Design and Characterization of an RF Applicator for In Vitro Tests of Electromagnetic Hyperthermia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Numerical Modelling
2.1.1. Electromagnetic Simulations
2.1.2. Thermal Simulations
2.2. Characterization and Measurement Set-Up
2.2.1. Reflection Measurements
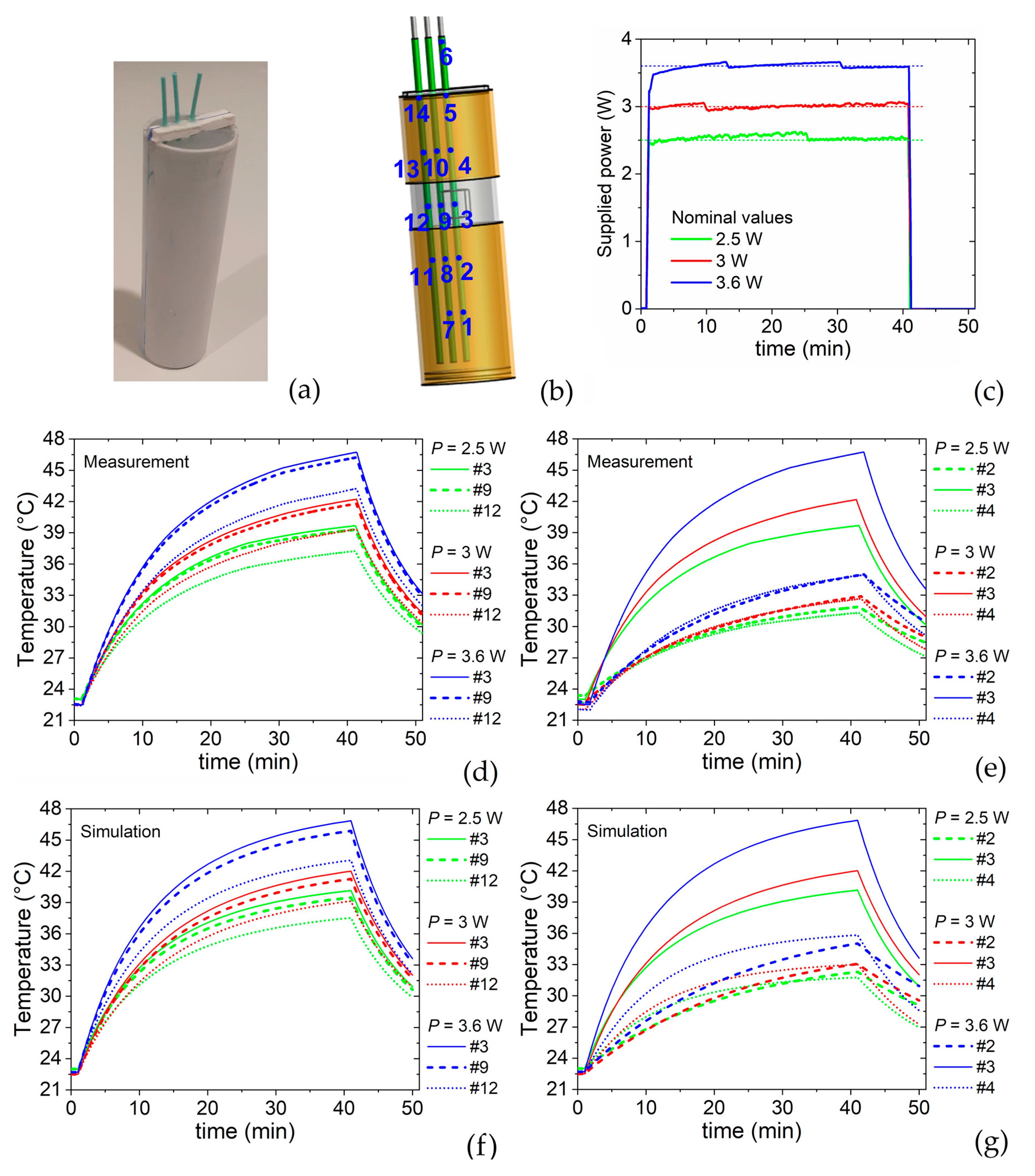
2.2.2. Power Supply and Monitoring
2.2.3. Phantom Preparation and Characterization
2.2.4. Measurement of Temperature Time Evolution
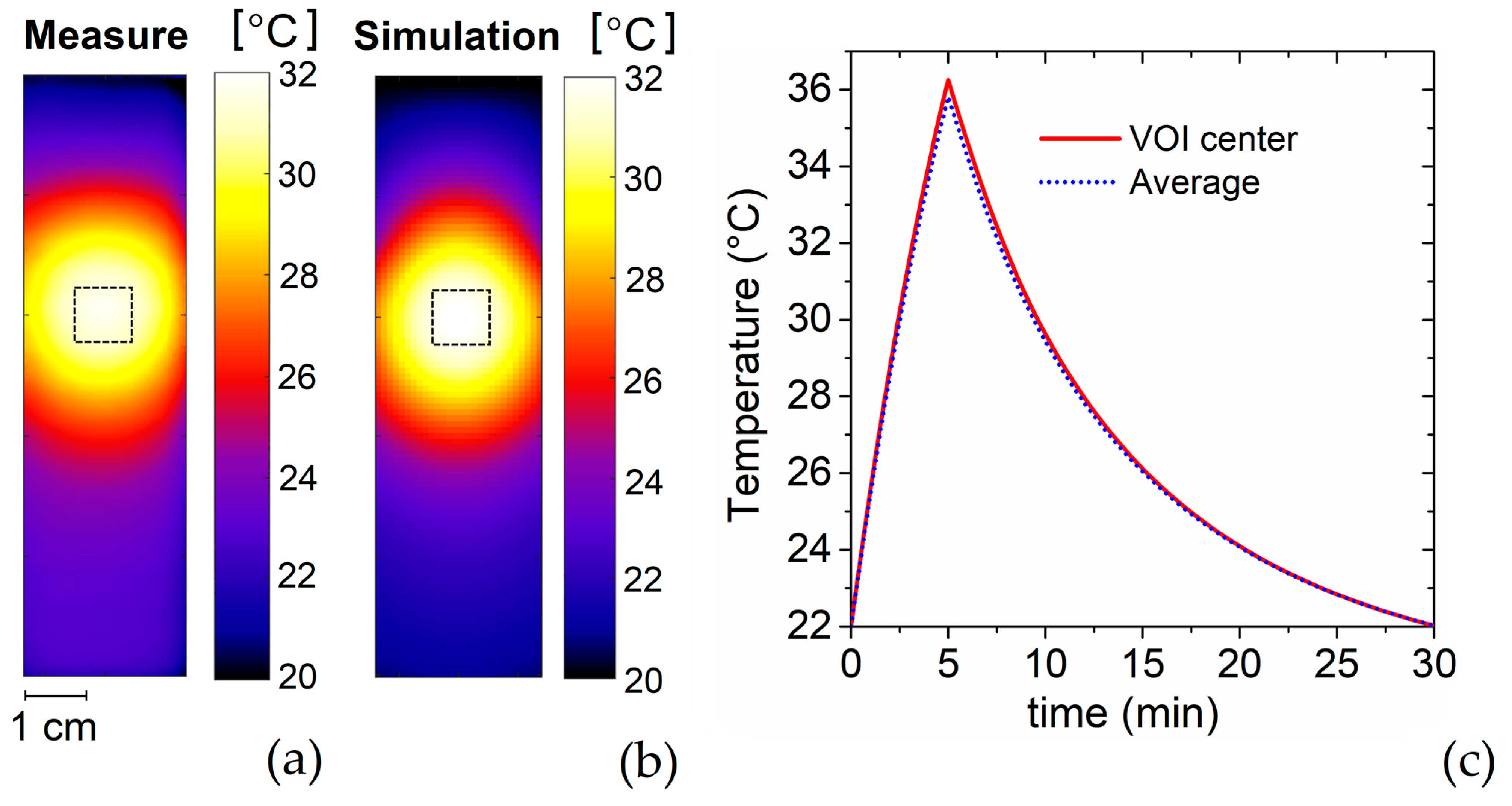
2.2.5. Measurement of Temperature Spatial Distribution
3. Design of the RF Applicator
3.1. Parametric Analysis
- (1)
- the aperture has to be properly dimensioned to guarantee the accommodation of standard vials for in vitro tests on 3D cell cultures;
- (2)
- the heating focus has to be positioned within a cubic target region of 1 cm3 volume (VOI), where a homogeneous power deposition should be obtained (schematic in Figure 1c);
- (3)
- under operating conditions, the applicator should match as close as possible a load with an impedance Z of 50 Ω, in order to minimize reflections and avoid the need of a matching network for its operation.
