Commercial Off-the-Shelf Components (COTS) in Realizing Miniature Implantable Wireless Medical Devices: A Review
Abstract
:1. Introduction
2. Macro Purview of COTS-Enabled IWMDs
2.1. History of Wireless Implants
2.2. Role of IWMDs in Healthcare Networks
2.3. Regulatory Guidelines for Medical Devices
2.4. Health and Safety
2.4.1. Effects of Electromagnetic (EM) Energy on Human Body
2.4.2. Encapsulation
2.4.3. Failure Due to Multiple Medical Steam Sterilization
3. Essential Building Blocks of COTS-Enabled IWMDs
3.1. Essential Blocks Description
3.1.1. Antenna
3.1.2. RF Transceiver
3.1.3. Power Management Unit
3.1.4. Microcontroller Unit
3.1.5. Analog Front-End (AFE) and Sensors
3.2. Consideration of the Number of COTS Components in IWMDs
3.3. Considerations of Power Supplies for IWMDs
3.3.1. Rechargeable Batteries for IWMD
3.3.2. Regular Batteries for IWMD
3.3.3. Battery-Free or Wirelessly Powered Transfer
4. Compilation of Recent Publications on RF Transceiver-Based COTS-Enabled IWMDs
- Near-field communications (NFC) and UHF RFID standards are suitable choices for battery-free IWMDs which operate over a very short-range (and contact-based) wireless connectivity and power transfer. In comparison with battery-operated MICS and BLE-enabled devices (ranging between −70 dBm and −100 dBm), implantable RFID devices have very poor RF receiver sensitivity levels (ranging between −10 and −20 dBm). This limits the operating range of battery-free RFID devices to a range of a few cm.
- Battery-operated COTS IWMDs can achieve wireless connectivity at distances greater than 1 m. However, they must contend with larger form factors and require rechargeable energy storage devices to operate over the desired application’s lifetime.
- A solar-powered implant potentially has an unlimited energy source and unlimited operating range [73] subject to the availability of direct sunlight. Additionally, the size of the solar cells to be used for IWMD is a concern.
| Compilation of Recent Publications on RF Transceiver-Based COTS-Enabled IWMDs | |||||
|---|---|---|---|---|---|
| Ref | COTS Component | Manufacturer (Vendor) | Wireless Standard | Power Source | Operating Range |
