Exploitation of SnO2/Polypyrrole Interface for Detection of Ammonia Vapors Using Conductometric and Optical Techniques: A Theoretical and Experimental Analysis
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Optimization of SnO2 and PPy Thicknesses
3.1.1. Varying Thickness of SnO2
3.1.2. Varying Thickness of Polypyrrole Thin Film
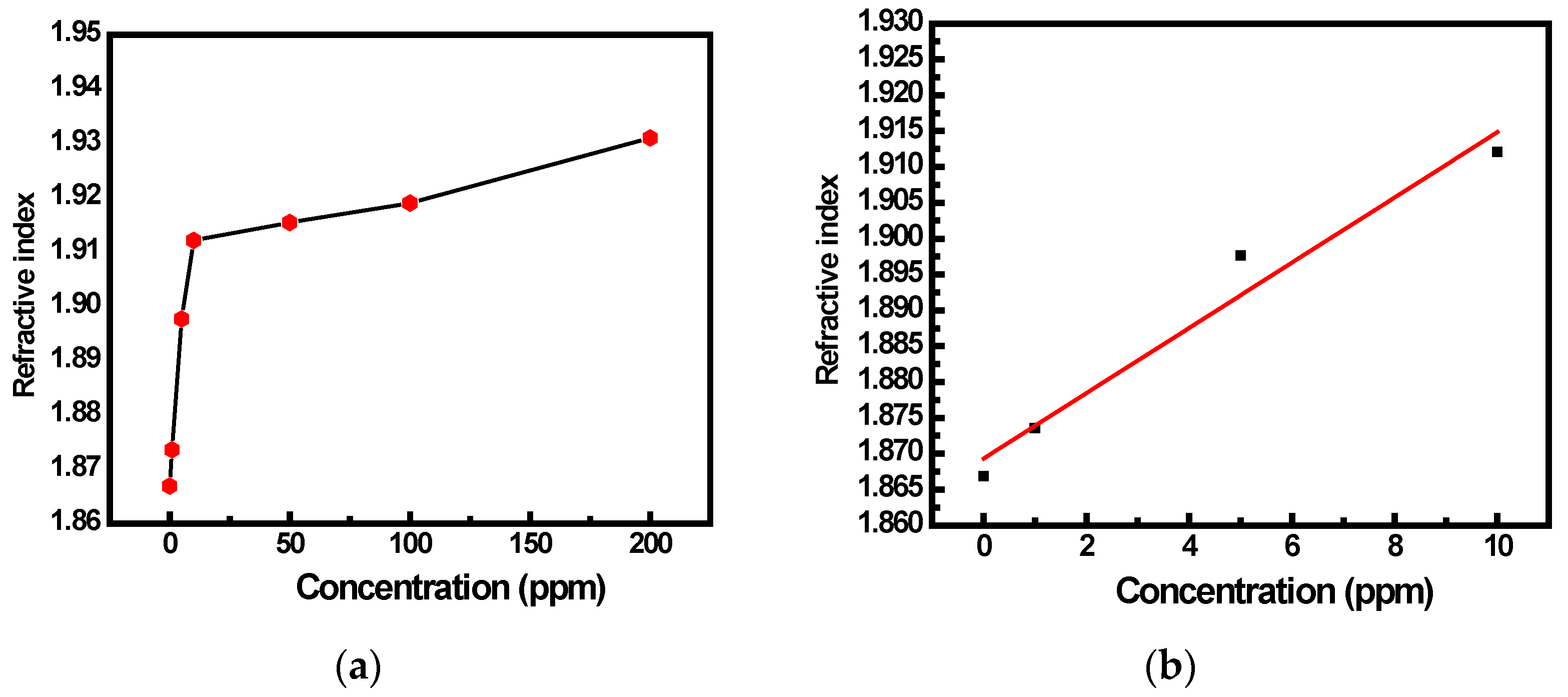
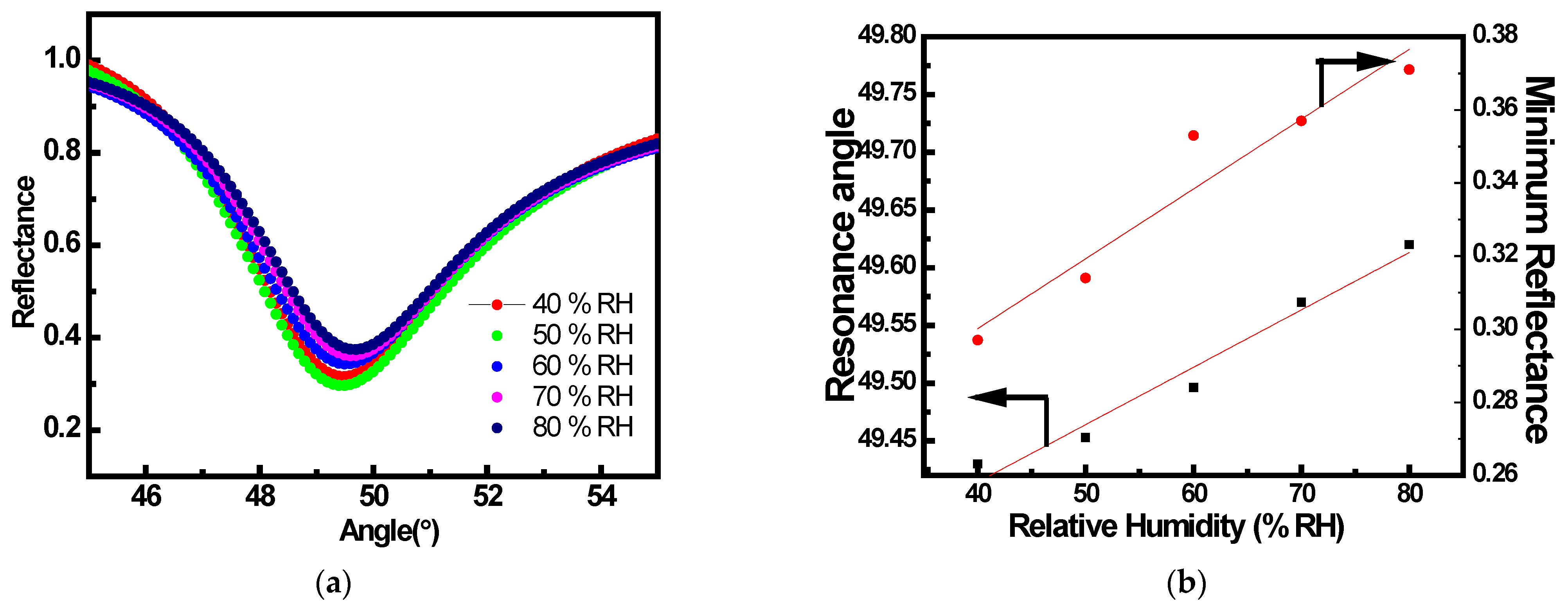
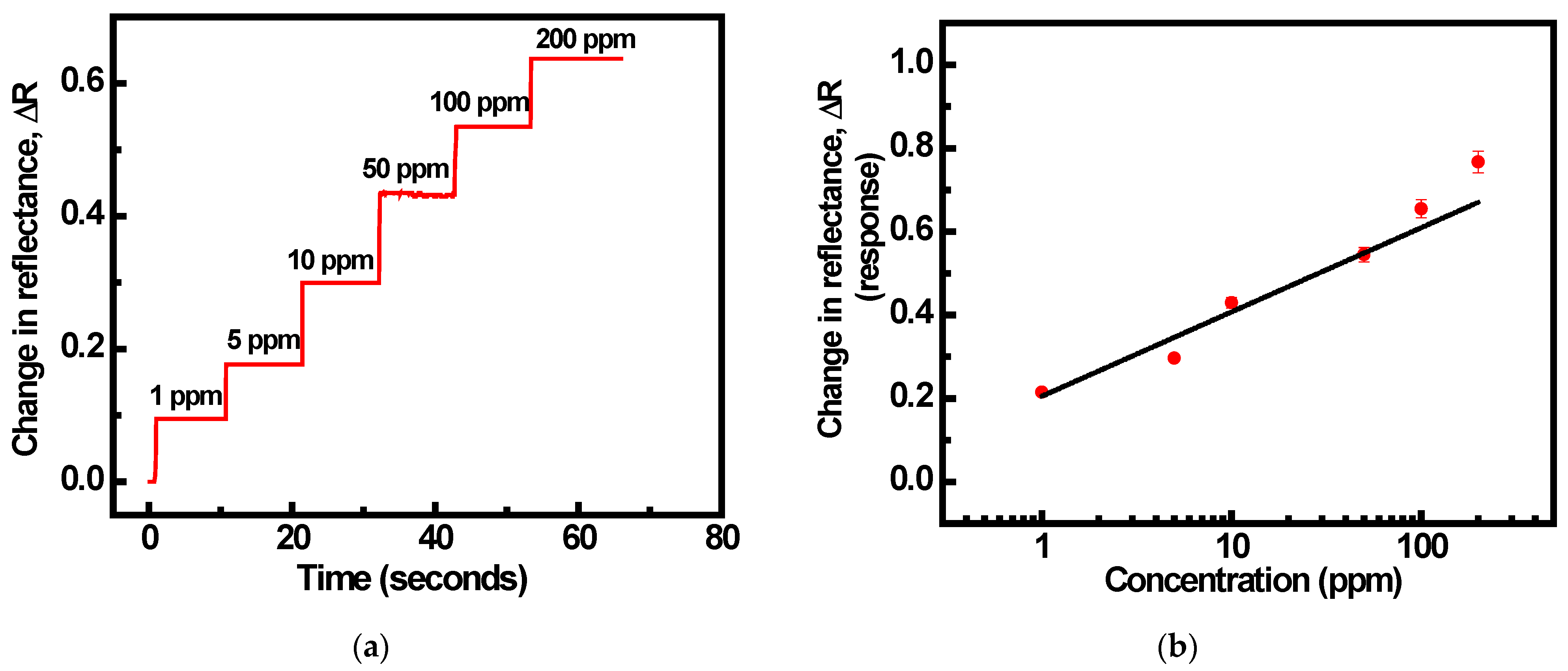
3.2. SPR Gas Sensing Experimental Studies
3.3. Sensing Mechanism
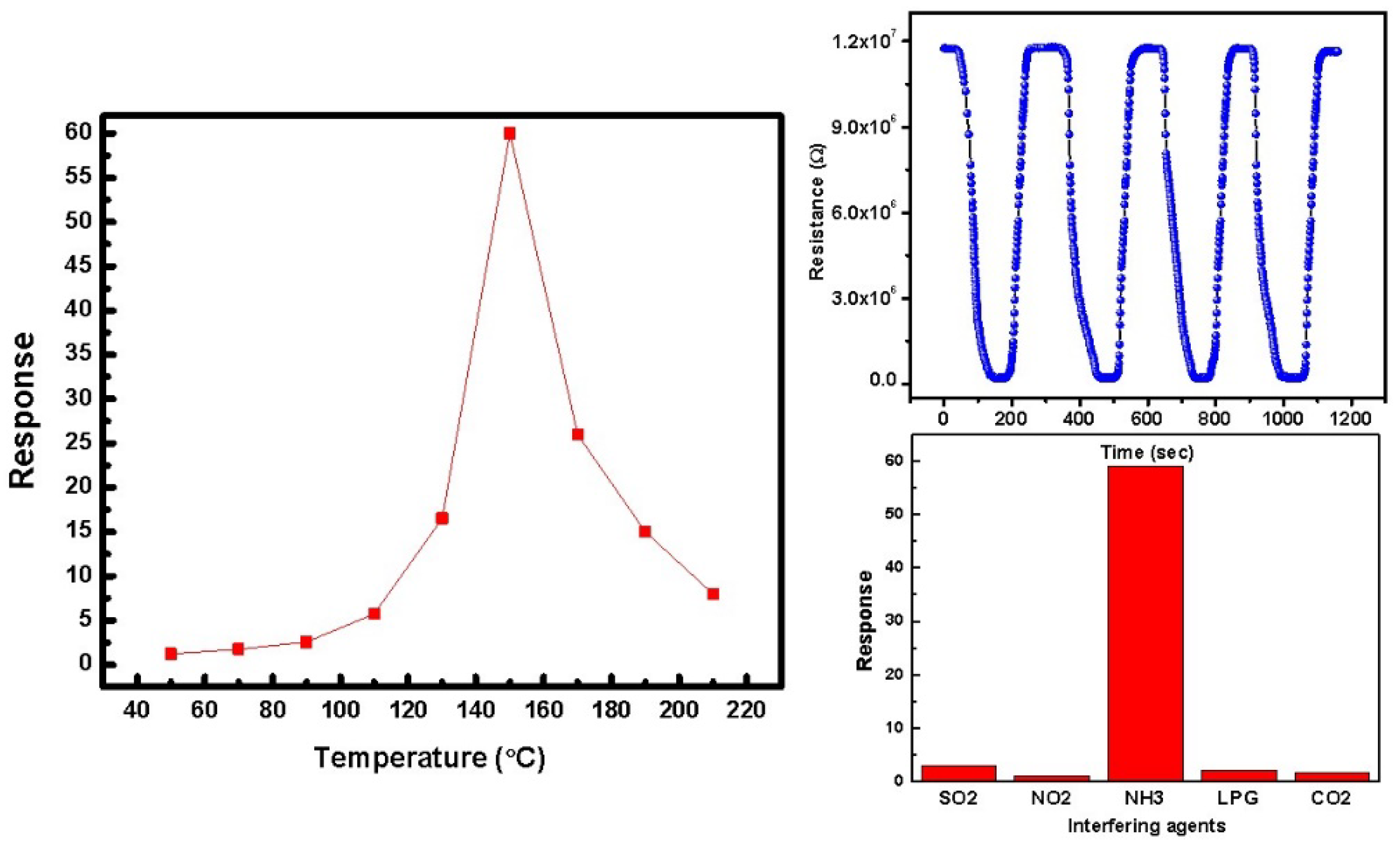
3.4. Conductometric Gas Sensing Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Richardson, T.; Smith, V.C.; Johnstone, R.A.W.; Sobral, A.J.F.N.; Rocha-Gonsalves, A.M.d’.A. Optical response of monolayer flimsof a metal-free sulfonamido-porphyrin. Thin Solid Film 1998, 327–329, 315–320. [Google Scholar] [CrossRef]
- Sberveglieri, G. Gas Sensors-Principles, Operation and Developments; Kluwer Academic Publisher: Dordrecht, The Netherlands, 1992. [Google Scholar]
- Manera, M.G.; Rella, R. Improved gas sensing performances in SPR sensors by transducers activation. Sens. Actuators B 2013, 179, 175–186. [Google Scholar] [CrossRef]
- Bae, M.K.; Lim, J.A.; Kim, S.; Song, Y.W. Ultra-highly sensitive optical gas sensors based on chemomechanical polymer-incorporated fiber interferometer. Opt. Exp. 2013, 21, 2018–2023. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, L.; Hu, Y.; Zhou, C.; Lan, W.; Fu, H.; She, Y. Nanomaterials as optical sensors for application in rapid detection of food contaminants, quality, and authenticity. Sens. Actuators B Chem. 2013, 329, 129135. [Google Scholar] [CrossRef]
