A New Analytical Method to Quantify Ammonia in Freshwater with a Bulk Acoustic Wave Sensor
Abstract
:1. Introduction
2. Materials and Methods
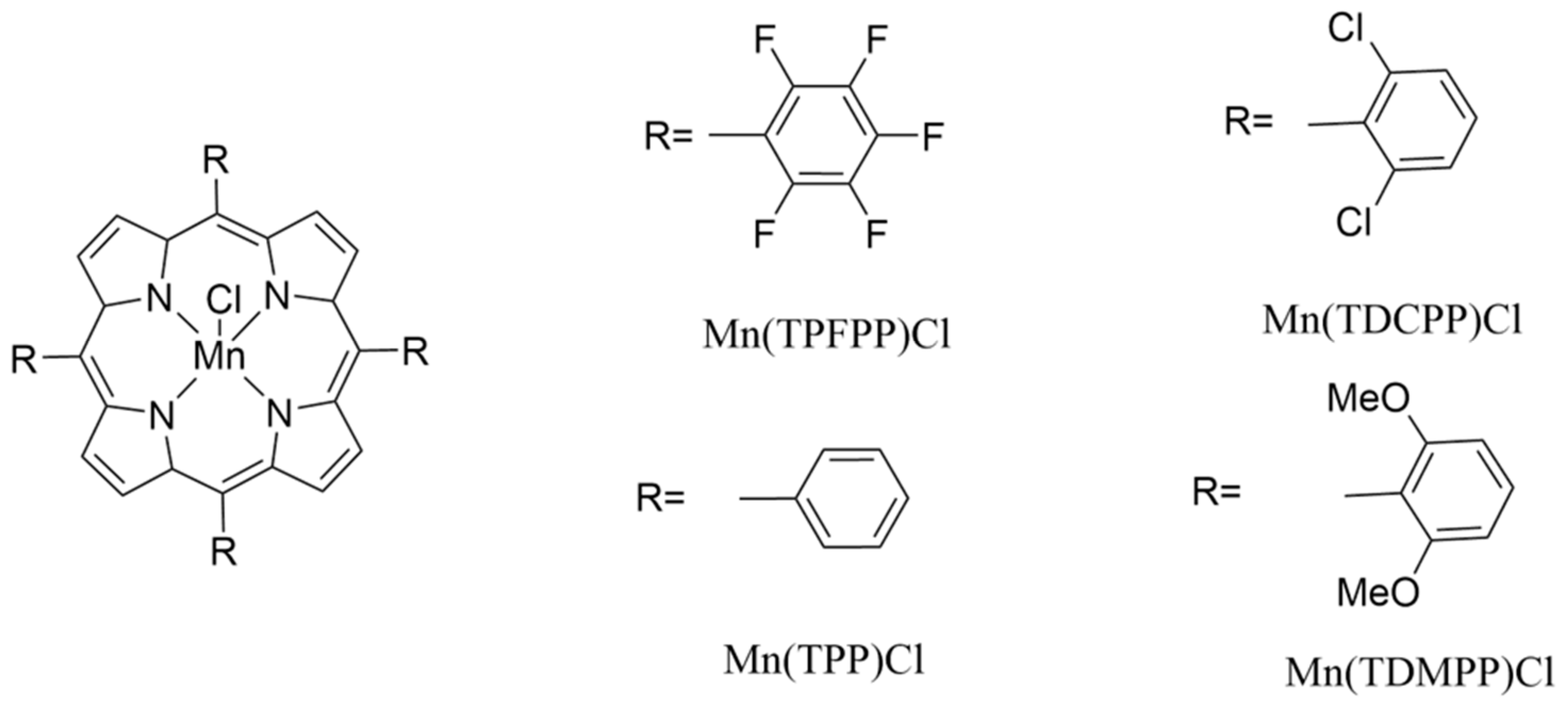
2.1. Reagents
- Reagents used in the sensor coating and testing
