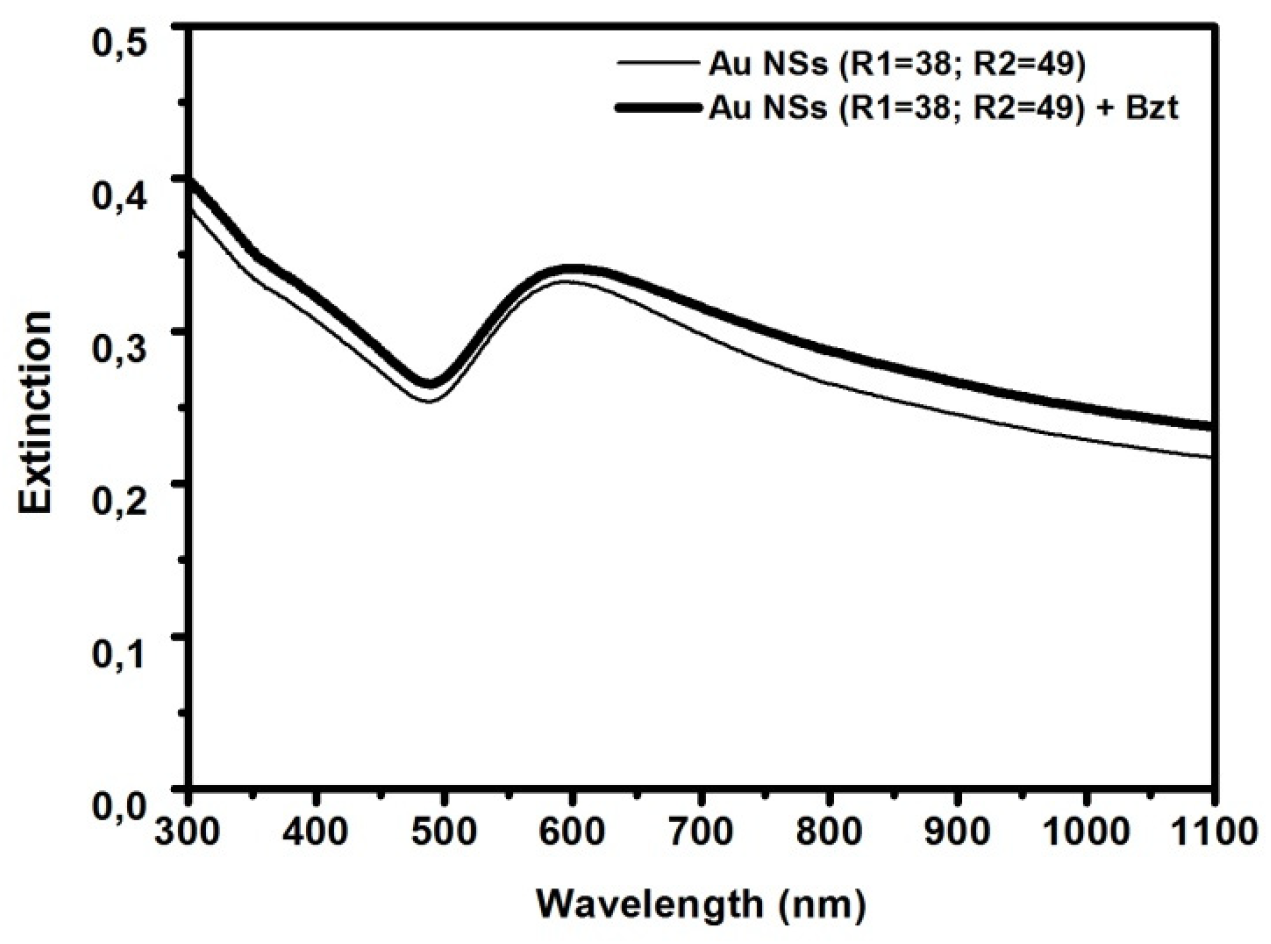
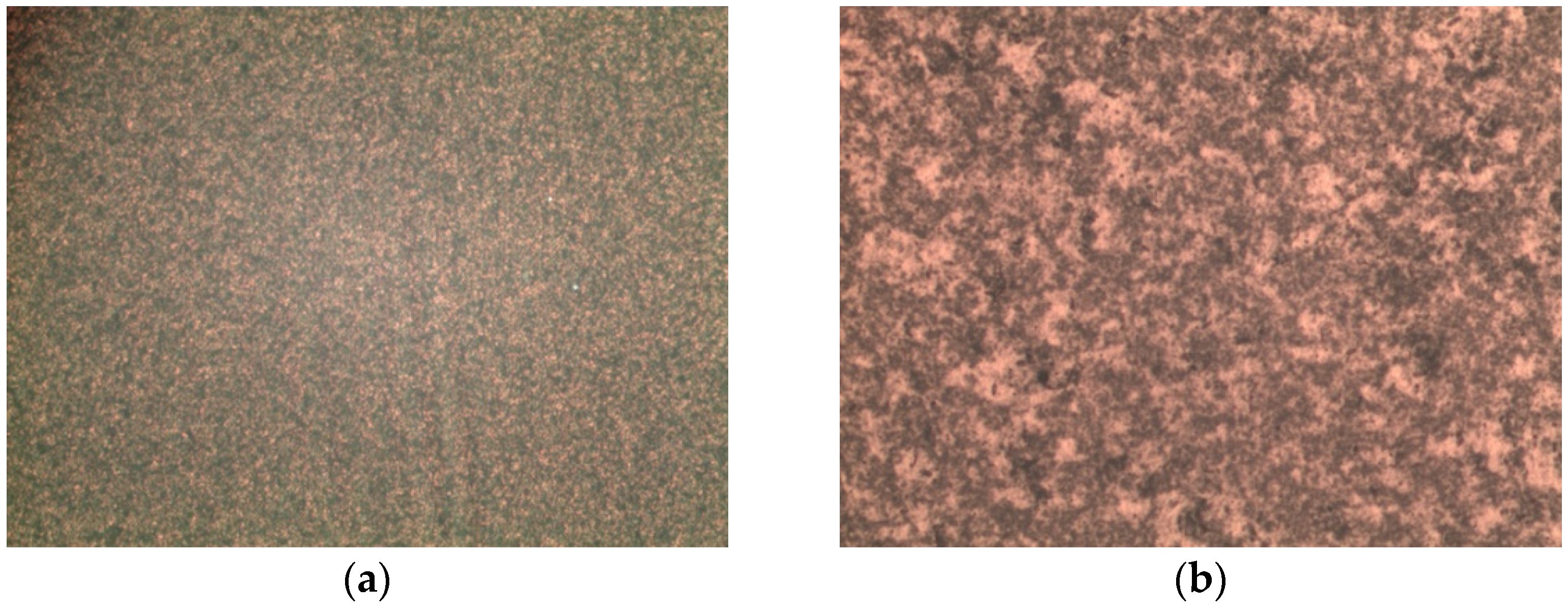



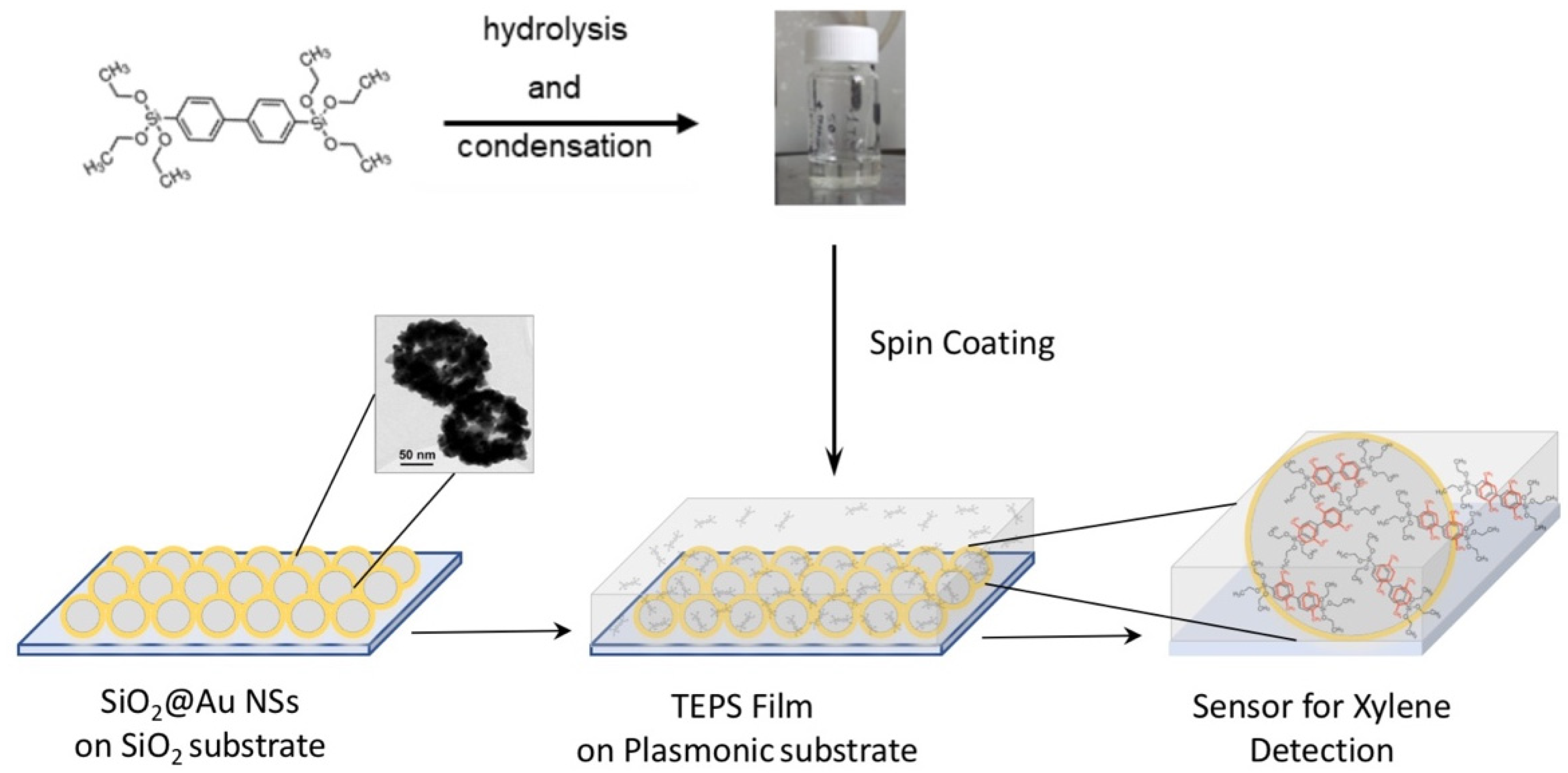


| Sample | Radius | AFM Radius [nm] | Shell Thickness [nm] | TEM Radius [nm] |
|---|---|---|---|---|
| SiO2@APTMS | R1 | 61 ± 5 | – | 46 ± 3 |
| Au NSs | R2 | 82 ± 7 | 21 ± 9 | 67 ± 9 |
| Raman Shift (cm−1) | Xylene Assignment [50,51,52] | TEPS-Film Assignment [37,38] |
|---|---|---|
| 722–730 | ν1 | |
| 780 | νs Si-C | |
| 810–827 | ν10a | |
| 997 | ν18b | |
| 1030 | ν1a ν1b | |
| 1050 | ν1b | |
| 1132 | νs Si-O | |
| 1204 | =C-H Out of plane ring def. vib. | |
| 1206 | =C-H Out of plane ring def. vib | |
| 1223 | ν1b | |
