A Near InfraRed Emissive Chemosensor for Zn2+ and Phosphate Derivatives Based on a Di-(2-picolyl)amine-styrylflavylium Push-Pull Fluorophore
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis
2.2. UV-Vis and Fluorescence Measurements
2.2.1. Minimum Inhibitory Concentration (MIC) Determination
2.2.2. Biocompatibility Assay in A549 Lung Carcinoma Cells
2.2.3. Microscopy Experiments
3. Results and Discussion
3.1. Synthesis and Photophysical Characterization
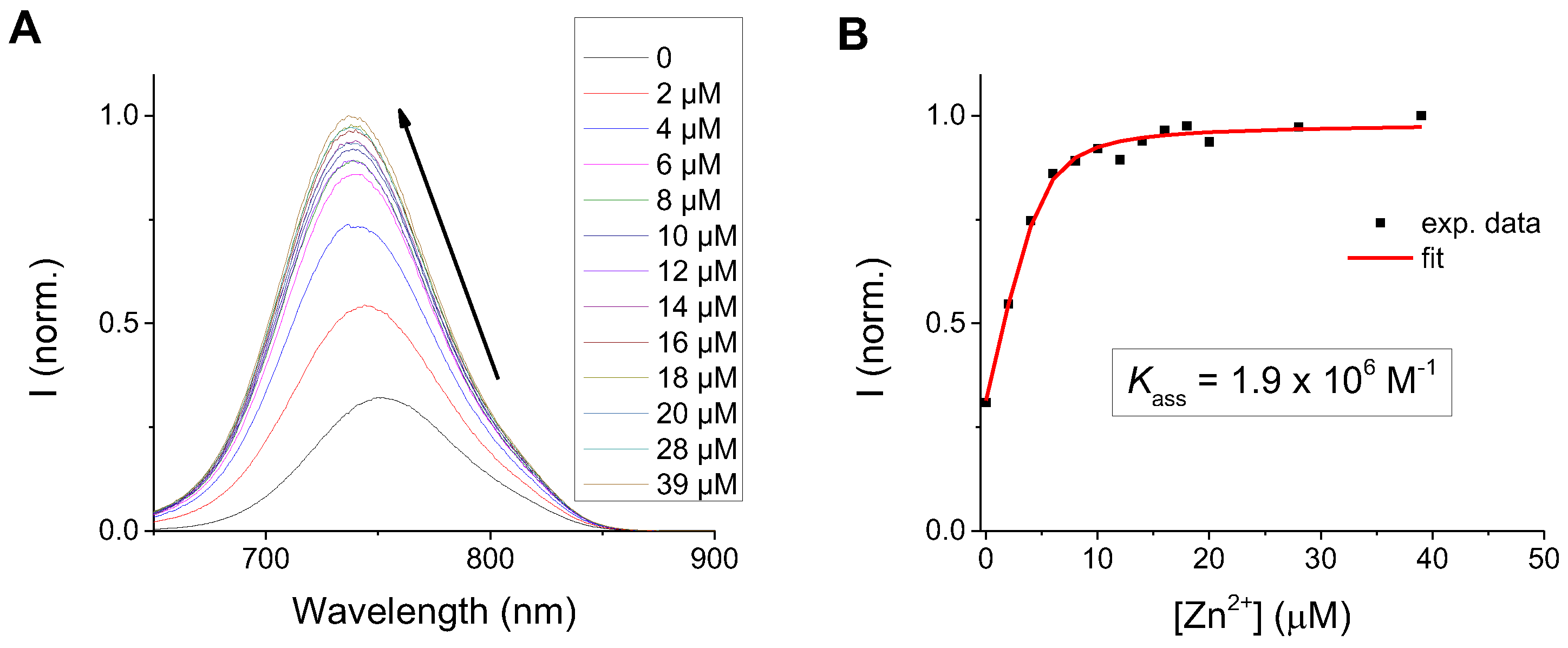
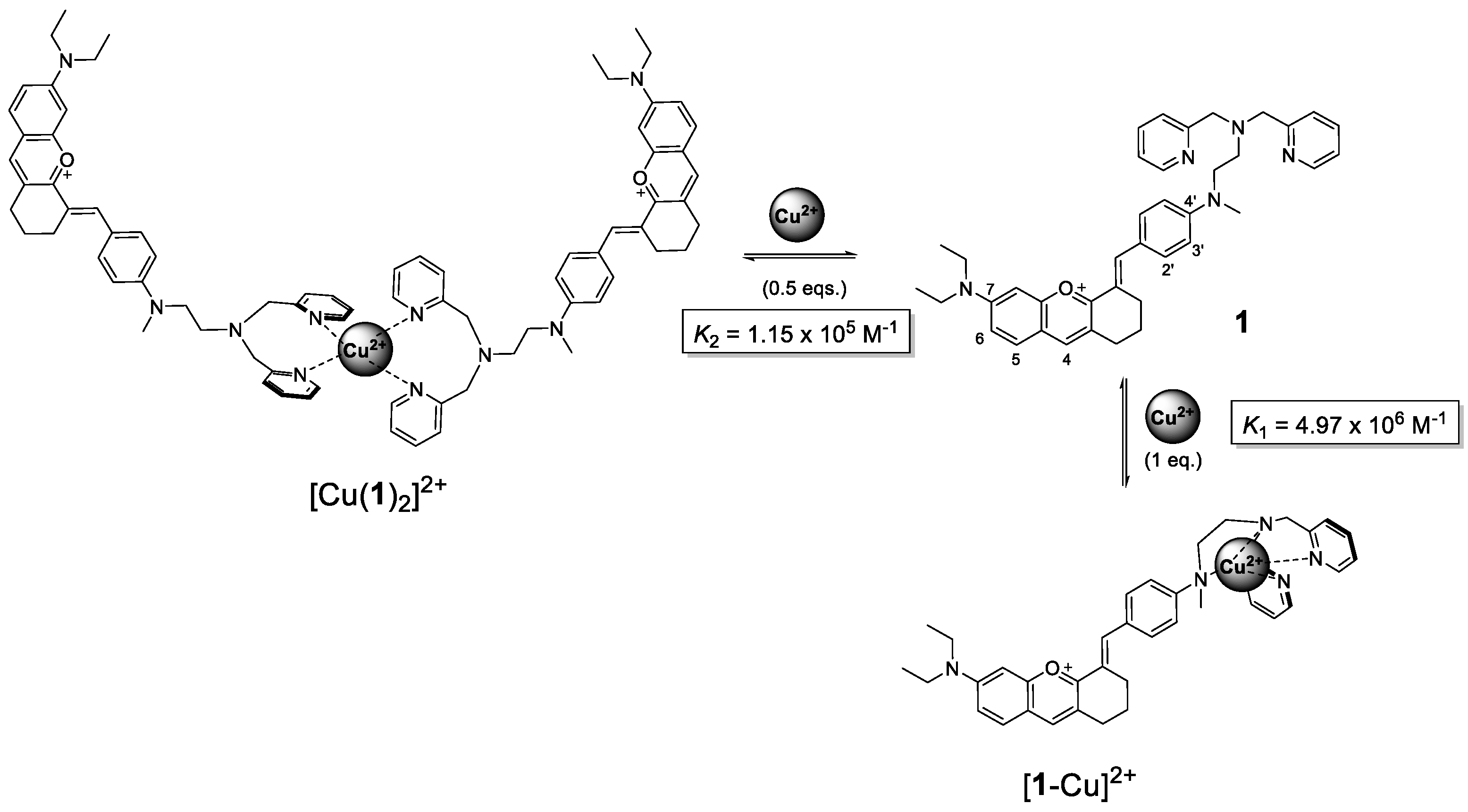
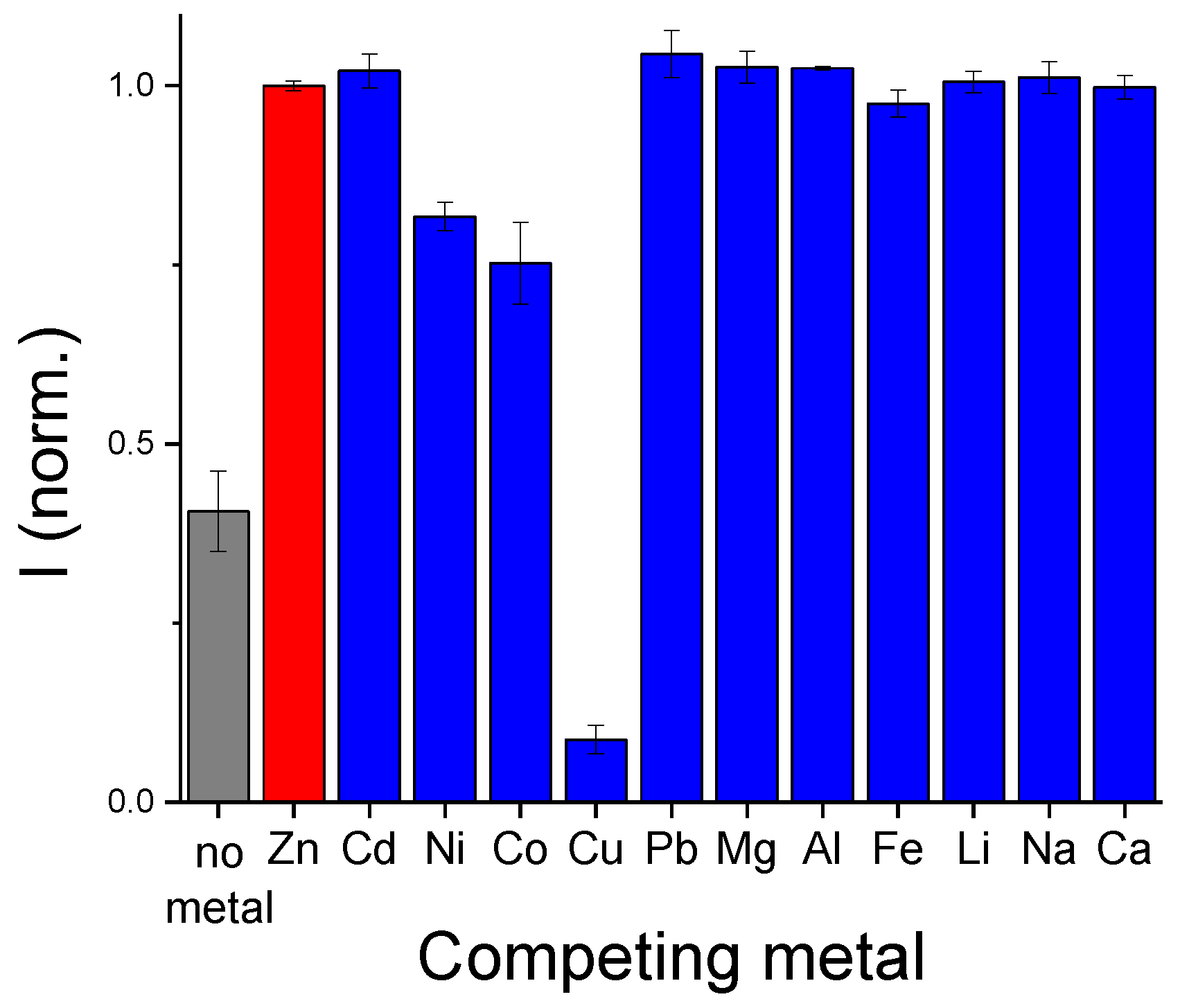
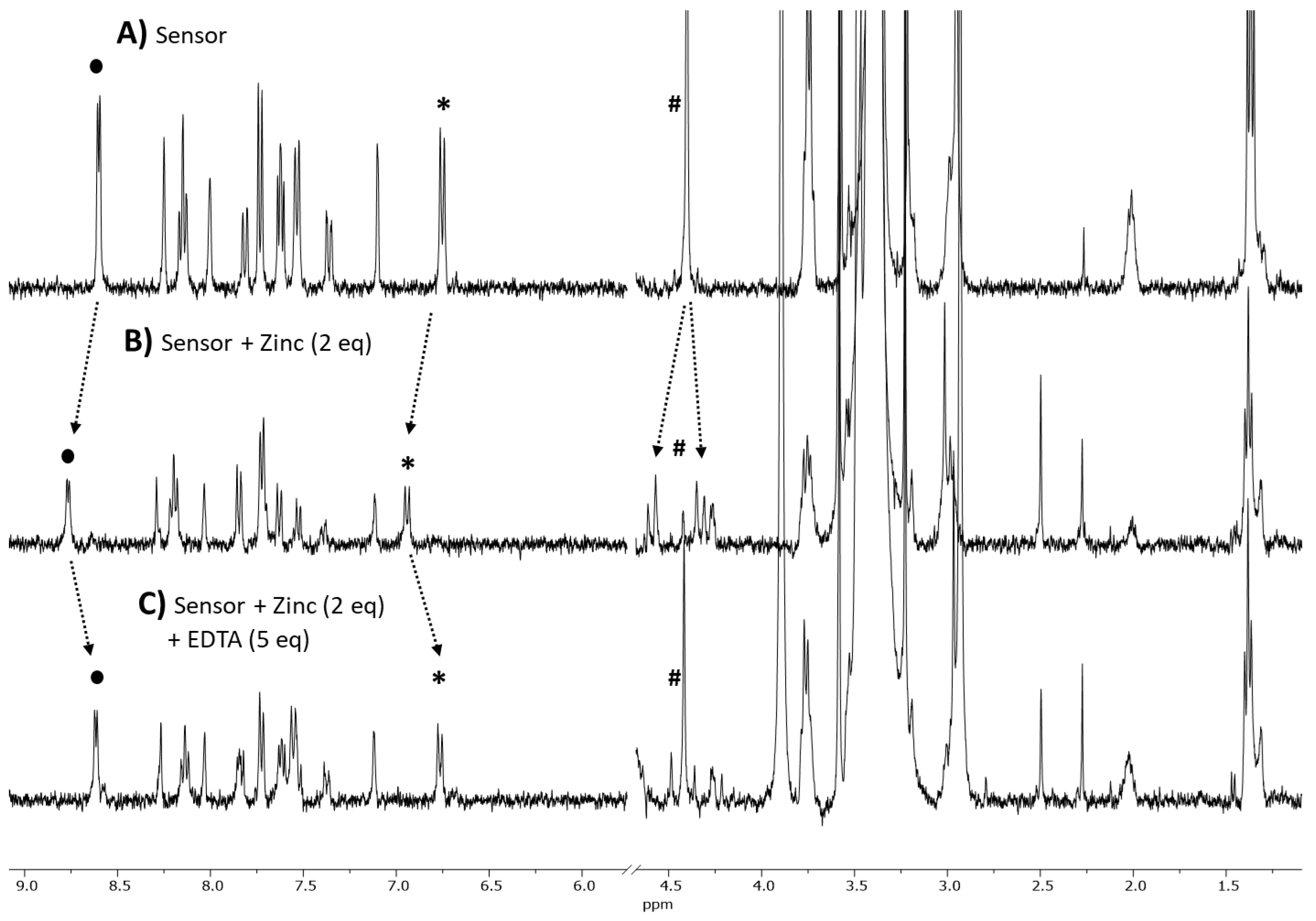
3.2. Metal Sensitivity/Selectivity
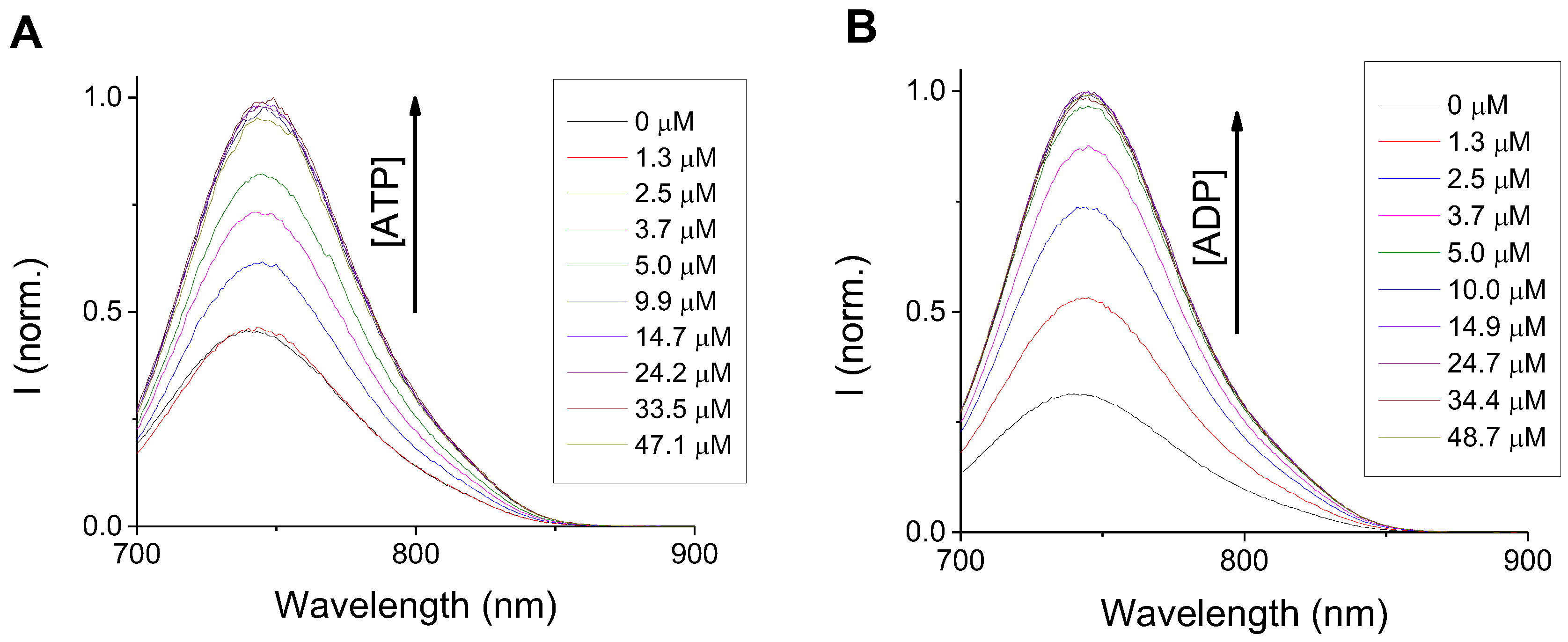
3.2.1. Anion Sensing
3.2.2. Biocompatibility and Live-Cell Imaging Studies Using with Chemosensor 1
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, D.; Sedgwick, A.C.; Gunnlaugsson, T.; Akkaya, E.U.; Yoon, J.; James, T.D. Fluorescent chemosensors: The past, present and future. Chem. Soc. Rev. 2017, 46, 7105–7123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, C.; Sedgwick, A.C.; Hirao, T.; Sessler, J.L. Supramolecular fluorescent sensors: An historical overview and update. Coord. Chem. Rev. 2021, 427, 213560. [Google Scholar] [CrossRef] [PubMed]
- Hang, Y.; Boryczka, J.; Wu, N. Visible-light and near-infrared fluorescence and surface-enhanced Raman scattering point-of-care sensing and bio-imaging: A review. Chem. Soc. Rev. 2022, 51, 329–375. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, H.; Zhang, Y.; Jia, C.; Ji, M. Development and application of several fluorescent probes in near infrared region. Dye. Pigment. 2021, 190, 109284. [Google Scholar] [CrossRef]