3.2. Final Prototype
4. Results from Characterization of the Experimental Set-Up
4.1. Phantom Electrical Properties
4.2. Power Reflection Coefficient
5. Results from Characterization of Heating Efficiency
5.1. Heating-Cooling Transients
5.2. Heating Focus
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Horsman, M.R.; Overgaard, J. Hyperthermia: A Potent Enhancer of Radiotherapy. Clin. Oncol. 2007, 19, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Oei, A.L.; Kok, H.P.; Oei, S.B.; Horsman, M.R.; Stalpers, L.J.A.; Franken, N.A.P.; Crezee, J. Molecular and biological rationale of hyperthermia as radio- and chemosensitizer. Adv. Drug Deliv. Rev. 2020, 163–164, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Overgaard, J. Simultaneous and sequential hyperthermia and radiation treatment of an experimental tumor and its surrounding normal tissue in vivo. Int. J. Radiat. Oncol. 1980, 6, 1507–1517. [Google Scholar] [CrossRef]
- Franckena, M.; Stalpers, L.J.A.; Koper, P.C.M.; Wiggenraad, R.G.J.; Hoogenraad, W.J.; van Dijk, J.D.P.; Wárlám-Rodenhuis, C.C.; Jobsen, J.J.; van Rhoon, G.C.; van der Zee, J. Long-Term Improvement in Treatment Outcome After Radiotherapy and Hyperthermia in Locoregionally Advanced Cervix Cancer: An Update of the Dutch Deep Hyperthermia Trial. Int. J. Radiat. Oncol. 2008, 70, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- VilasBoas-Ribeiro, I.; Van Rhoon, G.C.; Drizdal, T.; Franckena, M.; Paulides, M.M. Impact of Number of Segmented Tissues on SAR Prediction Accuracy in Deep Pelvic Hyperthermia Treatment Planning. Cancers 2020, 12, 2646. [Google Scholar] [CrossRef] [PubMed]
- Iero, D.A.M.; Crocco, L.; Isernia, T. Thermal and Microwave Constrained Focusing for Patient-Specific Breast Cancer Hyperthermia: A Robustness Assessment. IEEE Trans. Antennas Propag. 2014, 62, 814–821. [Google Scholar] [CrossRef]
- Kok, H.P.; Navarro, F.; Strigari, L.; Cavagnaro, M.; Crezee, J. Locoregional hyperthermia of deep-seated tumours applied with capacitive and radiative systems: A simulation study. Int. J. Hyperth. 2018, 34, 714–730. [Google Scholar] [CrossRef] [Green Version]
- Priester, M.I.; Curto, S.; van Rhoon, G.C.; ten Hagen, T.L.M. External Basic Hyperthermia Devices for Preclinical Studies in Small Animals. Cancers 2021, 13, 4628. [Google Scholar] [CrossRef]
- van Leeuwen, C.M.; Crezee, J.; Oei, A.L.; Franken, N.A.P.; Stalpers, L.J.A.; Bel, A.; Kok, H.P. 3D radiobiological evaluation of combined radiotherapy and hyperthermia treatments. Int. J. Hyperth. 2017, 33, 160–169. [Google Scholar] [CrossRef] [Green Version]