| [71] | SL900A RFID Sensory Tag | Austria Microsystems | UHF RFID | Battery-free | 60 cm |
| [98] | EM4325 RFID Sensory Tag | EM Microelectronics | UHF RFID | WPT: 6.78 MHz | Not mentioned |
| [99] | MONZA-4 RFID Chip | IMPINJ | UHF RFID | Battery-free | ≈35 cm |
| [100] | MONZA-4 RFID Die | IMPINJ | UHF RFID | Battery-free | ≈35 cm |
| [101] | G2X RFID Chip | NXP | UHF RFID | Battery-free | 20 cm |
| [102] | Alien Higgs-4 RFID Chip | Alien Technology | UHF RFID | Battery-free | 10 cm |
| [103] | Alien Higgs-4 RFID Chip | Alien Technology | UHF RFID | Battery-free | Not mentioned |
| [104] | Alien Higgs-4 RFID Chip | Alien Technology | UHF RFID | Battery-free | Not mentioned |
| [92] | CC430F5137 SoC, PIC12LF1552 | TI, Microchip | 433 MHz NFC RFID | WPT: 6.67 MHz, Rechargeable battery | 10 cm 8.8 cm |
| [105] | USRP B210 SDR | Ettus Research | UHF RFID | WPT: 13.56 MHz | 5 mm |
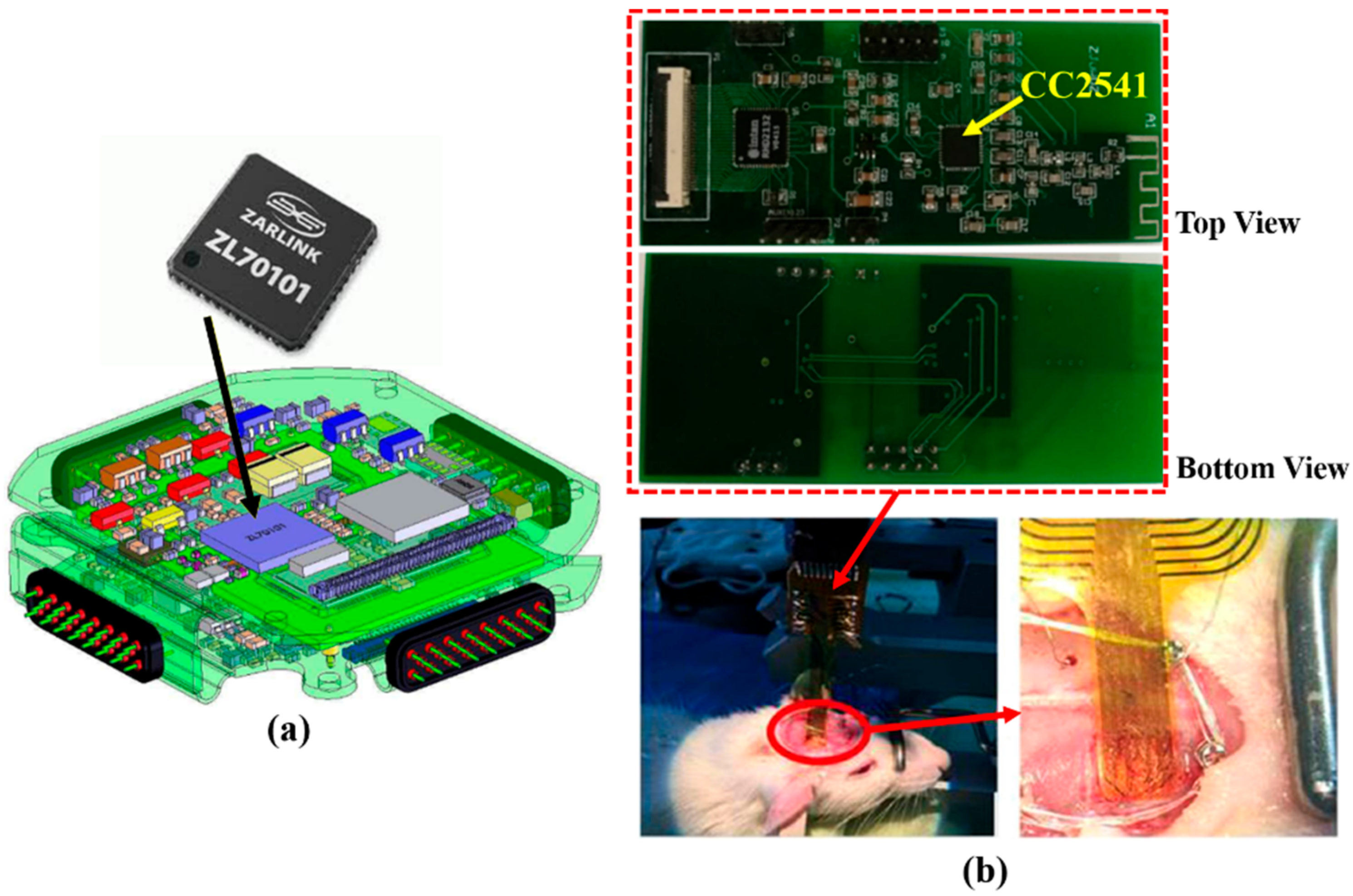
| [47] | ZL70101 | Microsemi | MICS 402 MHz | WPT 13.56 MHz | 2 m |
| [106] | ZL70102 | Microsemi | MICS 402 MHz | WPT TI: BQ51013 | 5 mm |
| [107] | ZL70101 | Microsemi | Implant 2.45 GHz WuR: 420 MHz | WPT: 13.56 MHz | ≈2 m |
| [108] | ZL70123 | Microsemi | MICS 402 MHz WuR: 420 MHz | 600 mAh Li-ion | ≈2.5 m |
| [75] | ZL70102 | Microsemi | MICS 402 MHz WuR: 2.45 GHz | WPT TI: BQ51013 | 1.98 m |
| [73] | RFD77101 | SIMBLEE | BLE: 2.45 GHz | 7 mAh rechargeable battery (VL1220) with solar panel | N/A |
| [96] | CC2640 | TI | BLE: 2.45 GHz | Battery | Not mentioned |
| [97] | nRF51822 | NORDIC | BLE: 2.45 GHz | Rechargeable Li-ion, WPT: 1.056 MHz | 10 mm |
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- John, O. Maintaining and sustaining a telehealth-based ecosystem. In Fundamentals of Telemedicine and Telehealth; Academic Press: Cambridge, MA, USA, 2020; pp. 127–143. [Google Scholar] [CrossRef]
- World Health Organization Telemedicine: Opportunities and Developments in Member States: Report on the Second Global Survey on eHealth. 2010. Available online: https://apps.who.int/iris/bitstream/handle/10665/44497/9789241564144_eng.pdf?sequence=1&isAllowed=y (accessed on 10 December 2021).
