Polymer Doping as a Novel Approach to Improve the Performance of Plasmonic Plastic Optical Fibers Sensors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. SPR Experimental Setup
2.3. MIP Synthesis and Platform Functionalization
2.4. SPR-Based Experimental Procedures
2.4.1. Optical Characterization
2.4.2. BSA Dose-Response Curve
2.5. Surface Characterization
3. A Novel SPR-POF Platform Based on a PMMA Doping Procedure
4. Experimental Results
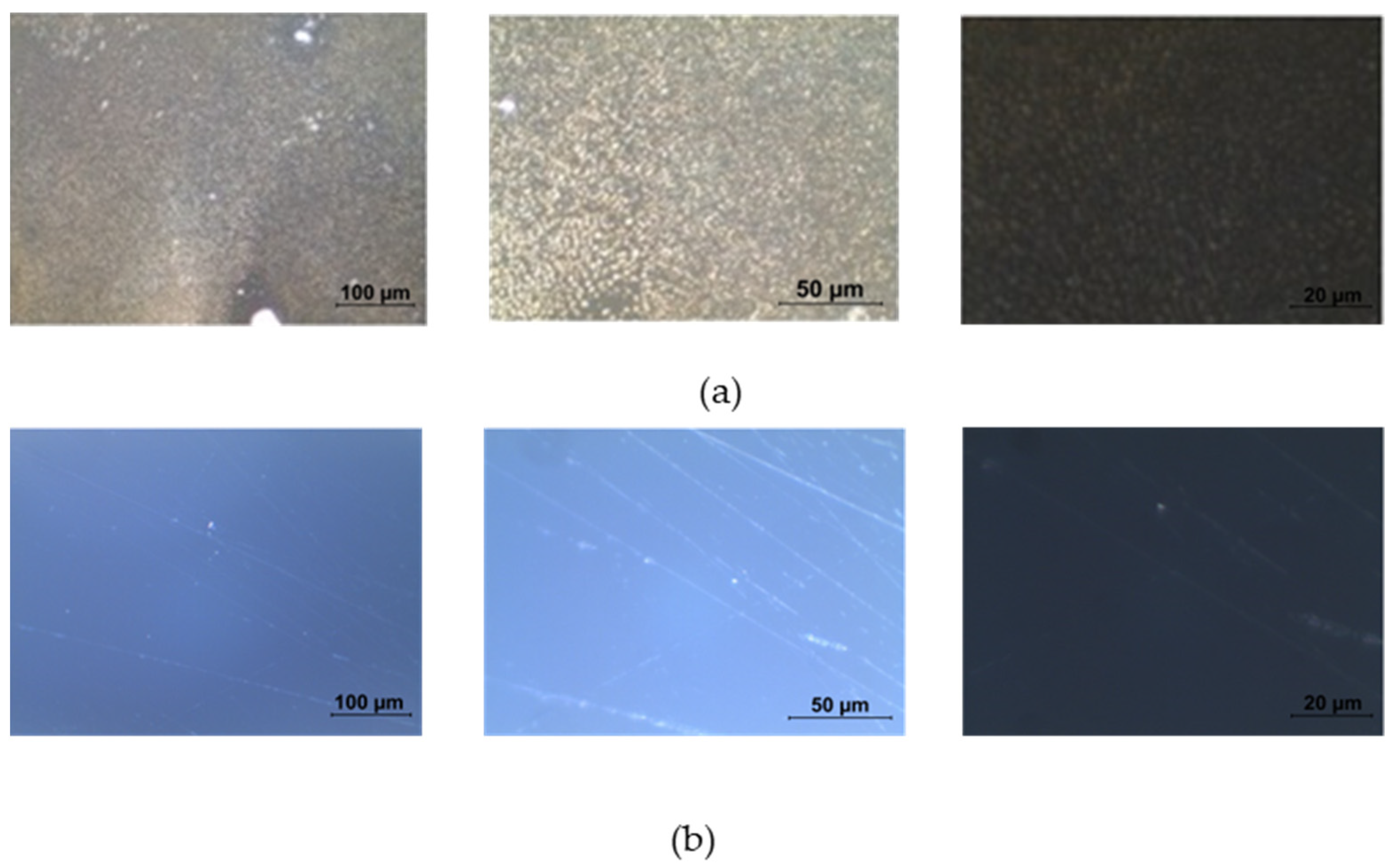