- Pyo, J.; Hong, S.M.; Kwon, Y.S.; Kim, M.S.; Cho, K.H. Estimation of heavy metals using deep neural network with visible and infrared spectroscopy of soil. Sci. Total Environ. 2020, 741, 140162. [Google Scholar] [CrossRef] [PubMed]
- Beck, U.; Hertwig, A.; Kormunda, M.; Krause, A.; Kruger, H.; Lohse, V.; Nooke, A.; Pavlik, J.; Steinbach, J. SPR enhanced ellipsometric gas monitoring on thin iron doped tin oxide layers. Sens. Actuators B 2011, 160, 609–615. [Google Scholar] [CrossRef]
- El-Basaty, A.B.; El-Brolossy, T.A.; Abdalla, S.; Negm, S.; Abdella, R.A.; Talaat, H. Surface Plasmon Sensors for NO2 gas. Surf. Interface Anal. 2008, 40, 1623–1626. [Google Scholar] [CrossRef]
- Wilde, J.N.; Petty, M.C.; Saffell, J.; Tempore, A.; Valli, L. Surface Plasmon Resonance Imaging for gas sensing. Meas. Control 1997, 30, 269–272. [Google Scholar] [CrossRef]
- Nikitin, P.I.; Beloglazov, A.A.; Kochergin, V.E.; Valeiko, M.V.; Ksenevich, T.I. Surface plasmon resonance interferometry for biological and chemical sensing. Sens. Actuators B 1999, 54, 43–50. [Google Scholar] [CrossRef]
- Bakhshpour, M.; Denizli, A. Highly sensitive detection of Cd (II) ions using ion-imprinted surface plasmon resonance sensors. Microchem. J. 2020, 159, 105572. [Google Scholar] [CrossRef]