- Zhao, J.; Ma, T.; Chang, B.; Fang, J. Recent Progress on NIR Fluorescent Probes for Enzymes. Molecules 2022, 27, 5922. [Google Scholar] [CrossRef]
- Jeong, Y.; Yoon, J. Recent progress on fluorescent chemosensors for metal ions. Inorg. Chim Acta 2012, 381, 2–14. [Google Scholar] [CrossRef]
- Komatsu, K.; Kikuchi, K.; Kojima, H.; Urano, Y.; Nagano, T. Selective zinc sensor molecules with various affinities for Zn2+, revealing dynamics and regional distribution of synaptically released Zn2+ in hippocampal slices. J. Am. Chem. Soc. 2005, 127, 10197–10204. [Google Scholar] [CrossRef]
- Goldsmith, C.R.; Lippard, S.J. 6-Methylpyridyl for pyridyl substitution tunes the properties of fluorescent zinc sensors of the Zinpyr family. Inorg. Chem. 2006, 45, 555–561. [Google Scholar] [CrossRef]
- Gwizdala, C.; Kennedy, D.P.; Burdette, S.C. ZinCast-1: A photochemically active chelator for Zn2+. Chem. Commun. 2009, 6967–6969. [Google Scholar] [CrossRef]
- Sivaraman, G.; Iniya, M.; Anand, T.; Kotla, N.G.; Sunnapu, O.; Singaravadivel, S.; Gulyani, A.; Chellappa, D. Chemically diverse small molecule fluorescent chemosensors for copper ion. Coord. Chem. Rev. 2018, 357, 50–104. [Google Scholar] [CrossRef]
- Ojida, A.; Mito-Oka, Y.; Inoue, M.A.; Hamachi, I. First artificial receptors and chemosensors toward phosphorylated peptide in aqueous solution. J. Am. Chem. Soc. 2002, 124, 6256–6258. [Google Scholar] [CrossRef] [PubMed]