- Kaushik, A.; Mujawar, M.A. Point of Care Sensing Devices: Better Care for Everyone. Sensors 2018, 18, 4303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghafar-Zadeh, E. Wireless integrated biosensors for point-of-care diagnostic applications. Sensors 2015, 15, 3236–3261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trautner, B.W.; Darouiche, R.O. Catheter-associated infections: Pathogenesis affects prevention. Arch. Intern. Med. 2004, 164, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.C.; Gambini, S.; Izyumin, I.; Moin, A.; Zhou, A.; Alexandrov, G.; Santacruz, S.R.; Rabaey, J.M.; Carmena, J.M.; Muller, R. An implanTable 700μW 64-channel neuromodulation IC for simultaneous recording and stimulation with rapid artifact recovery. In Proceedings of the 2017 Symposium on VLSI Circuits, Kyoto, Japan, 5–8 June 2017; pp. C48–C49. [Google Scholar] [CrossRef] [Green Version]
- Bhamra, H.; Tsai, J.W.; Huang, Y.W.; Yuan, Q.; Shah, J.V.; Irazoqui, P. A Subcubic Millimeter Wireless Implantable Intraocular Pressure Monitor Microsystem. IEEE Trans. Biomed. Circ. Syst. 2017, 11, 1204–1215. [Google Scholar] [CrossRef]
- Chow, E.Y.; Chlebowski, A.L.; Chakraborty, S.; Chappell, W.J.; Irazoqui, P.P. Fully wireless implantable cardiovascular pressure monitor integrated with a medical stent. IEEE Trans. Biomed. Eng. 2010, 57, 1487–1496. [Google Scholar] [CrossRef]
- Charthad, J.; Chang, T.C.; Liu, Z.; Sawaby, A.; Weber, M.J.; Baker, S.; Gore, F.; Felt, S.A.; Arbabian, A. A mm-Sized wireless implantable device for electrical stimulation of peripheral nerves. IEEE Trans. Biomed. Circuits Syst. 2018, 12, 257–270. [Google Scholar] [CrossRef]
- Bisht, R.; Mandal, A.; Mitra, A.K. Micro-and Nanotechnology-Based Implantable Devices and Bionics. In Emerging Nanotechnologies for Diagnostics, Drug Delivery and Medical Devices; Elsevier: Amsterdam, The Netherlands, 2017; pp. 249–290. [Google Scholar] [CrossRef]
- Meng, E.; Hoang, T. Micro- and nano-fabricated implantable drug-delivery systems. Ther. Deliv. 2012, 3, 1457–1467. [Google Scholar] [CrossRef] [Green Version]
- Kwong, D.-L. Bringing the Benefits of Moore’s Law to Medicine; Institute of Microelectronics, Agency for Science, Technology, and Research: Singapore, 2011. [Google Scholar]
- Kazmi, A.; Arslan, B.R.; Tulgar, M.; Pedersen, P.C.; Pahlavan, K.; Cengiz, B.; Arslan, A. Advances in wireless telemedicine: Improvements and challenges facing wireless ultrasound. In Proceedings of the 2000 IEEE EMBS International Conference on Information Technology Applications in Biomedicine, ITAB-ITIS, Arlington, VA, USA, 9–10 November 2000. [Google Scholar] [CrossRef]
- Fuller, J.L.; Gordon, T.M. The Radio Inductograph—A Device for Recording Physiological Activity in Unrestrained Animals. Science 1948, 108, 287–288. [Google Scholar] [CrossRef]
- Jacobson, B.; Mackay, R.S. A pH-endoradiosonde. Lancet 1957, 269, 1224. [Google Scholar] [CrossRef]
- Watson, B.W.; Ross, B.; Kay, A.W. Telemetering from within the body using a pressure-sensitive radio pill. Gut 1962, 3, 181. [Google Scholar] [CrossRef] [Green Version]
- Ko, W.H.; Neuman, M.R. Implant Biotelemetry and Microelectronics. Science 1967, 156, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Pipes, E.W. Miniaturuzed Radiotelemetry Device for Monitoring Temperatures; Union Carbide Corp.: Oak Ridge, TN, USA, 1966. [Google Scholar]
- Fryer, T. Implantable Biotelemetry Systems: A Report; NASA SP-5094; NASA: Pasadena, CA, USA, 1970. [Google Scholar]
- Troyk, P.R. Injectable electronic identification, monitoring, and stimulation systems. Annu. Rev. Biomed. Eng. 1999, 1, 177–209. [Google Scholar] [CrossRef] [PubMed]
- Fulford-Jones, T.; Welsh, M.; Moulton, S.; Malan, D. CodeBlue: An Ad Hoc Sensor Network Infrastructure for Emergency Medical Care. In Proceedings of the International Workshop on Wearable and Implantable Body Sensor Networks, London, UK, 6–7 April 2004. [Google Scholar]
- Xie, K.; Zhang, S.; Dong, S.; Li, S.; Yu, C.; Xu, K.; Chen, W.; Guo, W.; Luo, J.; Wu, Z. Portable wireless electrocorticography system with a flexible microelectrodes array for epilepsy treatment. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef]
- Faerber, J.; Cummins, G.; Pavuluri, S.K.; Record, P.; Rodriguez, A.R.A.; Lay, H.S.; McPhillips, R.; Cox, B.F.; Connor, C.; Gregson, R.; et al. In Vivo Characterization of a Wireless Telemetry Module for a Capsule Endoscopy System Utilizing a Conformal Antenna. IEEE Trans. Biomed. Circ. Syst. 2017, 12, 95–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avaltroni, P.; Nappi, S.; Marrocco, G.; Member, S. Antennifying Orthopedic Bone-Plate Fixtures for the Wireless Monitoring of Local Deep Infections. IEEE Sens. J. 2021, 21, 21012–21021. [Google Scholar] [CrossRef]
- Cohen, T. Medical and information technologies converge. IEEE Eng. Med. Biol. Mag. 2004, 23, 59–65. [Google Scholar] [CrossRef]
- Mahfouz, M.R.; Kuhn, M.J.; To, G. Wireless medical devices: A review of current research and commercial systems. In Proceedings of the IEEE Topical Conference on Biomedical Wireless Technologies, Networks, and Sensing Systems, Austin, TX, USA, 23–25 January 2013; pp. 16–18. [Google Scholar] [CrossRef]
- Elayan, H.; Shubair, R.M.; Kiourti, A. Wireless sensors for medical applications: Current status and future challenges. In Proceedings of the 11th European Conference on Antennas and Propagation (EUCAP), Paris, France, 19–24 March 2017; pp. 2478–2482. [Google Scholar] [CrossRef]
- WHO. A comprehensive guide to the Global Medical Device Nomenclature. 2010. Available online: https://www.who.int/medical_devices/innovation/GMDN_Agency_User_Guide_v120810.pdf (accessed on 14 July 2021).