- Yang, D.; Lu, H.H.; Chen, B.; Lin, C.W. Surface plasmon resonance of SnO2/Au bi-layer films for gas sensing applications. Sens. Actuators B 2010, 145, 832. [Google Scholar] [CrossRef]
- Bannov, A.G.; Popov, M.V.; Brester, A.E.; Kurmashov, P.B. Recent advances in ammonia gas sensors based on carbon nanomaterials. Micromachines 2021, 12, 186. [Google Scholar] [CrossRef] [PubMed]
- Bielecki, Z.; Stacewicz, T.; Smulko, J.; Wojtas, J. Ammonia gas sensors: Comparison of solid-state and optical methods. Appl. Sci. 2020, 10, 5111. [Google Scholar] [CrossRef]
- Available online: www.cdc.gov/niosh/docs (accessed on 1 January 2020).
- Maciak, E.; Opilski, Z.; Pustelny, T. Effect of humidity on NH3 gas sensitivity of Nafion/WO3 Sensing structure of SPR sensor. Mol. Quant. Acoust. 2005, 26, 205–215. [Google Scholar]
- Dolbec, R.; El Khakani, M.A. Pulsed laser deposited Platinum and gold nanoparticles as catalyst for enhancing the CO sensitivity of nanostructured SnO2 sensors. Sens. Lett. 2005, 3, 216–221. [Google Scholar] [CrossRef]
- Kishnani, V.; Verma, G.; Pippara, R.K.; Yadav, A.; Chauhan, P.S.; Gupta, A. Highly sensitive, ambient temperature CO sensor using tin oxide-based composites. Sens. Actuators A Phys. 2021, 332, 113111. [Google Scholar] [CrossRef]
- Wei, L.; Xia, Y.; Meng, J. A novel tin oxide sensor and gas detection performance in asphalt. J. Nanoelectron. Optoelectron. 2018, 13, 1290–1294. [Google Scholar] [CrossRef]