- Ojida, A.; Mito-Oka, Y.; Sada, K.; Hamachi, I. Molecular Recognition and Fluorescence Sensing of Monophosphorylated Peptides in Aqueous Solution by Bis(zinc(II)-dipicolylamine)-Based Artificial Receptors. J. Am. Chem. Soc. 2004, 126, 2454–2463. [Google Scholar] [CrossRef] [PubMed]
- Ngo, H.T.; Liu, X.; Jolliffe, K.A. Anion recognition and sensing with Zn(II)–dipicolylamine complexes. Chem. Soc. Rev. 2012, 41, 4928–4965. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, L.E.S.; Moragues, M.E.; Climent, E.; Agostini, A.; Martínez-Máñez, R.; Sancenón, F. Chromogenic and fluorogenic chemosensors and reagents for anions. A comprehensive review of the years 2010–2011. Chem. Soc. Rev. 2013, 42, 3489–3613. [Google Scholar] [CrossRef]
- Busschaert, N.; Caltagirone, C.; Van Rossom, W.; Gale, P.A. Applications of Supramolecular Anion Recognition. Chem. Rev. 2015, 115, 8038–8155. [Google Scholar] [CrossRef] [PubMed]
- Gale, P.A.; Howe, E.N.W.; Wu, X. Anion Receptor Chemistry. Chem 2016, 1, 351–422. [Google Scholar] [CrossRef] [Green Version]
- Feng, L.; Chen, W.; Ma, X.; Liu, S.H.; Yin, J. Near-infrared heptamethine cyanines (Cy7): From structure, property to application. Org. Biomol. Chem. 2020, 18, 9385–9397. [Google Scholar] [CrossRef]
- Kaur, P.; Singh, K. Recent advances in the application of BODIPY in bioimaging and chemosensing. J. Mater. Chem. C 2019, 7, 11361–11405. [Google Scholar] [CrossRef]
- Xia, G.; Wang, H. Squaraine dyes: The hierarchical synthesis and its application in optical detection. J. Photochem. Photobiol. C Photochem. Rev. 2017, 31, 84–113. [Google Scholar] [CrossRef]