- FDA Medical Device Databases. 2022. Available online: https://www.fda.gov/medical-devices/device-advice-comprehensive-regulatory-assistance/medical-device-databases (accessed on 14 July 2021).
- US Airforce Material Command: COTS Guide. 1993. Available online: https://nepp.nasa.gov/DocUploads/1219C61B-7337-48C4-8760E6456F861839/COTSguide.pdf (accessed on 14 July 2021).
- Texas Instruments INC Components for Space, Avionics and Defense. 2022. Available online: www.ti.com/SpaceAvionicsDefense (accessed on 14 July 2021).
- IEC/IEEE 62704-1. IEC/IEEE International Standard—Determining the Peak Spatial-Average Specific Absorption Rate (SAR) in the Human Body from Wireless Communications Devices, 30 MHz to 6 GHz—Part 1: General Requirements for Using the Finite-differ. 2017. Available online: https://standards.ieee.org/ieee/62704-1/5747 (accessed on 14 July 2021).
- Health Effects from Radiofrequency Electromagnetic Fields Report of the Independent Advisory Group on Non-Ionising Radiation. 2012. Available online: https://www.gov.uk/government/publications/radiofrequency-electromagnetic-fields-health-effects (accessed on 14 July 2021).
- Scenihr Opinion on Potential health effects of exposure to electromagnetic fields (EMF). EU Sci. Comm. 2015, 36, 480–484. [CrossRef]
- Madjar, H.M. Human radio frequency exposure limits: An update of reference levels in Europe, USA, Canada, China, Japan and Korea. IEEE Int. Symp. Electromagn. Compat. 2016, 2016, 467–473. [Google Scholar] [CrossRef]
- ICNIRP. Guidelines for Limiting Exposure to Electromagnetic Fields (100 kHz to 300 GHz). Health Phys. 2020, 118, 483–524. [Google Scholar] [CrossRef]
- Bocan, K.N.; Mickle, M.H.; Sejdic, E. Multi-Disciplinary Challenges in Tissue Modeling for Wireless Electromagnetic Powering: A Review. IEEE Sens. J. 2017, 17, 6498–6509. [Google Scholar] [CrossRef]
- Agarwal, A.; Shapero, A.; Rodger, D.; Humayun, M.; Tai, Y.C.; Emami, A. A wireless, low-drift, implantable intraocular pressure sensor with parylene-on-oil encapsulation. In Proceedings of the 2018 IEEE Custom Integrated Circuits Conference (CICC), San Diego, CA, USA, 8–11 April 2018. [Google Scholar] [CrossRef]
- LPS25H. MEMS Pressure Sensor: 260-1260 hPa Absolute Digital Output Barometer—STMicroelectronics. 2018. Available online: https://www.st.com/en/mems-and-sensors/lps25h.html (accessed on 7 March 2022).
- George, J.; Compagno, T.; Rodgers, K.; Waldron, F.; Barrett, J. Reliability of Plastic-Encapsulated Electronic Components in Supersaturated Steam Environments. IEEE Trans. Components Packag. Manuf. Technol. 2015, 5, 1423–1431. [Google Scholar] [CrossRef]
- 868 MHz Embedded Ceramic Loop Antenna for ISM/Lora/LPWAN/Sigfox. 2021. Available online: https://www.taoglas.com/product/530-5-ila-08-868mhz-embedded-ceramic-loop-antenna-ismloralpwansigfox (accessed on 14 July 2021).
- Johanson Technology. 868 MHz Antenna for Small Form Factor Applications. Available online: http://www.farnell.com/datasheets/2280483.pdf (accessed on 14 July 2021).
- 868/915 MHz. KIRBII, ISM SMD antenna—Antenova. 2016. Available online: http://antenova.com/wp-content/uploads/2015/11/Kirbii-A10472-PS-5.1.pdf?__hstc=176912634.486337ce428aefc1c912f83df14de3df.1626276596230.1626276596230.1626276596230.1&__hssc=176912634.1.1626276596231&__hsfp=2875711223&hsCtaTracking=459d335a-4319-4a90-bd00 (accessed on 14 July 2021).
- Surface Mount Ceramic Chip Antennas for 868 MHz. 2013. Available online: https://media.digikey.com/pdf/DataSheets/VishayVitramon/VJ5601M868MXBSR.pdf (accessed on 14 July 2021).
- WE-MCA Multilayer Chip Antenna. 2021. Available online: https://www.we-online.com/catalog/datasheet/7488910092.pdf (accessed on 19 April 2022).
- Cheng, K. Molex 2081420001-ISM 868/915 MHz Ceramic Antenna. 2020. Available online: https://www.molex.com/pdm_docs/ps/2081420001-PS.pdf (accessed on 19 April 2022).
- Charvet, G.; Foerster, M.; Filipe, S.; Porcherot, J.; Beche, J.F.; Guillemaud, R.; Audebert, P.; Regis, G.; Zongo, B.; Robinet, S.; et al. WIMAGINE: A wireless, low power, 64-channel ECoG recording platform for implantable BCI applications. In Proceedings of the 5th International IEEE/EMBS Conference on Neural Engineering, Cancun, Mexico, 27 April–1 May 2011; pp. 356–359. [Google Scholar] [CrossRef]
- 4-GHz BluetoothTM Low Energy and Proprietary System-on-Chip. 2013. Available online: https://www.ti.com/lit/ds/symlink/cc2541.pdf?ts=1640439778669&ref_url=https%253A%252F%252Fwww.google.com%252F (accessed on 3 December 2021).
