Detection of E. coli Bacteria in Milk by an Acoustic Wave Aptasensor with an Anti-Fouling Coating
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Aptamer Stock Solution
2.3. Cleaning and Surface Modification of Quartz Crystals
2.4. EMPAS Measurements
3. Results
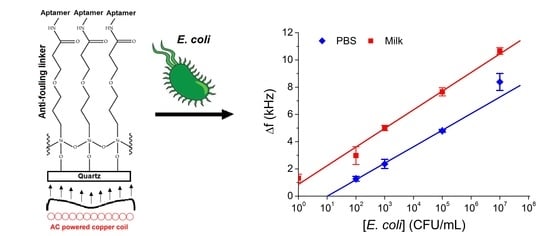
3.1. Sensing of E. coli in PBS and Milk
3.2. Testing the Selectivity of the Aptasensor
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Milk and Milk Product Statistics. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Milk_and_milk_product_statistics (accessed on 29 November 2021).
- World Health Organization; Food and Agriculture Organization of the United Nations. Shiga Toxin-Producing Escherichia coli (STEC) and Food: Attribution, Characterization, and Monitoring; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Ballem, A.; Gonçalves, S.; Garcia-Meniño, I.; Flament-Simon, S.C.; Blanco, J.E.; Fernandes, C.; Saavedra, M.J.; Pinto, C.; Oliveira, H.; Blanco, J.; et al. Prevalence and serotypes of Shiga toxin-producing Escherichia coli (STEC) in dairy cattle from Northern Portugal. PLoS ONE 2020, 15, e0244713. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Biological Hazards (EFSA BIOHAZ Panel). Scientific Opinion on the public health risks related to the consumption of raw drinking milk. EFSA J. 2015, 13, 3940. [Google Scholar] [CrossRef] [Green Version]
- Mir, R.A.; Kudva, I.T. Antibiotic-resistant Shiga toxin-producing Escherichia coli: An overview of prevalence and intervention strategies. Zoonoses Public Health 2019, 66, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milk Quality: Bacterial Contamination. Available online: https://www.teagasc.ie/media/website/animals/dairy/Bacteria.pdf#:~:text=EU%20legislation%20indicates%20that%20total%20bacterial%20count%20%28TBC%29,counts%20of%20greater%20than%201%2C000%2Fml%20are%20generally%20penalised (accessed on 29 November 2021).
- Chye, F.Y.; Abdullah, A.; Ayob, M.K. Bacteriological quality and safety of raw milk in Malaysia. Food Microbiol. 2004, 21, 535–541. [Google Scholar] [CrossRef]
- Ali, M.A.; Elsherbiny, M.E.; Emara, M. Updates on aptamer research. Int. J. Mol. Sci. 2019, 20, 2511. [Google Scholar] [CrossRef] [Green Version]
- Bu, S.J.; Wang, K.Y.; Bai, H.S.; Leng, Y.; Ju, C.J.; Wang, C.Y.; Liu, W.S.; Wan, J.Y. Immunoassay for pathogenic bacteria using platinum nanoparticles and a hand-held hydrogen detector as transducer. Application to the detection of Escherichia coli O157: H7. Mikrochim. Acta 2019, 186, 296. [Google Scholar] [CrossRef]
- Subjakova, V.; Oravczova, V.; Tatarko, M.; Hianik, T. Advances in electrochemical aptasensors and immunosensors for detection of bacterial pathogens in food. Electrochim. Acta 2021, 389, 138724. [Google Scholar] [CrossRef]
- Demirkol, D.O.; Timur, S. A sandwich-type assay based on quantum dot/aptamer bioconjugates for analysis of E. coli O157: H7 in microtiter plate format. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 85–90. [Google Scholar] [CrossRef]
- Denny, J.; Bhat, M.; Eckman, K. Outbreak of Escherichia coli O157:H7 associated with raw milk consumption in the Pacific northwest. Foodborne Path. Dis. 2008, 5, 321–328. [Google Scholar] [CrossRef]
- Yu, X.; Chen, F.; Wang, R.; Li, Y. Whole-bacterium SELEX of DNA aptamers for rapid detection of E. coli O157: H7 using a QCM sensor. J. Biotechnol. 2018, 266, 39–49. [Google Scholar] [CrossRef]
- Zou, Y.; Duan, N.; Wu, S.; Shen, M.; Wang, Z. Selection, identification, and binding mechanism studies of an ssDNA aptamer targeted to different stages of E. coli O157: H7. J. Agricult. Food Chem. 2018, 66, 5677–5682. [Google Scholar] [CrossRef] [PubMed]