- Ta, D.D.; Dzyuba, S.V. Squaraine-based optical sensors: Designer toolbox for exploring ionic and molecular recognitions. Chemosensors 2021, 9, 302. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Z.; Zhu, S.; Liu, Y.; Shi, P.; Tian, H.; Zhu, W.-H. Rational design of novel near-infrared fluorescent DCM derivatives and their application in bioimaging. J. Mater. Chem. B 2016, 4, 4683–4689. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.T.; Cai, Y.; Zhang, Z.Y.; Wang, C.Y.; Tang, Y.H.; Zhu, M.Q.; Wang, Y.-L. Progress of Dicyanomethylene-4H-Pyran Derivatives in Biological Sensing Based on ICT Effect. Front. Chem. 2022, 10, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.Q.; Liu, J.; Lv, X.; Liu, Y.; Zhao, Y.; Guo, W. Rhodamine-inspired far-red to near-infrared dyes and their application as fluorescence probes. Angew. Chemie. Int. Ed. 2012, 51, 7634–7636. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Du, W.; Hu, Z.; Uvdal, K.; Li, L.; Huang, W. Hybrid Rhodamine Fluorophores in the Visible/NIR Region for Biological Imaging. Angew. Chemie. Int. Ed. 2019, 58, 14026–14043. [Google Scholar] [CrossRef] [PubMed]
- Pina, F.; Melo, M.J.; Laia, C.A.T.; Parola, A.J.; Lima, J.C. Chemistry and applications of flavylium compounds: A handful of colours. Chem. Soc. Rev. 2012, 41, 869–908. [Google Scholar] [CrossRef]
- Basílio, N.; Pina, F. Chemistry and photochemistry of anthocyanins and related compounds: A thermodynamic and kinetic approach. Molecules 2016, 21, 1502. [Google Scholar] [CrossRef] [Green Version]
- Cruz, L.; Basílio, N.; Mateus, N.; De Freitas, V.; Pina, F. Natural and Synthetic Flavylium-Based Dyes: The Chemistry behind the Color. Chem. Rev. 2022, 122, 1416–1481. [Google Scholar] [CrossRef]