- NXP Semiconductors UCODE® 7/7m. 2019. Available online: https://www.nxp.com/products/rfid-nfc/ucode-rain-rfid-uhf/ucode-7-7m:SL3S1204 (accessed on 14 July 2021).
- Higgins, H. Two way wireless communication with implanted sensors and therapeutic devices. IET Semin. Dig. 2009, 2009, 4. [Google Scholar] [CrossRef]
- Higgins, H. Body implant communication—Is it a reality? IET Semin. Dig. 2007, 2007, 33–36. [Google Scholar] [CrossRef]
- Ghomashchi, A.; Zheng, Z.; Majaj, N.; Trumpis, M.; Kiorpes, L.; Viventi, J. A low-cost, open-source, wireless electrophysiology system. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 27–31 August 2014; Volume 2014, pp. 3138–3141. [Google Scholar]
- Sun, X.C.; Tao, W.J.; Zhu, C.Q.; Zhao, L.L.; Wang, M.; Lu, X.Y.; Wang, Z.G.; Fan, Z.N. System design and experimental research of lower esophageal sphincter stimulator for treatment of gastroesophageal reflux disease. In Proceedings of the IEEE Engineering in Medicine and Biology Society (EMBC), Jeju Island, Korea, 11–15 July 2017; pp. 2177–2180. [Google Scholar] [CrossRef]
- Gottardi, D.; Wienstroer, V.; Kronberger, R. Animal Skin Phantom for RFID UHF Transponder Development. In Proceedings of the 2018 International Symposium on Antennas and Propagation (ISAP), Busan, Korea, 23–26 October 2018. [Google Scholar]
- ZL70101 Medical Implantable RF Transceiver Features. 2015. Available online: https://www.microsemi.com/document-portal/doc_view/127877-zl70101-1645-19-fullds-datasheet (accessed on 14 July 2021).
- Silva, N.M.; Santos, P.M.; Ferreira, J.A.F.; Soares Dos Santos, M.P.; Ramos, A.; Simões, J.A.O.; Reis, M.J.C.S.; Morais, R. Power management architecture for smart hip prostheses comprising multiple energy harvesting systems. Sens. Actuators A Phys. 2013, 202, 183–192. [Google Scholar] [CrossRef]
- Pederson, D.J.; Quinkert, C.J.; Arafat, M.A.; Somann, J.P.; Williams, J.D.; Bercich, R.A.; Wang, Z.; Albors, G.O.; Jefferys, J.G.R.; Irazoqui, P.P. The Bionode. ACM Trans. Embed. Comput. Syst. 2019, 18, 400. [Google Scholar] [CrossRef]
- Serra, P.A.; Rocchitta, G.; Bazzu, G.; Manca, A.; Puggioni, G.M.; Lowry, J.P.; O’Neill, R.D. Design and construction of a low cost single-supply embedded telemetry system for amperometric biosensor applications. Sens. Actuators B 2007, 122, 118–126. [Google Scholar] [CrossRef] [Green Version]
- PIC12F683 8-Pin flash-based, 8-Bit CMOS Microcontrollers with NanoWatt Technology. 2004. Available online: http://ww1.microchip.com/downloads/en/devicedoc/41211b.pdf (accessed on 14 July 2021).
- Arshak, K.; Lyons, G.; Cavanagh, L.; Clifford, S. Front-end signal conditioning used for resistance-based sensors in electronic nose systems: A review. Sens. Rev. 2003, 23, 230–241. [Google Scholar] [CrossRef] [Green Version]
- Horn, G.V.D.; Huijsing, J.H. Integrated Smart Sensor Calibration. Analog. Integr. Circ. Sign. Process. 1997, 14, 207–222. [Google Scholar] [CrossRef]
- Wilson, A.D.; Baietto, M. Applications and Advances in Electronic-Nose Technologies. Sensors 2009, 9, 5099–5148. [Google Scholar] [CrossRef]
- Lee, J.H.; Seo, D.W. Development of ECG Monitoring System and Implantable Device with Wireless Charging. Micromachines 2019, 10, 38. [Google Scholar] [CrossRef] [Green Version]
- Bilodeau, G.; Gagnon-Turcotte, G.; Gagnon, L.L.; Keramidis, I.; Timofeev, I.; De Koninck, Y.; Ethier, C.; Gosselin, B. A Wireless Electro-Optic Platform for Multimodal Electrophysiology and Optogenetics in Freely Moving Rodents. Front. Neurosci. 2021, 15, 990. [Google Scholar] [CrossRef] [PubMed]
- Ukkonen, L.; Sydänheimo, L.; Ma, S.; Björninen, T. Backscattering-based wireless communication and power transfer to small biomedical implants. In Proceedings of the Microfluidics, BioMEMS, and Medical Microsystems XVIII, San Francisco, CA, USA, 1–4 February 2020; Volume 11235, p. 112350A. [Google Scholar] [CrossRef]
- Großmann, S.; Ott, R.; Kosub, R.; Schmitz, K.-P.; Siewert, S.; Schmidt, W.; Grabow, N. Numerical investigation of stent designs for wireless access to integrated sensors. Curr. Dir. Biomed. Eng. 2019, 5, 497–499. [Google Scholar] [CrossRef]
- Shapero, A.; Agarwal, A.; Martinez, J.C.; Emami, A.; Humayun, M.S.; Tai, Y.C. Wireless Implantable Intraocular Pressure Sensor with Parylene-Oil-Encapsulation and Forward-Angled RF Coil. In Proceedings of the IEEE 32nd International Conference on Micro Electro Mechanical Systems, Seoul, Korea, 27–31 January 2019; pp. 21–24. [Google Scholar] [CrossRef]
- Becker, J.; Pellhammer, D.; Preißner, P.; Glöggler, J.C.; Lapatki, B.G.; Ortmanns, M. An implant for wireless in situ measurement of lip pressure with 12 sensors. In Proceedings of the 2017 IEEE Biomedical Circuits and Systems Conference, Turin, Italy, 19–21 October 2018; pp. 1–4. [Google Scholar] [CrossRef]
- SL900A EPC Sensor Tag and Data Logger IC. 2018. Available online: https://ams.com/sl900a (accessed on 14 July 2021).