- Reagents used in the indophenol method
2.2. Apparatus
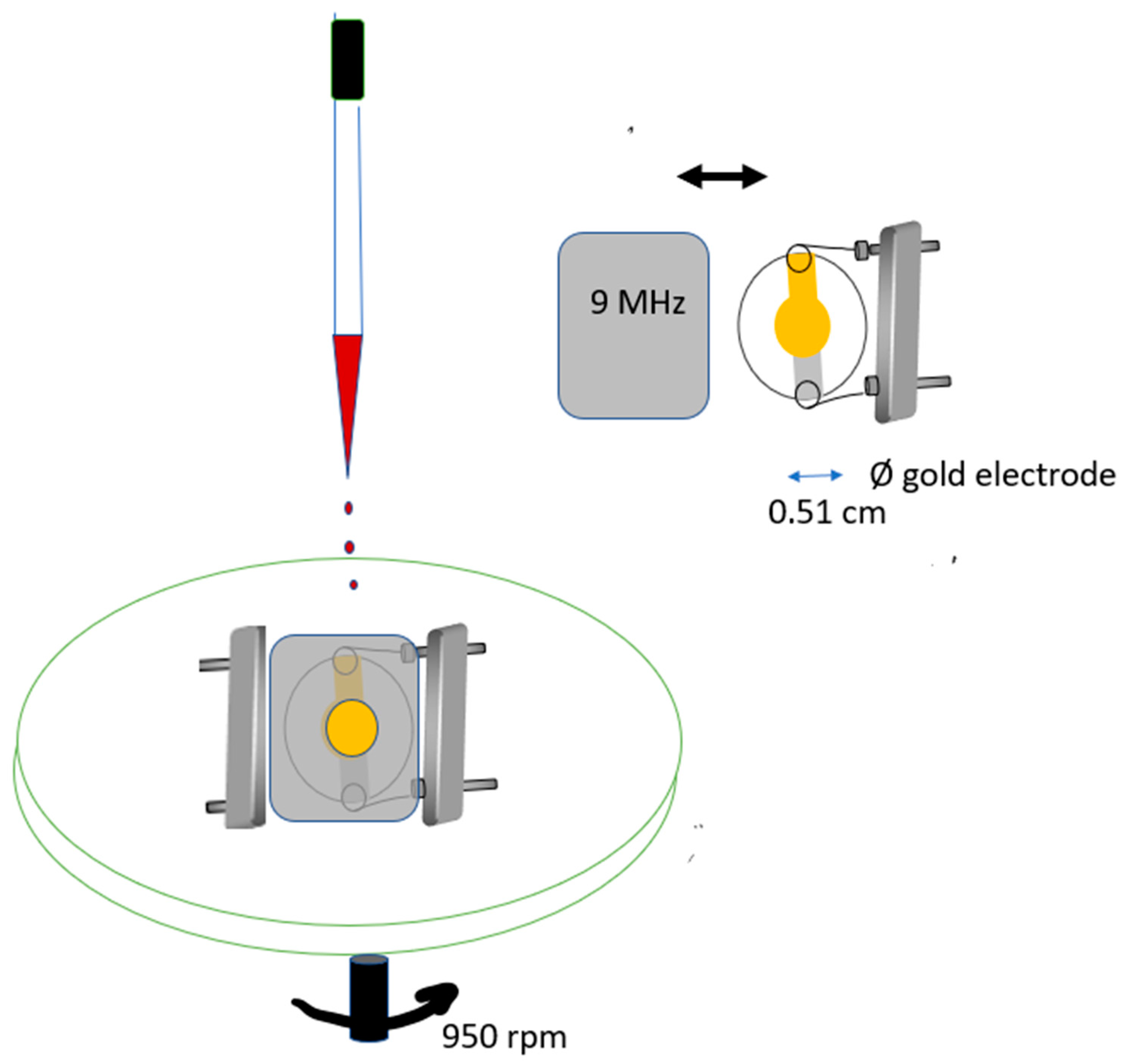
2.2.1. Apparatus for Coating
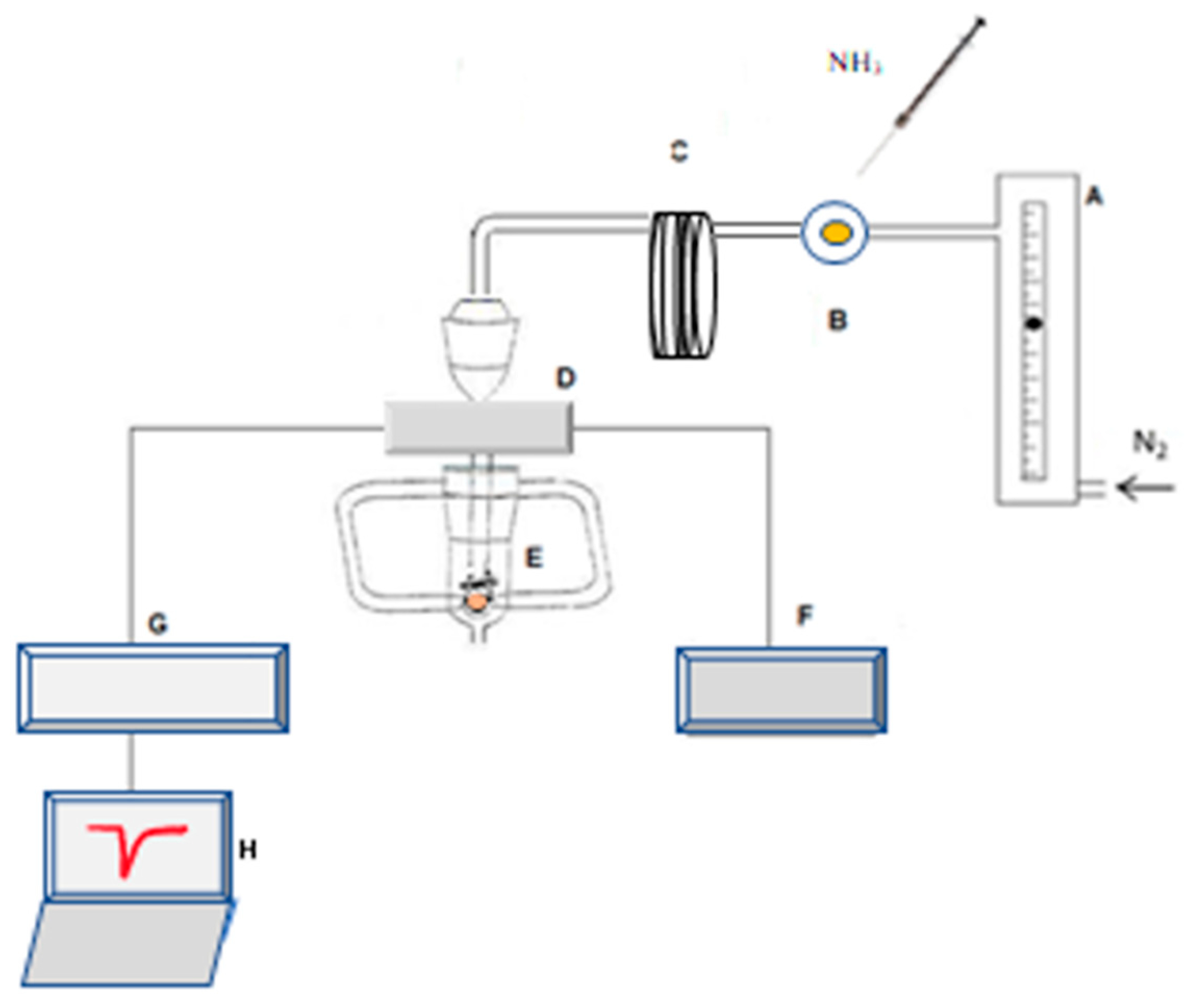
2.2.2. Apparatus for Coating Selection
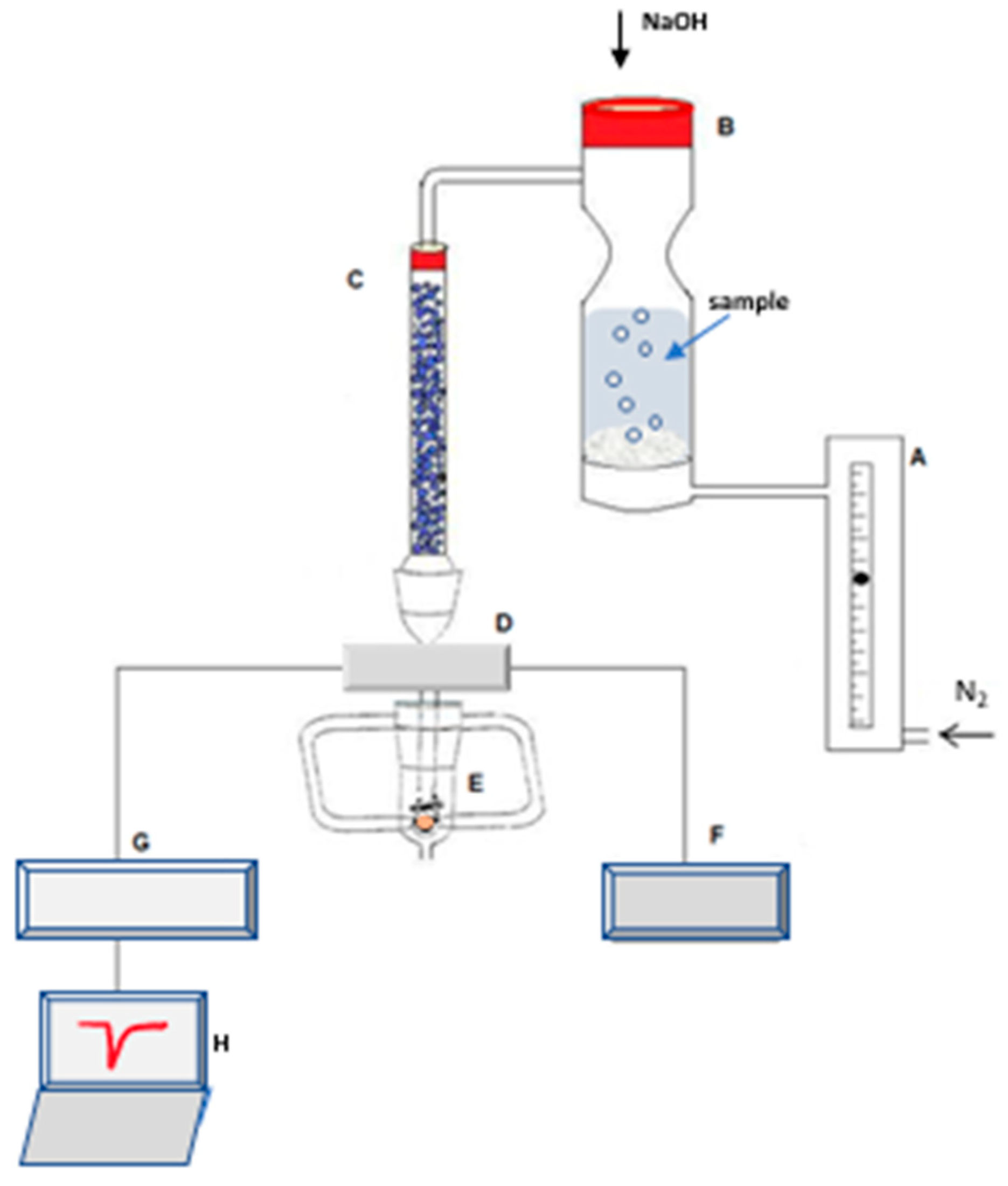