- Abdelrasoul, G.N.; Anwar, A.; MacKay, S.; Tamura, M.; Shah, M.A.; Khasa, D.P.; Montgomery, R.R.; Ko, A.I.; Chen, J. DNA aptamer-based non-faradaic impedance biosensor for detecting E. coli. Anal. Chim. Acta 2020, 1107, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-W.; Wang, H.-X.; Jia, G.-C.; Li, Z. Application of aptamer-based biosensor for rapid detection of pathogenic Escherichia coli. Sensors 2018, 18, 2518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melikishvili, S.; Piovarci, I.; Hianik, T. Advances in colorimetric assay based on AuNPs modified by proteins and nucleic acid aptamers. Chemosensors 2021, 9, 281. [Google Scholar] [CrossRef]
- Urmann, K.; Bahnemann, J.; Chikneyan, Z.; Kasmaee, L.M.; Hoffmann, M.R. Electromechanical detection of pathogens with self-assembled nucleic acid biosensors. TechConnect Briefs 2018, 2, 153–156. [Google Scholar]
- Khobragade, S.; Da Silva Granja, C.; Sandström, N.; Efimov, I.; Ostanin, V.P.; van der Wijngaart, W.; Klenerman, D.; Ghosh, S.K. Direct detection of whole bacteria using a nonlinear acoustic resonator. Sens. Actuat. B Chem. 2020, 316, 128086. [Google Scholar] [CrossRef]
- Ballantyne, S.M.; Thompson, M. Superior analytical sensitivity of electromagnetic excitation compared to contact electrode instigation of transverse acoustic waves. Analyst 2004, 129, 219–224. [Google Scholar] [CrossRef]
- Thompson, M.; Blaszykowski, C.; Sheikh, S.; Rodriguez-Emmenegger, C.; De Los Santos Pereira, A. Biological Fluid-Surface Interactions. Detection and Medical Devices; RSC Detection Science Series; Royal Society of Chemistry: Cambridge, UK, 2017. [Google Scholar]
- Banerjee, I.; Pangule, R.C.; Kane, R.S. Antifouling coatings: Recent developments in the design of surfaces that prevent fouling by proteins, bacteria, and marine organisms. Adv. Mater. 2011, 23, 690–718. [Google Scholar] [CrossRef]
- Blaszykowski, C.; Sheikh, S.; Thompson, M. Surface chemistry to minimize fouling from blood-based fluids. Chem. Soc. Rev. 2012, 41, 5599–5612. [Google Scholar] [CrossRef]
- Damodaran, V.B.; Murthy, N.S. Bio-inspired strategies for designing antifouling biomaterials. Biomater. Res. 2016, 20, 18. [Google Scholar] [CrossRef] [Green Version]
- Sheikh, S.; Yang, D.Y.; Blaszykowski, C.; Thompson, M. Single ether group in a glycol-based ultra-thin layer prevents surface fouling from undiluted serum. Chem. Commun. 2012, 48, 1305–1307. [Google Scholar] [CrossRef]
- Fedorov, K.; Jankowski, A.; Sheikh, S.; Blaszykowski, C.; Reheman, A.; Romaschin, A.; Ni, H.; Thompson, M. Prevention of surface-induced thrombogenesis on poly(vinyl chloride). J. Mater. Chem. B 2015, 3, 8623. [Google Scholar] [CrossRef] [PubMed]
- De La Franier, B.; Asker, D.; van den Berg, D.; Hatton, B.; Thompson, M. Reduction of microbial adhesion on polyurethane by a sub-nanometer covalently-attached surface modifier. Coll. Surf. B Biointerfaces 2021, 200, 111579. [Google Scholar] [CrossRef] [PubMed]
- De La Franier, B.; Jankowski, A.; Thompson, M. Functionalizable self-assembled trichlorosilyl-based monolayer for application in biosensor technology. Appl. Surf. Sci. 2017, 414, 435–441. [Google Scholar] [CrossRef]
- Spagnolo, S.; De La Franier, B.; Hianik, T.; Thompson, M. Surface probe linker with tandem anti-fouling properties for application in biosensor technology. Biosensors 2020, 10, 20. [Google Scholar] [CrossRef] [Green Version]