- Di Trocchio, L.; Carucci, C.; Sindhu, K.R.; Morel, C.; Lachaud, J.L.; Bichon, S.; Gounel, S.; Mano, N.; Boiziau, C.; Dejous, C.; et al. Wireless in Vivo Biofuel Cell Monitoring. IEEE J. Electromagn. RF Microw. Med. Biol. 2021, 5, 25–34. [Google Scholar] [CrossRef]
- Vena, A.; Sorli, B.; Charlot, B.; Naudi, S. An RFID-based implant for identification and pressure sensing of orthopedic prosthesis. In Proceedings of the 1st URSI Atlantic Radio Science Conference (URSI AT-RASC); Institute of Electrical and Electronics Engineers (IEEE), Gran Canaria, Spain, 16–24 May 2015. [Google Scholar]
- Lazaro, M.; Lazaro, A.; Villarino, R. Feasibility of backscatter communication using LoRAWAN signals for deep implanted devices and wearable applications. Sensors 2020, 20, 6342. [Google Scholar] [CrossRef]
- Wu, T.; Redouté, J.M.; Yuce, M.R. A Wireless Implantable Sensor Design With Subcutaneous Energy Harvesting for Long-Term IoT Healthcare Applications. IEEE Access 2018, 6, 35801–35808. [Google Scholar] [CrossRef]
- Bluetooth, S. SimbleeTM RFD77101 IoT for Connecting Everyone and Everything. 2015. Available online: https://cdn.sparkfun.com/datasheets/IoT/Simblee%20RFD77101%20Datasheet%20v1.0.pdf (accessed on 14 July 2021).
- Shon, A.; Chu, J.-U.; Jung, J.; Kim, H.; Youn, I. An Implantable Wireless Neural Interface System for Simultaneous Recording and Stimulation of Peripheral Nerve with a Single Cuff Electrode. Sensors 2017, 18, 1. [Google Scholar] [CrossRef] [Green Version]
- Bentler, C.; Stieglitz, T. Building wireless implantable neural interfaces within weeks for neuroscientists. In Proceedings of the 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Jeju, Korea, 11–15 July 2017; pp. 1078–1081. [Google Scholar] [CrossRef]
- Bock, D.C.; Marschilok, A.C.; Takeuchi, K.J.; Takeuchi, E.S. Batteries used to Power Implantable Biomedical Devices. Electrochim. Acta 2012, 84, 155–164. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, E.S.; Leising, R.A. Lithium Batteries for Biomedical Applications. MRS Bull. 2002, 27, 624–627. [Google Scholar] [CrossRef]
- Qualliion Medical Batteries: Powering Life. 2016. Available online: https://www.enersys.com/en/products/batteries/quallion/quallion-lithium (accessed on 14 July 2021).
- Young, D.J.; Cong, P.; Suster, M.A.; Damaser, M. Implantable wireless battery recharging system for bladder pressure chronic monitoring. Lab Chip 2015, 15, 4338. [Google Scholar] [CrossRef]
- J. Macsalka. Micro3—QL0003B Rechargeable Lithium Ion Battery. Available online: https://medical.enersys.com/products (accessed on 13 December 2021).