- Gomes, R.; Diniz, A.M.; Jesus, A.; Parola, A.J.; Pina, F. The synthesis and reaction network of 2-styryl-1-benzopyrylium salts: An unexploited class of potential colorants. Dye. Pigment. 2009, 81, 69–79. [Google Scholar] [CrossRef]
- Moro, A.; Diniz, A.M.; Petrov, V.; Pina, F. Chemistry of 7,8-dihydroxy-2-(4-dimethylaminostyryl)-1-benzopyrylium. A photochromic system switching from yellow to green. J. Photochem. Photobiol. A Chem. 2013, 263, 17–23. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, Q.; Tan, Y.; Lang, M.J.; Sun, H.; Liu, X. Rational Development of Near-Infrared Fluorophores with Large Stokes Shifts, Bright One-Photon, and Two-Photon Emissions for Bioimaging and Biosensing Applications. Chem. A Eur. J. 2017, 23, 8736–8740. [Google Scholar] [CrossRef]
- Zhang, G.; Quan, W.; Li, Y.; Song, W.; Lin, W. Near-Infrared Mitochondria-Targetable Single-Molecule probe for Dual-Response of viscosity and sulfur dioxide in vivo. Spectrochim. Acta. Part A Mol. Biomol. Spectrosc. 2022, 270, 120796. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.F.; Wu, M.X.; Wei, X.R.; Sun, R.; Xu, Y.J.; Ge, J.F. The fluorescent probe based on methyltetrahydroxanthylium skeleton for the detection of hydrazine. Talanta 2020, 218, 121164. [Google Scholar] [CrossRef] [PubMed]
- Kuba, M.; Kraus, T.; Pohl, R.; Hocek, M. Nucleotide-Bearing Benzylidene-Tetrahydroxanthylium Near-IR Fluorophore for Sensing DNA Replication, Secondary Structures and Interactions. Chem. A Eur. J. 2020, 26, 11950–11954. [Google Scholar] [CrossRef]
- Küster Friedrich, W.T.A. Tabelle per Le Analisi Chimiche e Chimico-Fisiche, 14th ed.; Hoepli: Milano, Italy, 1985. [Google Scholar]