- Ryu, S.; Brown, S.L.; Kim, S.-H.; Khil, M.S.; Kim, J.H. Preferential radiosensitization of human prostatic carcinoma cells by mild hyperthermia. Int. J. Radiat. Oncol. 1996, 34, 133–138. [Google Scholar] [CrossRef]
- Brüningk, S.C.; Ijaz, J.; Rivens, I.; Nill, S.; ter Haar, G.; Oelfke, U. A comprehensive model for heat-induced radio-sensitisation. Int. J. Hyperth. 2017, 34, 392–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Mendoza, A.M.; Michlíková, S.; Berger, J.; Karschau, J.; Kunz-Schughart, L.A.; McLeod, D.D. Mathematical model for the thermal enhancement of radiation response: Thermodynamic approach. Sci. Rep. 2021, 11, 5503. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Luo, D.; Zhou, X.; Sun, Q.; Shen, P.; Wang, S. Combined effects of hyperthermia and chemotherapy on the regulate autophagy of oral squamous cell carcinoma cells under a hypoxic microenvironment. Cell Death Discov. 2021, 7, 227. [Google Scholar] [CrossRef] [PubMed]
- Brüningk, S.C.; Rivens, I.; Box, C.; Oelfke, U.; ter Haar, G. 3D tumour spheroids for the prediction of the effects of radiation and hyperthermia treatments. Sci. Rep. 2020, 10, 1653. [Google Scholar] [CrossRef] [PubMed]
- Mouratidis, P.X.E.; Rivens, I.; Civale, J.; Symonds-Tayler, R.; ter Haar, G. ‘Relationship between thermal dose and cell death for “rapid” ablative and “slow” hyperthermic heating’. Int. J. Hyperth. 2019, 36, 228–242. [Google Scholar] [CrossRef] [Green Version]
- Mei, X.; Ten Cate, R.; Van Leeuwen, C.M.; Rodermond, H.M.; De Leeuw, L.; Dimitrakopoulou, D.; Stalpers, L.J.A.; Crezee, J.; Kok, H.P.; Franken, N.A.P.; et al. Radiosensitization by hyperthermia: The effects of temperature, sequence, and time interval in cervical cell lines. Cancers 2020, 12, 582. [Google Scholar] [CrossRef] [Green Version]
- Novák, P.; Moros, E.G.; Parry, J.J.; E Rogers, B.; Myerson, R.J.; Zeug, A.; E Locke, J.; Rossin, R.; Straube, W.L.; Singh, A.K. Experience with a small animal hyperthermia ultrasound system (SAHUS): Report on 83 tumours. Phys. Med. Biol. 2005, 50, 5127–5139. [Google Scholar] [CrossRef]
- Raaijmakers, E.A.L.; Mestrom, R.M.C.; Sumser, K.; Salim, G.; Van Rhoon, G.C.; Essers, J.; Paulides, M.M. An MR-compatible antenna and application in a murine superficial hyperthermia applicator. Int. J. Hyperth. 2018, 34, 697–703. [Google Scholar] [CrossRef] [Green Version]
- Salahi, S.; Maccarini, P.F.; Rodrigues, D.B.; Etienne, W.; Landon, C.D.; Inman, B.A.; Dewhirst, M.W.; Stauffer, P.R. Miniature microwave applicator for murine bladder hyperthermia studies. Int. J. Hyperth. 2012, 28, 456–465. [Google Scholar] [CrossRef] [Green Version]