- Bhatia, N.; El-Chami, M. Leadless pacemakers: A contemporary review. J. Geriatr. Cardiol. 2018, 15, 249. [Google Scholar] [CrossRef]
- Liu, G.; Mao, L.; Chen, L.; Xie, S. Locatable-Body Temperature Monitoring Based on Semi-Active UHF RFID Tags. Sensors 2014, 14, 5952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Salmerón, J.; Rivadeneyra, A.; Martínez-Martí, F.; Capitán-Vallvey, L.F.; Palma, A.J.; Carvajal, M.A. Passive UHF RFID Tag with Multiple Sensing Capabilities. Sensors 2015, 15, 26769–26782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rennane, A.; Fonseca, N.; Abdelnour, A.; Benmahmoud, F.; Kaddour, D.; Touhami, R.; Tedjini, S. Passive UHF RFID Sensor Tag for Pressure and Temperature Conditions Monitoring. In Proceedings of the 2nd URSI Atlantic Radio Science Meeting (AT-RASC), Gran Canaria, Spain, 28 May–1 June 2018. [Google Scholar] [CrossRef]
- De Donno, D.; Catarinucci, L.; Tarricone, L. RAMSES: RFID augmented module for smart environmental sensing. IEEE Trans. Instrum. Meas. 2014, 63, 1701–1708. [Google Scholar] [CrossRef]
- Jung, J.Y.; Kim, H.; Lee, H.S.; Yeom, K.W. An UHF RFID tag with long read range. In Proceedings of the 2009 European Microwave Conference (EuMC), Rome, Italy, 29 September–1 October 2009; pp. 1113–1116. [Google Scholar] [CrossRef]
- Khan, S.R.; Desmulliez, M.P.Y. Implementation of a Dual Wireless Power Transfer and Rotation Monitoring System for Prosthetic Hands. IEEE Access 2019, 7, 1. [Google Scholar] [CrossRef]
- Khan, S.R.; Desmulliez, M.P.Y. Towards a Miniaturized 3D Receiver WPT System for Capsule Endoscopy. Micromachines 2019, 10, 545. [Google Scholar] [CrossRef] [Green Version]
- Biswas, D.K.; Tasneem, N.T.; Mahbub, I. Optimization of Miniaturized Wireless Power Transfer System to Maximize Efficiency for Implantable Biomedical Devices. In Proceedings of the IEEE Texas Symposium on Wireless and Microwave Circuits and Systems (WMCS), Waco, TX, USA, 28–29 March 2019. [Google Scholar] [CrossRef]
- Ahmadi, M.M.; Sarbandi-Farahani, M. A Class-E Power and Data Transmitter with On–Off Keying Data Modulation for Wireless Power and Data Transmission to Medical Implants. Circ. Syst. Signal Process. 2020, 39, 4174–4186. [Google Scholar] [CrossRef]
- Bakula, M.; Pelgrims, P.; Puers, R. Wireless powering and communication for implants, based on a Royer oscillator with radio and near-field links. Sens. Actuators A 2016, 250, 273–280. [Google Scholar] [CrossRef]
- Seckler, T.; Jagielski, K.; Stunder, D. Assessment of Electromagnetic Interference with Active Cardiovascular Implantable Electronic Devices (CIEDs) Caused by the Qi A13 Design Wireless Charging Board. Int. J. Environ. Res. Public Health 2015, 12, 5886–5904. [Google Scholar] [CrossRef] [Green Version]
- Wireless Power Consortium. 2018. Available online: https://www.wirelesspowerconsortium.com (accessed on 14 July 2021).
- WCT1001A/WCT1003A. Automotive A13 Wireless Charging Application User’s Guide. 2014. Available online: https://www.nxp.com/docs/en/user-guide/WCT100XAWCAUG_V3.5.pdf (accessed on 14 July 2021).
- Zhou, L.; Chen, X.; Li, Y.; Li, J. Bluetooth low energy 4. 0-based communication method for implants. In Proceedings of the 10th International Congress on Image and Signal Processing, BioMedical Engineering and Informatics CISP-BMEI, Beijing, China, 13–15 October 2018; pp. 1–5. [Google Scholar] [CrossRef]
- Su, Y.; Routhu, S.; Moon, K.S.; Lee, S.Q.; Youm, W.; Ozturk, Y. A Wireless 32-Channel Implantable Bidirectional Brain Machine Interface. Sensors 2016, 16, 1582. [Google Scholar] [CrossRef]
- Kracek, J.; Švanda, M.; Mazanek, M.; Machac, J. Implantable Semi-Active UHF RFID Tag with Inductive Wireless Power Transfer. IEEE Antennas Wirel. Propag. Lett. 2016, 15, 1657–1660. [Google Scholar] [CrossRef]
- Lodato, R.; Marrocco, G. Close Integration of a UHF-RFID Transponder into a Limb Prosthesis for Tracking and Sensing. IEEE Sens. J. 2016, 16, 1806–1813. [Google Scholar] [CrossRef]
- Lodato, R.; Lopresto, V.; Pinto, R.; Marrocco, G. Numerical and experimental characterization of through-the-body UHF-RFID links for passive tags implanted into human limbs. IEEE Trans. Antennas Propag. 2014, 62, 5298–5306. [Google Scholar] [CrossRef]
- Occhiuzzi, C.; Contri, G.; Marrocco, G. Design of implanted RFID tags for passive sensing of human body: The STENTag. IEEE Trans. Antennas Propag. 2012, 60, 3146–3154. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Miquel, A.; Medina-Rodriguez, B.; Vidal, N.; Ramos, F.M.; Roca, E.; Lopez-Villegas, J.M. Design and characterization of a miniaturized implantable UHF RFID tag based on LTCC technology. In Proceedings of the 11th European Conference on Antennas and Propagation (EUCAP), Paris, France, 19–24 March 2017; pp. 1024–1026. [Google Scholar] [CrossRef]