- McNaught, A.D.; Wilkinson, A. International Union of Pure Appl. Chem. Compendium of Chemical Terminology (“The Gold Book”); Blackwell Science: West Sussex, UK, 1997. [Google Scholar]
- Dempster, D.N.; Morrow, T.; Rankin, R.; Thompson, G.F. Photochemical Characteristics of the mode-locking dyes 1,1′-diethyl-4,4′ carbocyanine iodide (cryptocayanine, DCI) and 1,1′-diethyl-2,2′ dicarb. Chem. Phys. Lett. 1973, 18, 488–492. [Google Scholar] [CrossRef]
- Thordarson, P. Determining association constants from titration experiments in supramolecular chemistry. Chem. Soc. Rev. 2011, 40, 1305–1323. [Google Scholar] [CrossRef]
- Giard, D.J.; Aaronson, S.A.; Todaro, G.J.; Arnstein, P.; Kersey, J.H.; Parks, W.P. In vitro cultivation of human tumors: Establishment of cell lines derived from a series of solid tumors. J. Natl. Cancer Inst. 1973, 51, 1417–1423. [Google Scholar] [CrossRef]
- Fey, P.D.; Endres, J.L.; Yajjala, V.K.; Yajjala, K.; Widhelm, T.J.; Boissy, R.J.; Bose, J.L.; Bayles, K.W. A Genetic Resource for Rapid and Comprehensive Phenotype. mBio 2013, 4, e00537-12. [Google Scholar] [CrossRef] [Green Version]
- Monk, I.R.; Shah, I.M.; Xu, M.; Tan, M.W.; Foster, T.J. Transforming the untransformable: Application of direct transformation to manipulate genetically Staphylococcus aureus and Staphylococcus epidermidis. mBio 2012, 3, e00277. [Google Scholar] [CrossRef] [Green Version]
- Gomes, L.J.; Moreira, T.; Rodríguez, L.; Moro, A.J. Chalcone-based fluorescent chemosensors as new tools for detecting Cu2+ ions. Dye. Pigment. 2022, 197, 109845. [Google Scholar] [CrossRef]