- Bruno, J.G.; Carrillo, M.P.; Phillips, T.; Andrews, C.J. A novel screening method for competitive FRET-aptamers applied to E. coli assay development. J. Fluoresc. 2010, 20, 1211–1223. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Zhang, H.; Wang, X.; Wei, W.; Shen, Y.; Yu, J.; Liang, J.; Zheng, J.; Shen, Y. Facile synthesis of tunable plasmonic silver core/magnetic Fe3O4 shell nanoparticles for rapid capture and effective photothermal ablation of bacterial pathogens. New J. Chem. 2017, 41, 10155–10164. [Google Scholar] [CrossRef]
- Fang, W.; Han, C.; Zhang, H.; Wei, W.; Liu, R.; Shen, Y. Preparation of amino-functionalized magnetic nanoparticles for enhancement of bacterial capture efficiency. RSC Adv. 2016, 6, 67875–67882. [Google Scholar] [CrossRef]
- Xing, J.; Ma, L.; Cheng, X.; Ma, J.; Wang, R.; Xu, K.; Mymryk, J.S.; Zhang, Z. Expression and functional analysis of the argonaute protein of thermus thermophilus (TtAgo) in E. coli BL21 (DE3). Biomolecules 2021, 11, 524. [Google Scholar] [CrossRef]
- de los Santos Pereira, A.; Sheikh, S.; Blaszykowski, C.; Pop-Georgievski, O.; Fedorov, K.; Thompson, M.; Rodriguez-Emmenegger, C. Antifouling polymer brushes displaying antithrombogenic surface properties. Biomacromolecules 2016, 17, 1179–1185. [Google Scholar] [CrossRef]
- Wilson, B.D.; Soh, H.T. Re-evaluation the conventional wisdom about binding assays. Trends Biochem. Sci. 2020, 45, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Shorie, M.; Sharma, M.; Ganguli, A.K.; Sabherwal, P. Bridged rebar graphene functionalized aptasensor for pathogenic E. coli O78:K80:H11 detection. Biosens. Bioelectron. 2017, 98, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Shorie, M.; Sabherwal, P. Electrochemical aptasensor using boron-carbon nanorods decorated by nickel nanoparticles for detection of E. coli O157:H7. Microchim. Acta 2020, 187, 461. [Google Scholar] [CrossRef] [PubMed]
- Brosel-Oliu, S.; Ferreira, F.; Uria, N.; Abramova, N.; Gargallo, R.; Muñoz-Pascual, F.Y.; Bratov, A. Novel impedimetric aptasensor for label-free detection of Escherichia coli O157:H7. Sens. Actuat. B Chem. 2018, 255, 2988–2995. [Google Scholar] [CrossRef] [Green Version]
- Raj, P.; Oh, M.H.; Han, K.; Lee, T.Y. Label-free electrochemical biosensor based on Au@MoS2–PANI for Escherichia coli detection. Chemosensors 2021, 9, 49. [Google Scholar] [CrossRef]
- Zelada-Guillén, G.A.; Bhosale, S.V.; Riu, J.; Rius, F.X. Real-time potentiometric detection of bacteria in complex samples Anal. Chem. 2010, 82, 9254–9260. [Google Scholar] [CrossRef]
- Bu, S.; Wang, K.; Li, Z.; Wang, C.; Hao, Z.; Liu, W.; Wan, J. An electrochemical biosensor based on methylene blue-loaded nanocomposites as signal-amplifying tags to detect pathogenic bacteria. Analyst 2020, 145, 4328. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, M.; Zhong, Y.; Yang, J.; Zhao, Y.; Wu, W.; Ye, W.; Wen, J.; Wang, Q.; Lu, J. An aptamer-based biosensor for colorimetric detection of Escherichia coli O157:H7. PLoS ONE 2012, 7, e48999. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.H.; Li, M.; Wang, Y.; Ouyang, H.X.; Wang, L.; Li, C.X.; Cao, Y.C.; Meng, Q.H.; Lu, J.X. Aptasensors for rapid detection of Escherichia coli O157:H7 and Salmonella typhimurium. Nanosci. Res. Lett. 2012, 7, 658–665. [Google Scholar] [CrossRef] [Green Version]
- Ledlod, S.; Areekit, S.; Santiwatanakul, S.; Chansiri, K. Colorimetric aptasensor for detecting Salmonella spp., Listeria monocytogenes, and Escherichia coli in meat samples. Food Sci. Technol. Int. 2020, 26, 430–443. [Google Scholar] [CrossRef]
- Duan, N.; Yang, W.; Wu, S.; Zou, Y.; Wang, Z. A visual and sensitive detection of Escherichia coli based on aptamer and peroxidase-like mimics of copper-metal organic framework nanoparticles. Food Anal. Meth. 2020, 13, 1433–1441. [Google Scholar] [CrossRef]