- Tabish, M.; Malik, M.U.; Khan, M.A.; Yasin, G.; Asif, H.M.; Anjum, M.J.; Nazir, M.T. Construction of NiCo/graphene nanocomposite coating with bulges-like morphology for enhanced mechanical properties and corrosion resistance performance. J. Alloys Compd. 2021, 867, 159138. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, X.; Huang, Y.; Zhai, H.; Liu, Z. Ammonia sensing properties of perylene diimides: Effect of core- substituted chiral groups. Sens. Actuators B Chem. 2018, 254, 805–810. [Google Scholar] [CrossRef]
- Ali, S.; Jameel, M.A.; Oldham, G.; Gupta, A.; Shafiei, M.; Langford, S.J. Enhancement in room temperature ammonia sensing properties of naphthalene diimides through core expansion. J. Mater. Chem. C 2022, 10, 1326–1333. [Google Scholar] [CrossRef]
- Miller, D.R.; Akbar, S.A.; Morris, P.A. Nanoscale metal oxide-based heterojunctions for gas sensing: A review. Sens. Actuators B Chem. 2014, 204, 250–272. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, L.; Shen, J.; Chen, Z.; Shi, G.; Zhang, B. Synthesis property and field-emission behaviour of amorphous polypyrrole nanowires. Nanotechnology 2006, 17, 3446–3450. [Google Scholar] [CrossRef]
- Cho, J.H.; Yu, J.B.; Kim, J.S.; Sohn, S.O.; Lee, D.D.; Huh, J.S. Sensing behaviours of polypyrrole sensor under humidity condition. Sens. Actuator B 2005, 108, 389–392. [Google Scholar] [CrossRef]
- Arora, K.; Chaubey, A.; Singhal, R.; Singh, R.P.; Pandey, M.K.; Samanta, S.B.; Malhotra, B.D.; Chand, S. Application of electrochemically prepared polypyrrole–polyvinyl sulphonate films to DNA biosensor. Biosens. Bioelectron. 2006, 21, 1777–1783. [Google Scholar] [CrossRef]