2.2.3. Apparatus for the Analysis of Aqueous Standards and Water Samples
2.3. Procedure
2.3.1. Sample Collection and Preservation
2.3.2. Crystal Coating
2.3.3. Analysing Standards and Samples
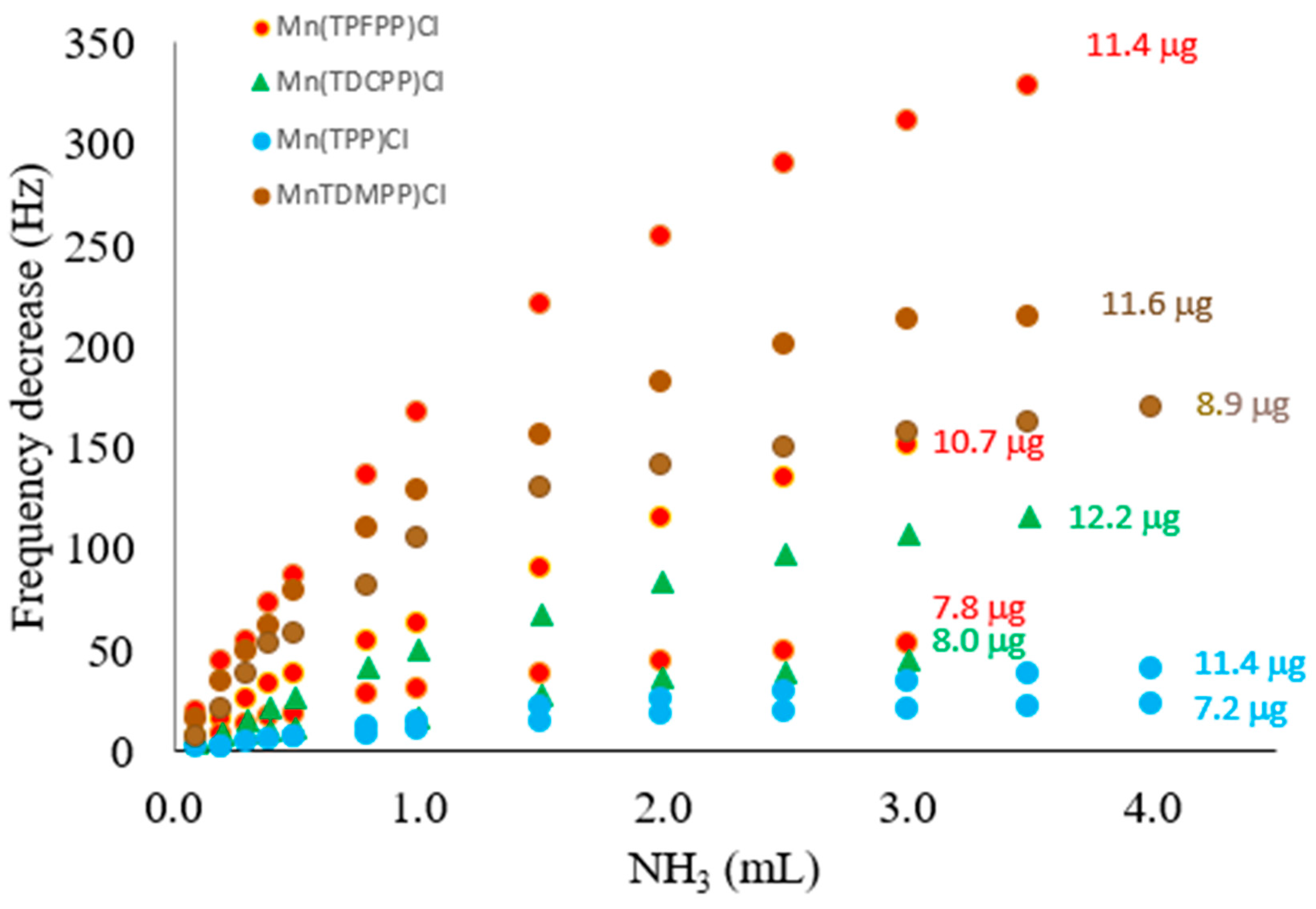
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gibb, S.W. Ammonia. In Handbook of Water Analysis; Nollet, L.M.L., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 2000; pp. 223–259. [Google Scholar]
- Decreto-Lei nº 236/98, Diário de República—Série -A nº176, 1-8-1998. Lisbon, Portugal, 1998. (In Portuguese).