- Moncada, M.C.; Parola, J.; Lodeiro, C.; Folgosa, F.; Pina, F. Multistate properties of 7-(N,N-diethylamino)-4 ′-hydroxyflavylium. An example of an unidirectional reaction cycle driven by pH. Org. Biomol. Chem. 2004, 2, 2802. [Google Scholar] [CrossRef]
- Avó, J.; Petrov, V.; Basílio, N.; Jorge Parola, A.; Pina, F. Evidence against the Twisted Intramolecular Charge Transfer (TICT) model in 7-aminoflavylium derivatives. Dye. Pigment. 2016, 135, 86–93. [Google Scholar] [CrossRef]
- Fan, J.; Wu, Y.; Peng, X. A naphthalimide fluorescent sensor for Zn2+ based on photo-induced electron transfer. Chem. Lett. 2004, 33, 1392–1393. [Google Scholar] [CrossRef]
- Louie, M.W.; Liu, H.W.; Lam, M.H.C.; Lau, T.C.; Lo, K.K.W. Novel luminescent tricarbonylrhenium(I) polypyridine tyraminederived dipicolylamine complexes as sensors for zinc(II) and cadmium(II) Ions. Organometallics 2009, 28, 4297–4307. [Google Scholar] [CrossRef]
- Lu, X.; Zhu, W.; Xie, Y.; Li, X.; Gao, Y.; Li, F.; Tian, H. Near-IR core-substituted naphthalenediimide fluorescent chemosensors for zinc ions: Ligand effects on PET and ICT channels. Chem. A Eur. J. 2010, 16, 8355–8364. [Google Scholar] [CrossRef]
- Moro, A.J.; Cywinski, P.J.; Körsten, S.; Mohr, G.J. An ATP fluorescent chemosensor based on a Zn(ii)-complexed dipicolylamine receptor coupled with a naphthalimide chromophore. Chem. Commun. 2010, 46, 1085. [Google Scholar] [CrossRef]