- Prasad, B.; Kim, S.; Cho, W.; Kim, S.; Kim, J.K. Effect of tumor properties on energy absorption, temperature mapping, and thermal dose in 13.56-MHz radiofrequency hyperthermia. J. Therm. Biol. 2018, 74, 281–289. [Google Scholar] [CrossRef]
- Rodrigues, H.F.; Capistrano, G.; Bakuzis, A.F. In vivo magnetic nanoparticle hyperthermia: A review on preclinical studies, low-field nano-heaters, noninvasive thermometry and computer simulations for treatment planning. Int. J. Hyperth. 2020, 37, 76–99. [Google Scholar] [CrossRef] [PubMed]
- Manzin, A.; Ferrero, R.; Vicentini, M. From Micromagnetic to in Silico Modeling of Magnetic Nanodisks for Hyperthermia Applications. Adv. Theory Simul. 2021, 4, 2100013. [Google Scholar] [CrossRef]
- Dutz, S.; Hergt, R. Magnetic particle hyperthermia—A promising tumour therapy? Nanotechnology 2014, 25, 452001. [Google Scholar] [CrossRef] [PubMed]
- Vilas-Boas, V.; Carvalho, F.; Espiña, B. Magnetic Hyperthermia for Cancer Treatment: Main Parameters Affecting the Outcome of In Vitro and In Vivo Studies. Molecules 2020, 25, 2874. [Google Scholar] [CrossRef] [PubMed]
- Sapozink, M.D.; Gibbs, F.A., Jr.; Gates, K.S.; Stewart, J.R. Regional hyperthermia in the treatment of clinically advanced, deep seated malignancy: Results of a pilot study employing an annular array applicator. Int. J. Radiat. Oncol. Biol. Phys. 1984, 10, 775–786. [Google Scholar] [CrossRef]
- Diederich, C.J.; Hynynen, K. Ultrasound technology for hyperthermia. Ultrasound Med. Biol. 1999, 25, 871–887. [Google Scholar] [CrossRef]
- Ohguri, T.; Kuroda, K.; Yahara, K.; Nakahara, S.; Kakinouchi, S.; Itamura, H.; Morisaki, T.; Korogi, Y. Optimization of the Clinical Setting Using Numerical Simulations of the Electromagnetic Field in an Obese Patient Model for Deep Regional Hyperthermia of an 8 MHz Radiofrequency Capacitively Coupled Device in the Pelvis. Cancers 2021, 13, 979. [Google Scholar] [CrossRef]
- Turner, P.F. Regional Hyperthermia with an Annular Phased Array. IEEE Trans. Biomed. Eng. 1984, 31, 106–114. [Google Scholar] [CrossRef]
- Lagendijk, J.J.W. A New Coaxial TEM Radiofrequency/Microwave Applicator for Non-Invasive Deep-Body Hyperthermia. J. Microw. Power 1983, 18, 367–375. [Google Scholar] [CrossRef]
- De Leeuw, A.A.C.; Lagendijk, J.J.W. Design of a clinical deep-body hyperthermia system based on the ‘coaxial TEM’ applicator. Int. J. Hyperth. 1987, 3, 413–421. [Google Scholar] [CrossRef]
- De Leeuw, A.A.C.; Mooibroek, J.; Lagendijk, J.J.W. Specific absorption rate steering by patient positioning in the ‘Coaxial TEM’ system: Phantom investigation. Int. J. Hyperth. 1991, 7, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Sim4Life. Available online: https://zmt.swiss/sim4life/ (accessed on 9 May 2022).