- Searle, P.L. The Berthelot or indophenol reaction and its use in the analytical chemistry of nitrogen. Analyst 1984, 109, 549–568. [Google Scholar] [CrossRef]
- Weatherburn, M.W. Phenol-hypochlorite reaction for determination of ammonia. Anal. Chem. 1967, 39, 971–974. [Google Scholar] [CrossRef]
- Park, G.-E.; Oh, H.N.; Ahn, S. Improvement of the ammonia analysis by the phenate method in water and wastewater. Bull. Korean Chem. Soc. 2009, 30, 2032–2038. [Google Scholar] [CrossRef] [Green Version]
- Aminot, A.; Kirkwood, D.S.; Kérouel, R. Determination of ammonia in seawater by the indophenol-blue method: Evaluation of the ICES NUTS I/C 5 questionnaire. Mar. Chem. 1997, 56, 59–75. [Google Scholar] [CrossRef]
- Shen, H.; Cardwell, T.J.; Catrall, R.W. Determination of ammonia in waste waters by a differential pH method using flow injection potentiometry and a nonactin-based sensor. Analyst 1997, 122, 89–93. [Google Scholar] [CrossRef]
- Ghauri, M.S.; Thomas, J.D.R. Evaluation of an ammonium ionophore for use in poly(vinyl chloride) membrane ion-selective electrodes: Solvent mediator effects. Analyst 1994, 119, 2323–2326. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Zhong, T.; Guan, H.; Liu, F.; Lu, G.; Quan, B. Ammonia sensor based on NASICON and Cr2O3 electrode. Sens. Actuators B Chem. 2009, 136, 479–483. [Google Scholar] [CrossRef]
- Hassan, S.M.; Marei, S.A.; Badr, I.H.; Arida, H.A. Novel solid-state ammonium io potentiometric sensor based on zirconium titanium phosphate ion exchanger. Anal. Chim. Acta 2001, 427, 21–28. [Google Scholar] [CrossRef]
- Lobnik, A.; Wolfbeis, O.S. Sol-gel based optical sensor for dissolved ammonia. Sens. Actuators B Chem. 1998, 51, 203–207. [Google Scholar] [CrossRef]
- Ozawa, S.; Hauser, P.C.; Seiler, K.; Tan, S.S.; Morf, W.E.; Simon, W. Ammonia-gas-selective optical sensors based on neutral ionophores. Anal. Chem. 1991, 63, 640–644. [Google Scholar] [CrossRef]
- Lau, K.T.; Edwards, S. Solid-state ammonia sensor based on Berthelots reaction. Sens. Actuators B Chem. 2004, 98, 12–17. [Google Scholar] [CrossRef]
- Raimundo, I.M., Jr.; Narayanaswamy, R. Simultaneous determination of relative humidity and ammonia in aior emplying na optical fibre sensor and artificial network. Sens. Actuators B Chem. 2001, 74, 60–68. [Google Scholar] [CrossRef]
- Nivens, D.A.; Schiza, M.V.; Angel, S.M. Multilayer sol-gel membranes for optical sensing applications: Single layer pH and dual layer CO2 and NH3 sensors. Talanta 2002, 58, 543–550. [Google Scholar] [CrossRef]
- Tao, S.; Xu, L.; Fanguy, J.C. Optical fibre ammonia sensing probes using reagent immobilized porous silica coating as transducers. Sens. Actuators B Chem. 2006, 115, 158–163. [Google Scholar] [CrossRef]
- Waish, K.; Borisov, S.; Mayr, T.; Klimant, I. Dual lifetime reference trace ammonia sensors. Sens. Actuators B Chem. 2009, 139, 132–138. [Google Scholar] [CrossRef]
- Korposh, S.; Kodaira, S.; Selyanchyn, R.; Ledezma, F.H.; James, S.W.; Lee, S.-W. Porphyrin-nanoassembled fiber-optic gas sensor fabrication: Optimization of parameters for sensitive ammonia gas detection. Opt. Laser Tchnol. 2018, 101, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Zilberman, Y.; Chen, Y.; Sonkusale, R. Dissolved ammonia sensing in complex mixtures using metalloporphyrin-based optoelectronic sensor ans sepectrscopic detection. Sens. Actuators B Chem. 2014, 202, 976–983. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, M.; Ding, H.; Xie, Z. Colloidal silica beads modified with quantum dots and zinc(II) tetraphenylporphyrin for colorimetric sensing. Microchim. Acta 2013, 180, 85–91. [Google Scholar] [CrossRef]
- Timmer, B.; Olthuis, W.; van den Berg, A. Ammonia sensors and their application—A review. Sens. Actuators B Chem. 2005, 107, 666–677. [Google Scholar] [CrossRef]
- Karunagaran, B.; Uthirakumar, S.J.; Chang, S.J.; Velumani, S.; Suh, E.-K. TiO2 thin film gas sensor for monitoring ammonia. Mater. Charact. 2007, 58, 680–684. [Google Scholar] [CrossRef]