- Xu, J.; Sato, H.; Motoyoshi, M.; Suematu, N.; Kanetaka, H.; Yasui, K.; Chen, Q. Development of Denture Implanted RFID Tag Antennas. In Proceedings of the IEEE International Workshop on Electromagnetics: Applications and Student Innovation Competition (iWEM), Nagoya, Japan, 29–31 August 2018. [Google Scholar] [CrossRef]
- Rajagopalan, H.; Rahmat-Samii, Y. Ingestible RFID bio-capsule tag design for medical monitoring. In Proceedings of the IEEE Antennas and Propagation Society International Symposium, Toronto, ON, Canada, 11–17 July 2010. [Google Scholar] [CrossRef]
- Kampianakis, E.; Sharma, A.; Arenas, J.; Reynolds, M.S. A dual-band wireless power transfer and backscatter communication approach for implantable neuroprosthetic devices. IEEE J. Radio Freq. Identif. 2017, 1, 100–107. [Google Scholar] [CrossRef]
- Rotermund, D.; Pistor, J.; Hoeffmann, J.; Schellenberg, T.; Boll, D.; Tolstosheeva, E.; Gauck, D.; Stemmann, H.; Peters-Drolshagen, D.; Kreiter, A.K.; et al. Open Hardware: Towards a Fully-Wireless Sub-Cranial Neuro-Implant for Measuring Electrocorticography Signals. bioRxiv 2017, 15, 036855. [Google Scholar] [CrossRef] [Green Version]
- Fang, Q.; Lee, S.Y.; Permana, H.; Ghorbani, K.; Cosic, I. Developing a wireless implantable body sensor network in MICS band. IEEE Trans. Inf. Technol. Biomed. 2011, 15, 567–576. [Google Scholar] [CrossRef]
- Tantin, A.; Letourneau, A.; Zgaren, M.; Hached, S.; Clausen, I.; Sawan, M. Implantable MICS-based wireless solution for bladder pressure monitoring. In Proceedings of the 2017 IEEE Biomedical Circuits and Systems Conference (BioCAS), Torino, Italy, 19–21 October 2017; pp. 1–4. [Google Scholar] [CrossRef]











| Year | Application | Reference |
|---|---|---|
| 1948 | Radio Inductograph | [14] |
| 1957 | pH-Endoradiosonde | [15] |
| 1962 | Telemetry pill | [16] |
| 1967 | Electrocardiogram (ECG), electroencephalogram (EEG), wireless biotelemetry | [17] |
| Year | Application | Reference |
|---|---|---|
| 1966 | A radio-telemetry device for monitoring temperature | [18] |
| 1970 | Implantable bio-telemetry systems | [19] |
| 1999 | Injectable electronic identification, monitoring and stimulation systems | [20] |
| 2000 | Advances in wireless telemedicine | [13] |
| 2002 | Wireless network for emergency medical care | [21] |
| 2011 | Wireless and implantable electrocorticogram recording system | [22] |
| 2018 | Wireless telemetry ingestible capsule | [23] |
| 2021 | Wireless monitoring of local deep infection | [24] |
| 868 MHz DRAs | ||
|---|---|---|
| Reference | Gain (dBi) | Size |
| [41] | −0.5 | 5 mm× 3 mm × 0.5 mm |
| [42] | −1 | 7 mm × 2 mm × 1.2 mm |
| [43] | +0.7 | 10 mm × 3 mm × 2 mm |
| [44] | +1.73 | 15.5 mm × 10.5 mm × 1.2 mm |
| [45] | −0.7 | 11 mm × 5.1 mm × 1.5 mm |
| [46] | 1.5 | 9 mm × 3 mm × 0.63 mm |
| Parameters | ZL70101 | CC2541 | Ucode7 |
|---|---|---|---|
| Manufacturer | Microsemi | TI | NXP |
| Standard | *** MICS | ** BLE 4.0 | * UHF RFID |
| Frequency | 402 to 405 MHz | 2.4 GHz | 860 to 960 MHz |
| Transmit current (mA) | 5 | 18 | Battery-free |
| Distance (m) free space | 1000 | 100 | 15 |
| Form factor | 7 mm × 7 mm × 0.9 mm | 6 mm × 6 mm × 1 mm | 1.5 mm × 1 mm × 0.5 mm |
| Application | ECG | ECoG, EEG, GERD | Tracking |
| References | [47,50,51] | [22,52,53] | [54] |
| Bill of Materials | |||
|---|---|---|---|
| VENDOR | Part Number | Description | Block |
| Intan Tech | RHD2164 | 64 CH. Chip | Recorder |
| Analog Devices | AD5863 | 16-bit DAC | Stimulus |
| ADG506A | Multiplexer | ||
| LT1638 | Opamp | ||
| N/A | N/A | 1 Zener Diode | Power Supply |
| N/A | 5 Capacitors | ||
| N/A | 4 Diodes | ||
| Lattice Semi | LCMX03LF-1300E | FPGA | Radio/MCU |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, S.R.; Mugisha, A.J.; Tsiamis, A.; Mitra, S. Commercial Off-the-Shelf Components (COTS) in Realizing Miniature Implantable Wireless Medical Devices: A Review. Sensors 2022, 22, 3635. https://doi.org/10.3390/s22103635
Khan SR, Mugisha AJ, Tsiamis A, Mitra S. Commercial Off-the-Shelf Components (COTS) in Realizing Miniature Implantable Wireless Medical Devices: A Review. Sensors. 2022; 22(10):3635. https://doi.org/10.3390/s22103635
Chicago/Turabian StyleKhan, Sadeque Reza, Andrew J. Mugisha, Andreas Tsiamis, and Srinjoy Mitra. 2022. "Commercial Off-the-Shelf Components (COTS) in Realizing Miniature Implantable Wireless Medical Devices: A Review" Sensors 22, no. 10: 3635. https://doi.org/10.3390/s22103635