- Yee, K.S.; Chen, J.S. The finite-difference time-domain (FDTD) and the finite-volume time-domain (FVTD) methods in solving Maxwell’s equations. IEEE Trans. Antennas Propag. 1997, 45, 354–363. [Google Scholar] [CrossRef]
- Joines, W.T.; Zhang, Y.; Li, C.; Jirtle, R.L. The measured electrical properties of normal and malignant human tissues from 50 to 900 MHz. Med. Phys. 1994, 21, 547–550. [Google Scholar] [CrossRef] [PubMed]
- Balidemaj, E.; de Boer, P.; van Lier, A.L.H.M.W.; Remis, R.F.; Stalpers, L.J.A.; Westerveld, G.H.; Nederveen, A.J.; van den Berg, C.A.T.; Crezee, J. In vivo electric conductivity of cervical cancer patients based on B1+ maps at 3T MRI. Phys. Med. Biol. 2016, 61, 1596–1607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakker, J.F.; Paulides, M.M.; Westra, A.H.; Schippers, H.; Van Rhoon, G.C. Design and test of a 434 MHz multi-channel amplifier system for targeted hyperthermia applicators. Int. J. Hyperth. 2010, 26, 158–170. [Google Scholar] [CrossRef]
- Ferrero, R.; Barrera, G.; Celegato, F.; Vicentini, M.; Sözeri, H.; Yıldız, N.; Dinçer, C.A.; Coïsson, M.; Manzin, A.; Tiberto, P. Experimental and Modelling Analysis of the Hyperthermia Properties of Iron Oxide Nanocubes. Nanomaterials 2021, 11, 2179. [Google Scholar] [CrossRef]
- Rubia-Rodríguez, I.; Santana-Otero, A.; Spassov, S.; Tombácz, E.; Johansson, C.; De La Presa, P.; Teran, F.; Morales, M.D.P.; Veintemillas-Verdaguer, S.; Thanh, N.; et al. Whither Magnetic Hyperthermia? A Tentative Roadmap. Materials 2021, 14, 706. [Google Scholar] [CrossRef]



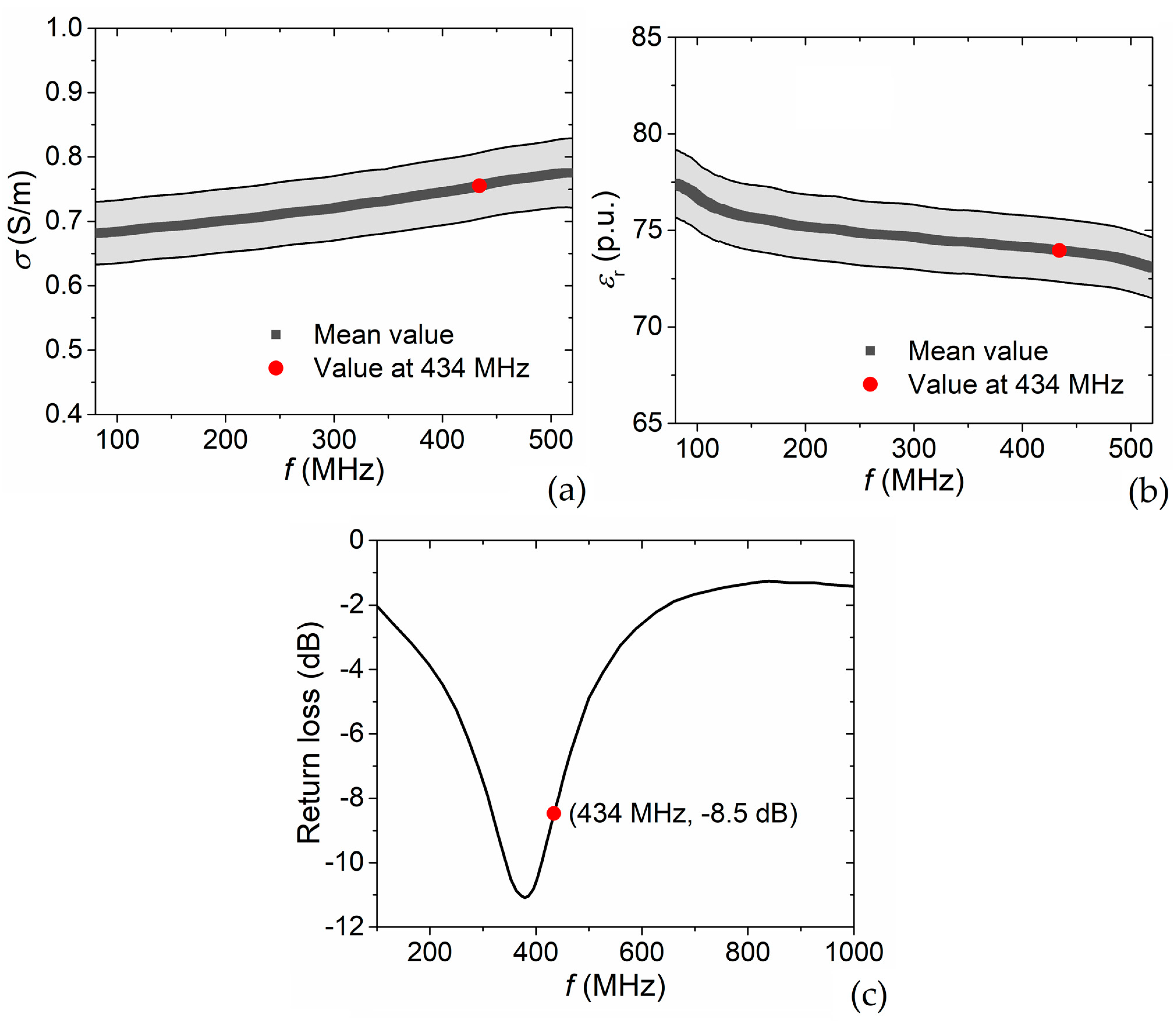

| Material | ρ [kg/m3] | σ [S/m] | εr 1 | Cp [J/(kg·K)] | k [W/(m·K)] |
|---|---|---|---|---|---|
| HDPE 1 | 950 | 0.00001 | 2.1 | 1900 | 0.5 |
| PVC 1 | 1380 | 0.0001 | 3 | 1250 | 0.2 |
| Agar gel | 1000 | 0.755 | 74 | 4181 | 0.563 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrero, R.; Androulakis, I.; Martino, L.; Nadar, R.; van Rhoon, G.C.; Manzin, A. Design and Characterization of an RF Applicator for In Vitro Tests of Electromagnetic Hyperthermia. Sensors 2022, 22, 3610. https://doi.org/10.3390/s22103610
Ferrero R, Androulakis I, Martino L, Nadar R, van Rhoon GC, Manzin A. Design and Characterization of an RF Applicator for In Vitro Tests of Electromagnetic Hyperthermia. Sensors. 2022; 22(10):3610. https://doi.org/10.3390/s22103610
Chicago/Turabian StyleFerrero, Riccardo, Ioannis Androulakis, Luca Martino, Robin Nadar, Gerard C. van Rhoon, and Alessandra Manzin. 2022. "Design and Characterization of an RF Applicator for In Vitro Tests of Electromagnetic Hyperthermia" Sensors 22, no. 10: 3610. https://doi.org/10.3390/s22103610