- Nanto, H.; Minami, S.; Tanaka, S. Zinc-oxide thin-film ammonia gas sensors with high sensitivity and excellent selectivity. J. Appl. Phys. 1986, 60, 482–484. [Google Scholar] [CrossRef]
- Khan, H.; Malook, K.; Shah, M. Highly selective ammonia sensor using polypyrrole/V2O5 composites. J. Mater. Sci. Mater. Electron. 2017, 28, 13873–13879. [Google Scholar] [CrossRef]
- Patil, D.R.; Patil, L.A.; Patil, P.P. Cr2O3-activated ZnO thick film resistors for ammonia gas sensing operable at room temperature. Sens. Actuators B Chem. 2007, 126, 368–374. [Google Scholar] [CrossRef]
- Tshabalala, Z.; Shinange, K.; Cummings, F.R.; Ntwaeaborwa, O.M.; Mhlongo, G.H.; Motaung, D.E. Ultra-sensitive and selective NH3 room temperature gas sensing induced by manganese-doped titanium dioxide nanoparticles. J. Colloid Interface Sci. 2017, 504, 371–386. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.; Tao, J. Novel highly-selective NH3 sensor based on potassium trisoxalateferrate(II) complex. Sens. Actuators B Chem. 2008, 129, 339–344. [Google Scholar] [CrossRef]
- Penza, M.; Milella, E.; Alba, M.B.; Quirini, A.; Vasanelli, L. Selective NH3 gas sensor based on Langmuir-Blodgett polypyrrole film. Sens. Actuators B Chem. 1997, 40, 205–209. [Google Scholar] [CrossRef]
- Upadhye, D.S.; Bagul, S.B.; Huse, N.P.; Dive, A.S.; Sharma, R. Room temperature ammonia sensing properties of nanostructured polyaniline salt and polyaniline base thin films. Ferroelectrics 2017, 518, 137–145. [Google Scholar] [CrossRef]
- Kumar, L.; Rawal, I.; Kaur, A.; Annapoorni, S. Flexible room temperature ammonia sensor based on polyaniline. Sens. Actuators B Chem. 2017, 240, 408–416. [Google Scholar] [CrossRef]
- Kuol, S.; Chandra, R.; Dhawan, S.K. Conductive polyaniline composite: A reusable sensor material for ammonia. Sens. Actuators B Chem. 2001, 75, 151–159. [Google Scholar] [CrossRef]
- Eising, M.; Cava, C.E.; Salvatierra, R.V.; Zarbi, A.J.G.; Roman, L.S. Doping effect on self-assembled films of polyaniline and carbon nanotube applied as ammonia gas sensor. Sens. Actuators B Chem. 2017, 240, 408–416. [Google Scholar] [CrossRef]
- Gavgani, J.N.; Hasani, A.; Nouri, R.V.; Zarbi, M.; Mahyari, M.; Salehi, A. Highly sensitive and flexible ammonia sensor based on S and N co-doped graphene quantum dots/polyaniline hybrid at room temperature. Sens. Actuators B Chem. 2016, 229, 239–248. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, Y.; Liu, B.; Duan, Z.; Pan, H.; Yuan, Z.; Guangzhong, X.; Wang, J.; Fang, Z.; Tai, H. Ultrathin Nb2CTx nanosheets-supported polyaniline nanocomposite ultrasensitive NH3 detection. Sens. Actuators B Chem. 2021, 343, 130069. [Google Scholar] [CrossRef]
- Matsugushi, M.; Nakamae, T.; Fijisada, R.; Shiba, S. A highly sensitive ammonia gas sensor using mirometer-sized cor-ahell-type spherical polyaniline perticles. Sensors 2021, 21, 7522. [Google Scholar] [CrossRef] [PubMed]
- Matindoust, S.; Farzi, A.; Nejad, M.B.; Abadi, M.H.S.; Zou, Z.; Zheng, L.-R. Ammoia gas sensor based on flexible polyaniline films for rapid detection of spoilage in protein-rich foods. J. Mater. Sci. Mater. Electron. 2017, 28, 7760–7768. [Google Scholar] [CrossRef]
- Fu, T. Determination of ammonia in air using a novel ammonia sensor based on nickel(II) macrocyclic complex. J. Electroanal. Chem. 2008, 618, 94–99. [Google Scholar] [CrossRef]
- Fu, T. Research on gas-sensing properties of lead sulfide-based sensor for detection of NO2 and NH3 at room temperature. Sen. Actuators B Chem. 2009, 140, 116–121. [Google Scholar] [CrossRef]
- Bendahan, M.; Lauque, P.; Lambert-Mauriat, C.; Carnachano, H.; Seguin, J.-L. Sputtered thin films of CuBr for ammonia microsensors: Morphology, composition and ageing. Sens. Actuators B Chem. 2002, 84, 6–11. [Google Scholar] [CrossRef]
- Raj, V.B.; Nimal, A.T.; Parmar, Y.; Sharma, M.U.; Sreenivas, K.; Gupta, V. Cross-sensitivity and selectivity studies on ZnO surface acoustic wave ammonia sensor. Sens. Actuators B Chem. 2010, 147, 517–524. [Google Scholar] [CrossRef]