- Wang, H.; Wu, H.; Xue, L.; Shi, Y.; Li, X. A naphthalimide fluorophore with efficient intramolecular PET and ICT Processes: Application in molecular logic. Org. Biomol. Chem. 2011, 9, 5436–5444. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, Y.; Feng, P.; Cao, J. Development of a borondipyrromethene-based Zn 2+ fluorescent probe: Solvent effects on modulation sensing ability. Dalt. Trans. 2012, 41, 831–838. [Google Scholar] [CrossRef]
- Lee, S.K.; Choi, M.G.; Choi, J.; Chang, S.K. Fluorescence signaling of Zn2+ levels in synthetic urine by dipicolylamine-armed hydroxynaphthalimide. Sens. Actuators B Chem. 2015, 207, 303–307. [Google Scholar] [CrossRef]
- Nowack, B. Environmental chemistry of aminopolycarboxylate chelating agents. Environ. Sci. Technol. 2002, 36, 4009–4016. [Google Scholar] [CrossRef]
- Nirody, J.A.; Budin, I.; Rangamani, P. ATP synthase: Evolution, energetics, and membrane interactions. J. Gen. Physiol. 2020, 152, 1–18. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, L.J.; Carrilho, J.P.; Pereira, P.M.; Moro, A.J. A Near InfraRed Emissive Chemosensor for Zn2+ and Phosphate Derivatives Based on a Di-(2-picolyl)amine-styrylflavylium Push-Pull Fluorophore. Sensors 2023, 23, 471. https://doi.org/10.3390/s23010471
Gomes LJ, Carrilho JP, Pereira PM, Moro AJ. A Near InfraRed Emissive Chemosensor for Zn2+ and Phosphate Derivatives Based on a Di-(2-picolyl)amine-styrylflavylium Push-Pull Fluorophore. Sensors. 2023; 23(1):471. https://doi.org/10.3390/s23010471
Chicago/Turabian StyleGomes, Liliana J., João P. Carrilho, Pedro M. Pereira, and Artur J. Moro. 2023. "A Near InfraRed Emissive Chemosensor for Zn2+ and Phosphate Derivatives Based on a Di-(2-picolyl)amine-styrylflavylium Push-Pull Fluorophore" Sensors 23, no. 1: 471. https://doi.org/10.3390/s23010471