- Gupta, R.; Kumar, A.; Kumar, S.; Kumar Pinnaka, A.; Kumar Singhal, N. Naked eye colorimetric detection of Escherichia coli using aptamer conjugated graphene oxide enclosed gold nanoparticles. Sens. Actuat. B Chem. 2021, 329, 129100. [Google Scholar] [CrossRef]
- Xie, Y.; Huang, Y.; Li, J.; Wu, J. A trigger-based aggregation of aptamer-functionalized gold nanoparticles for colorimetry: An example on detection of Escherichia coli O157:H7. Sens. Actuat. B Chem. 2021, 339, 129865. [Google Scholar] [CrossRef]





| Crystal Coating | E. coli (CFU/mL) | Δf in PBS (kHz) | Δf in Milk (kHz) |
|---|---|---|---|
| Bare | 1 × 108 | 17 ± 4 | 27 ± 2 |
| MEG-Cl | 1 × 108 | 0.5 ± 0.8 | N/A |
| MEG-Aptamer | 0 | N/A | 1.3 ± 0.3 |
| 1 × 102 | 1.2 ± 0.2 | 3.0 ± 0.7 | |
| 1 × 103 | 2.4 ± 0.4 | 5.0 ± 0.2 | |
| 1 × 105 | 4.8 ± 0.1 | 7.6 ± 0.3 | |
| 1 × 107 | 8.4 ± 0.6 | 10.7 ± 0.2 |
| E. coli Strain | Material Platform | Method of Detection | LOD, CFU/mL | Linear Range, CFU/mL | Food Sample/Recovery % | Reference |
|---|---|---|---|---|---|---|
| Acoustic biosensors | ||||||
| 8739 (Crooks strain) | Gold-thiolated aptamer | QCM | 107 | NA | NA | [18] |
| O157:H7 | Gold-MHDA-streptavidin, biotinylated aptamer | QCM | 1.46 × 103 | 5 × 102–5 × 105 | NA | [13] |
| KCTC 2571 | Gold-biotinylated thiol-streptavidin-biotinylated aptamer | QCM | 104 | 105–108 | NA | [19] |
| DH5α | SiO2-MEG-NH2-Aptamer | EMPAS | 8 | 10–107 | Not diluted milk/127.4 | This work |
| Electrochemical biosensors | ||||||
| O78:K80:H11 | SCPE/MWCNTs/TPA | EIS | 10 | 10–106 | Water, guava, litchi, mango juices, milk/NA | [36] |
| O157:H7 | SCPE/BC –Ni | EIS | 10 | 1–105 | Tap water, vegetable, carrot, orange juices, stool/NA | [37] |
| O157:H7 | 3D-IDEA | EIS | 2.9 × 102 | 10–105 | Water/92.3 | [38] |
| BL21 | IDE | EIS | 9 | 25–103 | NA | [15] |
| NA | Au@MoS2–PANI nanocomposite | EIS, DPV | 10 | 10–107 | Urine/90–110 | [39] |
| CECT 675 | SWCNTs-RNA aptamers | Potentiometry | 6–26 | 4–2.4 × 104 | Filtrated milk, apple juice/NA | [40] |
| O157:H7 | Gold-Aptamer/MCH/MB@MI nanocomposite | DPV | 32 | 102–107 | Milk/88.1–115.3 | [41] |
| Optical biosensors | ||||||
| O157:H7 | CdSe/ZnS-QD | Fluorescence | 102 | 102–107 | NA | [11] |
| O157:H7 | PDA vesicles | Colorimetry | 104 | 104–108 | Fecal samples in PBS/NA | [42] |
| O157:H7 | AuNPs | Colorimetry | 105 | 106–107 | NA | [43] |
| ATCC 25922 | AuNPs | Colorimetry | 105 | 105–108 | Meat/NA | [44] |
| O157:H7 | Cu-MOF NPs/sandwich assay with two aptamers | Colorimetry | 2 | 16–1.6 × 106 | Milk/filtrated/20x diluted with water/96–102.6 | [45] |
| MTCC 1698 | GO-AuNPs | Colorimetry | 10 | 10–104 | Coconut water, litchi juice (both 10x diluted), bread homogenized and filtrated/NA | [46] |
| O157:H7 | AuNPs | Colorimetry | 147 | 1.2 × 102–9 × 103 | Tap water/90.2–96.7 | [47] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spagnolo, S.; De La Franier, B.; Davoudian, K.; Hianik, T.; Thompson, M. Detection of E. coli Bacteria in Milk by an Acoustic Wave Aptasensor with an Anti-Fouling Coating. Sensors 2022, 22, 1853. https://doi.org/10.3390/s22051853
Spagnolo S, De La Franier B, Davoudian K, Hianik T, Thompson M. Detection of E. coli Bacteria in Milk by an Acoustic Wave Aptasensor with an Anti-Fouling Coating. Sensors. 2022; 22(5):1853. https://doi.org/10.3390/s22051853
Chicago/Turabian StyleSpagnolo, Sandro, Brian De La Franier, Katharina Davoudian, Tibor Hianik, and Michael Thompson. 2022. "Detection of E. coli Bacteria in Milk by an Acoustic Wave Aptasensor with an Anti-Fouling Coating" Sensors 22, no. 5: 1853. https://doi.org/10.3390/s22051853