- Tang, Y.-L.; Li, Z.-J.; Ma, J.-Y.; Guo, Y.-J.; Fu, Y.-Q.; Zu, X.-T. Ammonia gas sensors based on ZnO/SiO2 bi-layer nanofilms ST-cut quartz surface acoustic wave devices. Sens. Actuators B Chem. 2014, 201, 114–121. [Google Scholar] [CrossRef]
- Brannelly, N.T.; Killard, A.J. An electrochemical sensr device for measuring blood ammonia at the point of care. Talanta 2017, 167, 296–301. [Google Scholar] [CrossRef]
- Karmarkar, K.H.; Guilbault, G.G. The detection of ammonia and nitrogen dioxide at the parts per billion level with coated piezoelectric quartz crystal. Anal. Chim. Acta 1975, 75, 111–117. [Google Scholar] [CrossRef]
- Webber, L.M.; Guilbault, G.G. Coated piezoelectric crystal detector for selective detection of ammonia in the atmosphere. Anal. Chem. 1976, 48, 2244–2247. [Google Scholar] [CrossRef] [PubMed]
- Jlavay, J.; Guilbault, G.G. Detection of ammonia in ambient air with coated piezoelectric crystal detector. Anal. Chem. 1978, 50, 1044–1046. [Google Scholar]
- Shen, C.-Y.; Huang, C.-P.; Chuo, H.-C. The improved ammonia gas sensors constructed by L-glutamic acid hydrochloride on surface acoustic wave devices. Sens. Actuators B Chem. 2002, 84, 231–236. [Google Scholar] [CrossRef]
- Fraser, S.M.; Edmonds, T.E.; West, T.S. Development of a multi-sensor system using coated piezoelectric crystal detectors. Analyst 1986, 111, 1183–1188. [Google Scholar] [CrossRef]
- Mirmohseni, A.; Oladegaragoze, A. Construction of a sensor for determination of ammonia and aliphatic amines using polyvinylpyrrolidone coated quartz crystal microbalance. Sens. Actuators B Chem. 2003, 89, 164–172. [Google Scholar] [CrossRef]
- Aoki, K.; Brousseau, L.C.; Mallouk, T.E. Metal phosphonate-based quartz crystal microbalance sensors for amines and ammonia. Sens. Actuators B Chem. 1993, 13–14, 703–704. [Google Scholar] [CrossRef]
- Brousseau, L.C.; Mallouk, T.E. Molecular design of intercalation-based sensors. 1. Ammonia sensing with quartz crystal microbalances modified by copper biphenyl(phosphonate) thin films. Anal. Chem. 1997, 69, 679–687. [Google Scholar] [CrossRef]
- Penza, M.; Milella, E.; Anisimkin, V.I. Monitoring of NH3 gas by LB polypyrrole-based SAW sensor. Sens. Actuators B Chem. 1998, 47, 218–224. [Google Scholar] [CrossRef]
- Slater, J.M.; Watt, E.J. Examination of ammonia-poly(pyrrole) interactions of piezoelectric and conductivity measurements. Analyst 1991, 116, 1125–1130. [Google Scholar] [CrossRef]
- Xu, Y.S.; Si, S.; Lu, C.; Nie, L.; Yao, S. Ammonia selective bulk acoustic wave sensor based on neutral ionophpres. Anal. Chim. Acta 1995, 312, 9–13. [Google Scholar] [CrossRef]
- Schramm, U.; Meinhold, D.; Winter, S.; Heil, C.; Müller-Albrecht, J.; Wächter, L.; Hoff, H.; Roesky, C.E.O.; Rechenbach, T.; Boeker, P.L.; et al. QMB-based temperature-modulated ammonia sensor for humid air. Sens. Actuators B Chem. 2000, 67, 219–226. [Google Scholar] [CrossRef]
- Schramm, U.; Roesky, C.E.O.; Winter, S.; Rechenbach, T.; Boeker, P.; Lammers, P.S.; Weber, E.; Bargon, J. Temperature dependence of an ammonia sensor in humid air based on a cryptophane-coated quartz microbalance. Sens. Actuators B Chem. 1999, 57, 233–237. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Zhu, Z. Ammonia sensing characteristics of ZnO nanowires studied by quartz crystal microbalance. Appl. Surf. Sci. 2006, 252, 2404–2411. [Google Scholar] [CrossRef]
- Ding, B.; Kim, J.; Miyazaki, Y.; Shiratori, S. Electrospun nanofibrous membranes coatec quartz crystal microbalance as gas sensor for NH3 detection. Sens. Actuators B Chem. 2004, 101, 373–380. [Google Scholar] [CrossRef]
- Shen, C.-Y.; Liou, S.Y. Surface acoutic wave gas monitor for ppm ammonia detection. Talanta 2008, 131, 673–679. [Google Scholar] [CrossRef]
- Mérian, T.; Debarnot, D.; Rouessac, V.; Poncin-Epaillard, F. Ammonia absorption stdy of pulsed-plasma polyaniline by quartz crystal microgravimetry and UV/vis spectrometry. Talanta 2010, 81, 602–608. [Google Scholar] [CrossRef]
- Lv, D.; Chen, W.; Shen, W.; Peng, M.; Zhang, X.; Wang, R.; Xu, L.; Xu, W.; Song, W.; Tan, R. Enhanced flexible room temparature ammonia sensor based on PEDOT:PSS thin film with FeCl3 additives prepared by inkjet printing. Sens. Actuators B Chem. 2019, 298, 126890. [Google Scholar] [CrossRef]