- Yadav, A.A.; Lokhande, A.C.; Kim, J.H.; Lokhande, C.D. Enhanced sensitivity and selectivity of CO2 gas sensor based on modified La2O3 nanorods. J. Alloys Comp. 2017, 723, 880–886. [Google Scholar] [CrossRef]
- Heavens, S. Optical Properties of Thin Solid Films; Butterworth: London, UK, 1955; Chapter 4. [Google Scholar]
- Paliwal, A.; Sharma, A.; Tomar, M.; Gupta, V. Optical properties of WO3 thin films using surface plasmon resonance technique. J. App. Phys. 2014, 115, 043104. [Google Scholar] [CrossRef]
- Chougule, M.A.; Dalavi, D.S.; Mali, S.; Patil, P.S.; Moholkar, A.V.; Agawane, G.L.; Kim, J.H.; Sen, S.; Patil, V.B. Novel method for fabrication of room temperature polypyrrole-ZnO nanocomposite No2 sensor. Measurement 2012, 45, 1989–1996. [Google Scholar] [CrossRef]
- Singh, N.K.; Shrivastava, S.; Rath, S.; Annapoorni, S. Optical and room temperature sensing properties of highly oxygen deficient flower-like ZnO nanostructures. Surf. Sci. 2010, 257, 1544–1549. [Google Scholar] [CrossRef]
- Hieu, N.V.; Quang, V.V.; Hoa, N.D.; Kim, D. Preparing large-scale WO3 nanowire-like structures for high sensitivity NH3 gas sensor through a simple route. Curr. Appl. Phys. 2011, 11, 657–661. [Google Scholar] [CrossRef]
- Kanan, S.M.; El-Kadri, O.M.; Abu-Yousef, I.A.; Kanan, M.C. Semiconducting metal oxide based sensors for selective gas pollutant detection. Sensors 2009, 9, 8158–8196. [Google Scholar] [CrossRef]