| 1250 | =C-H In plane ring def. vib. | |
| 1286 | =C-H In plane ring def. vib. | |
| 1378 | νs C-H (metyl) | |
| 1602 | νs C = C |
| Sample | Peak Frequency [cm−1] | Peak Area |
|---|---|---|
| Au NSs-TEPS substrate | 995 | 1407 |
| Au NSs substrate | 996 | 741 |
| TEPS film | 997 | 107 |
| Glass | 997 | 33 |
| Analyte | SERS Substrate | LOD [mM] | Ref. |
|---|---|---|---|
| Toluene | Au NP monolayer | 0.5 | xxxiv |
| Au NP monolayer/PDMS | 0.005 | ||
| Toluene, Xylene | Ag nanowires/hydrophobic sponge | n.d. | xxxv |
| Xylene | Au NP monolayer/TEPS | 40 | This paper |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weber, V.; Brigo, L.; Brusatin, G.; Mattei, G.; Pedron, D.; Pilot, R.; Signorini, R. Hybrid Sol-Gel Surface-Enhanced Raman Sensor for Xylene Detection in Solution. Sensors 2021, 21, 7912. https://doi.org/10.3390/s21237912
Weber V, Brigo L, Brusatin G, Mattei G, Pedron D, Pilot R, Signorini R. Hybrid Sol-Gel Surface-Enhanced Raman Sensor for Xylene Detection in Solution. Sensors. 2021; 21(23):7912. https://doi.org/10.3390/s21237912
Chicago/Turabian StyleWeber, Verena, Laura Brigo, Giovanna Brusatin, Giovanni Mattei, Danilo Pedron, Roberto Pilot, and Raffaella Signorini. 2021. "Hybrid Sol-Gel Surface-Enhanced Raman Sensor for Xylene Detection in Solution" Sensors 21, no. 23: 7912. https://doi.org/10.3390/s21237912