- Sett, A.; Majunder, S.; Bhattacharyya, T.K. Flexible room temperature ammonia gas sensor based on low-temperature tuning of functional groups in graphene. IEEE Trans. Electron. Devices 2021, 68, 3181–3188. [Google Scholar] [CrossRef]
- Kumar, R.; Goel, N.; Hojamberdiev, M.; Kumar, M. Transition metal dichalcogenides-based flexible gas sensors. Sens. Actuators A 2020, 303, 111875. [Google Scholar] [CrossRef]
- Yu, X.; Chen, X.; Chen, X.; Zhao, X.; Yu, X.; Ding, X. Digital ammonia gas sensor based on quartz resonator tuned by interdigital electrode coated with polyaniline film. Org. Electron. 2020, 76, 105413. [Google Scholar] [CrossRef]
- Lv, D.; Shen, W.; Chen, W.; Tan, R.; Xu, L.; Song, W. PSS-PANI/PVDF composite based flexible NH3 sensors with sub-ppm detection at room temperature. Sens. Actuators B Chem. 2021, 328, 129085. [Google Scholar] [CrossRef]
- Hofstetter, D.; Fabian, E.; Monti, D.; Lorenzoni, A.G. Ammonia generation system for poultry health research using arduino. Sensors 2021, 117, 6664. [Google Scholar] [CrossRef]
- Paolesse, R.; Nardis, S.; Monti, D.; Stefanelli, M.; Di Natale, C. Porphyrinoids for chemical sensor applications. Chem. Rev. 2017, 117, 2517–2583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, J.M.M.; Farinha, A.S.F.; Muteto, P.V.; Woranovicz-Barreira, S.M.; Paz, F.A.A.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S.; Tomé, A.C.; Gomes, M.T.S.R.; Sessler, J.L.; et al. New porphyrin derivatives for phosphate anion sensing in both organic and aqueous media. ChemComm 2014, 50, 1359–1361. [Google Scholar] [CrossRef] [PubMed]
- Morales-Bahnik, A.; Czolk, R.; Ache, H.J. An optochemical ammonia sensor based on immibilized metalloporphyrin. Sens. Act. B 1994, 18–19, 493–496. [Google Scholar] [CrossRef]
- Sauerbrey, G.Z. Verwendung von Schwingquarzen zur Wägung dünner Schichten und zur Mikrowägung. Z. Physik 1959, 155, 206–222. [Google Scholar] [CrossRef]
- Gomes, M.T.S.R. Is a quartz crystal microbalance really a mass sensor? Curr. Top. Anal. Chem. 2001, 2, 187–193. [Google Scholar]
- Gonsalves, A.M.A.R.; Varejão, J.M.T.B.; Pereira, M.M. Some new aspects related to the synthesis of meso-substituted porphyrins. J. Heterocycl. Chem. 1991, 28, 635–640. [Google Scholar] [CrossRef]
- Adler, A.D.; Longo, F.R.; Kampas, F.L. On the preparation of metalloporphyrins. J. Inorg. Nucl. Chem. 1970, 32, 2443–2445. [Google Scholar] [CrossRef]
- Buchler, J.W. The Porphyrins; Dolphin, D., Ed.; Academic: New York, NY, USA, 1978; Volume 1, p. 398. [Google Scholar]
- Gomes, M.T.S.R.; Duarte, A.C.; Oliveira, J.A.B.P. Critical Assessment of the Parameters that Affect the Selection of Coating Compounds for Piezoelectric Quartz Crystal Microbalances. Talanta 1999, 48, 81–89. [Google Scholar] [CrossRef]


| Date | Calibration Line |
|---|---|
| 26 May | −ΔF = 0.599 [NH3] − 1.455 |
| 8 December | −ΔF = 0.608 [NH3] − 0.746 |
| 6 January | −ΔF = 0.609 [NH3] + 0.453 |
| 25 January | −ΔF = 0.607 [NH3] + 0.364 |
| 1 February | −ΔF = 0.612 [NH3] + 1.104 |
| Sample | A1 | A2 | V1 | V2 | V3 | V4 | V5 | |
|---|---|---|---|---|---|---|---|---|
| NH3 (µg L−1) | Sensor | 42.6 ± 1.4 | 20.1 ± 1.4 | 20.7 ± 1.3 | 20.4 ± 1.5 | 25.1 ± 1.9 | 22.0 ± 1.4 | 29.7 ± 1.8 |
| Indophenol Method | 42.3 ± 1.3 | 20.0 ± 1.7 | 20.7 ± 1.5 | 20.4 ± 1.6 | 25.9 ± 1.6 | 21.9 ± 1.7 | 29.6 ± 1.5 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antunes, V.L.M.; Gomes, M.T.S.R. A New Analytical Method to Quantify Ammonia in Freshwater with a Bulk Acoustic Wave Sensor. Sensors 2022, 22, 1528. https://doi.org/10.3390/s22041528
Antunes VLM, Gomes MTSR. A New Analytical Method to Quantify Ammonia in Freshwater with a Bulk Acoustic Wave Sensor. Sensors. 2022; 22(4):1528. https://doi.org/10.3390/s22041528
Chicago/Turabian StyleAntunes, Vera Lúcia M., and Maria Teresa S. R. Gomes. 2022. "A New Analytical Method to Quantify Ammonia in Freshwater with a Bulk Acoustic Wave Sensor" Sensors 22, no. 4: 1528. https://doi.org/10.3390/s22041528