- Mishra, S.K.; Tripathi, S.N.; Choudhary, V.; Gupta, B.D. SPR based fibre optic ammonia gas sensor utilising nanocomposite film of PMMA/educed graphene oxide prepared by in situ polymerization. Sens. Actuators B Chem. 2014, 199, 190–200. [Google Scholar] [CrossRef]
- Mishra, S.K.; Bhardwaj, S.; Gupta, B.D. Surface plasmon resonance-based fibre optic sensor for the detection of low concentrations of ammonia gas. IEEE Sens. J. 2014, 15, 1235–1239. [Google Scholar] [CrossRef]





| Thickness of SnO2 (in nm) | Full Width Half Maximum (FWHM) |
|---|---|
| 100 | 1.2 |
| 200 300 400 | 2.0 3.8 7.2 |
| Thickness of PPy (in nm) | Full Width Half Maximum (FWHM) |
|---|---|
| 100 | 2.1 |
| 200 300 400 | 3.0 1.5 4.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gahlot, A.P.S.; Paliwal, A.; Kapoor, A. Exploitation of SnO2/Polypyrrole Interface for Detection of Ammonia Vapors Using Conductometric and Optical Techniques: A Theoretical and Experimental Analysis. Sensors 2022, 22, 7252. https://doi.org/10.3390/s22197252
Gahlot APS, Paliwal A, Kapoor A. Exploitation of SnO2/Polypyrrole Interface for Detection of Ammonia Vapors Using Conductometric and Optical Techniques: A Theoretical and Experimental Analysis. Sensors. 2022; 22(19):7252. https://doi.org/10.3390/s22197252
Chicago/Turabian StyleGahlot, Ajay Pratap Singh, Ayushi Paliwal, and Avinashi Kapoor. 2022. "Exploitation of SnO2/Polypyrrole Interface for Detection of Ammonia Vapors Using Conductometric and Optical Techniques: A Theoretical and Experimental Analysis" Sensors 22, no. 19: 7252. https://doi.org/10.3390/s22197252