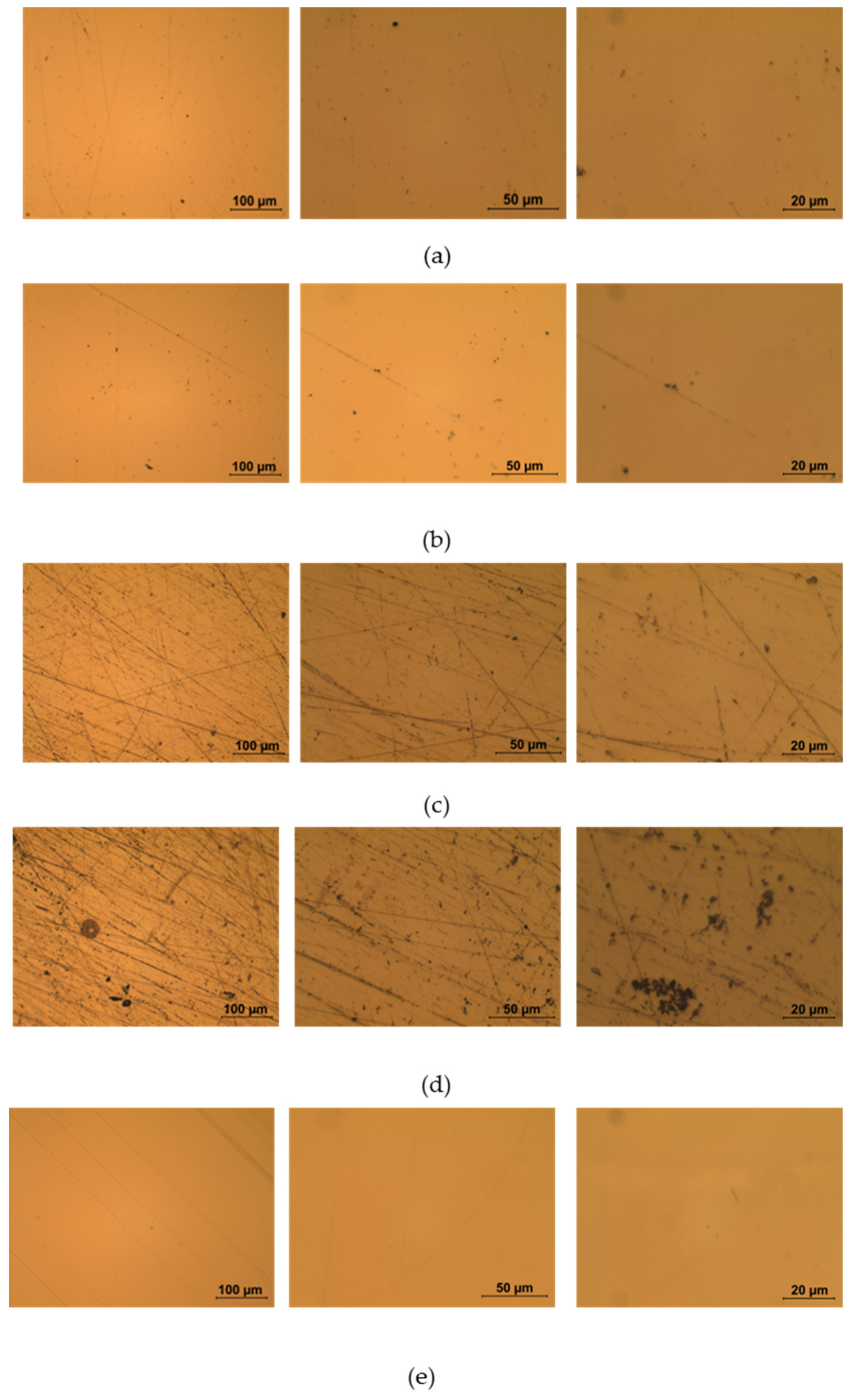
4.1. Surface Characterization of the Doped PMMA
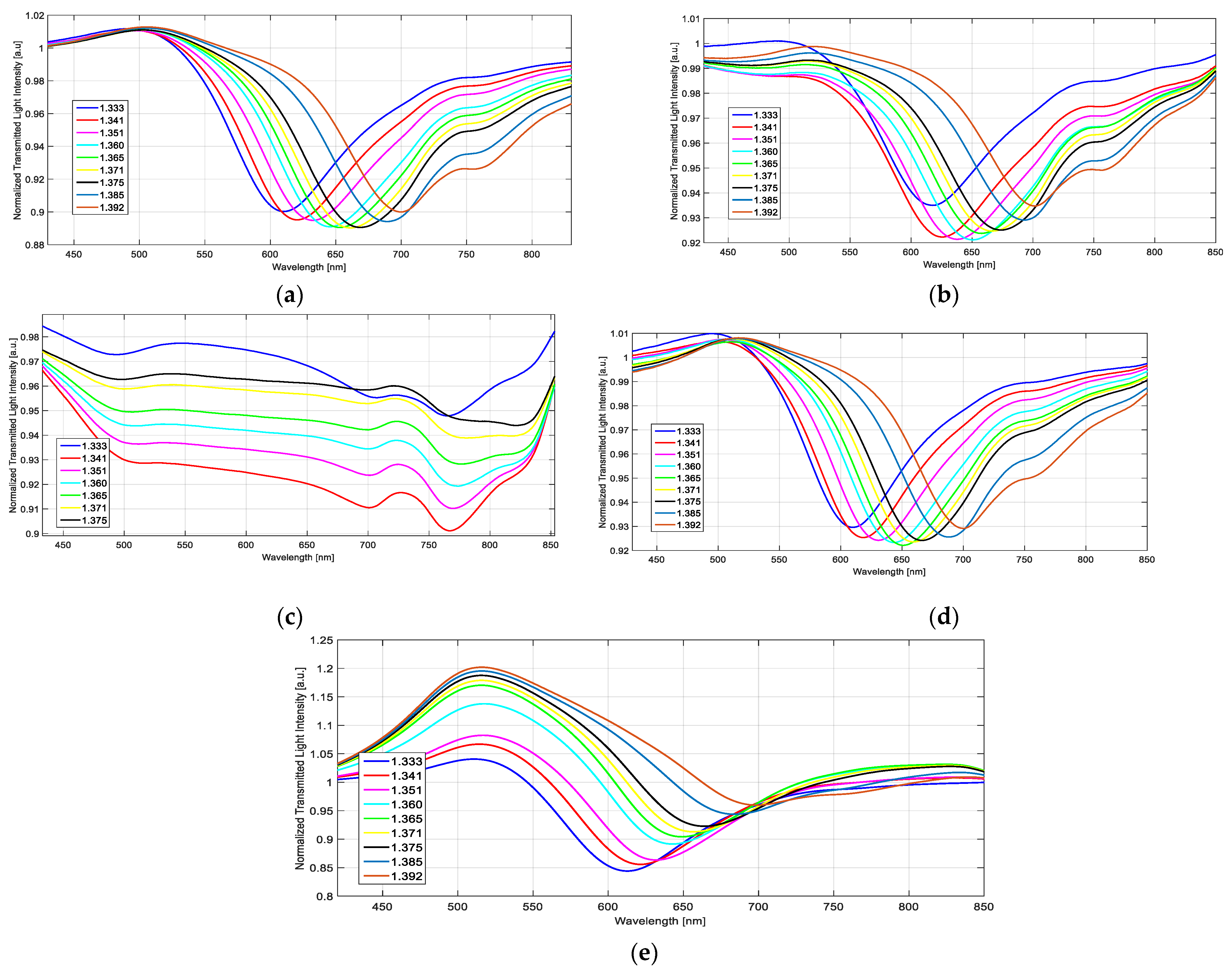
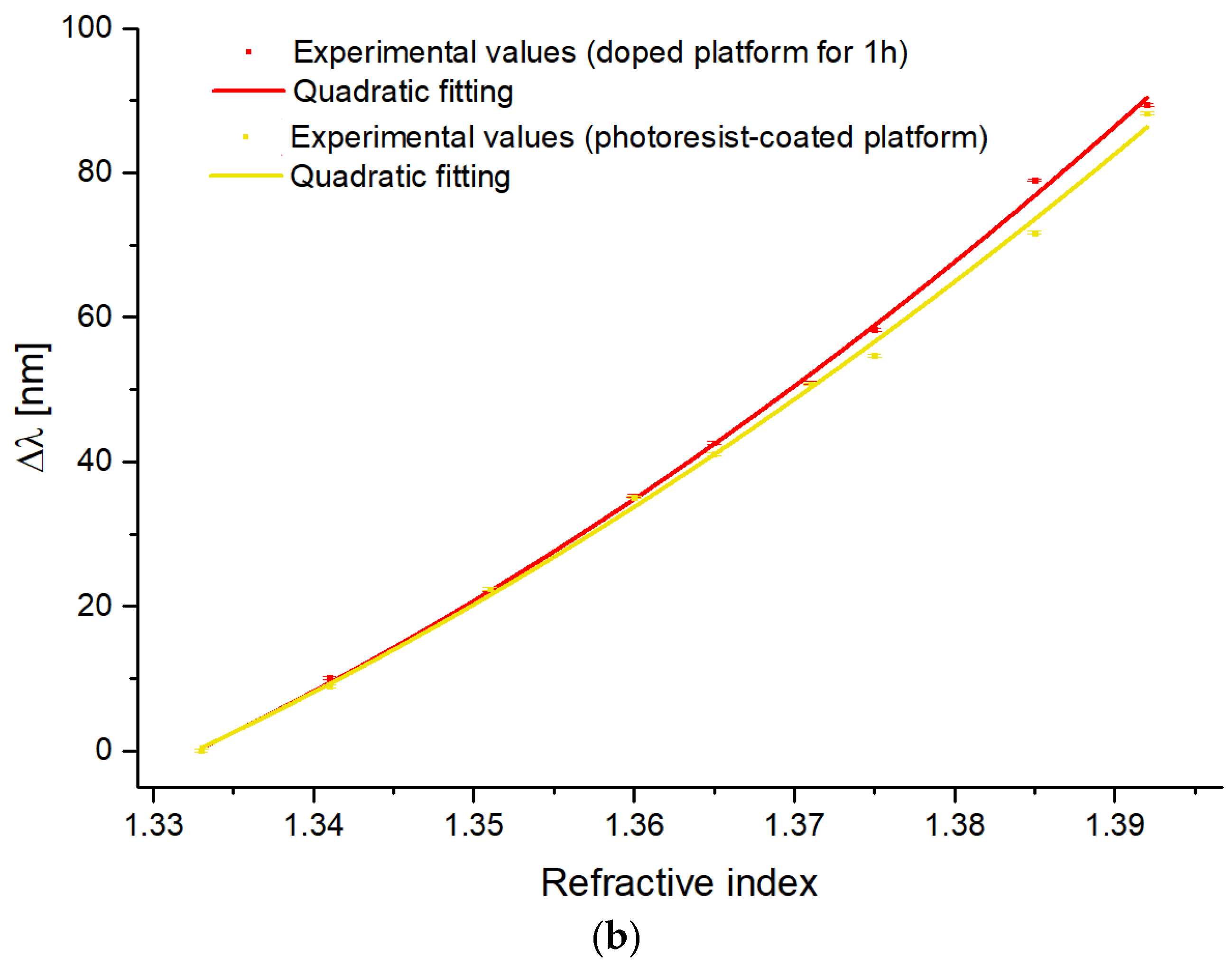
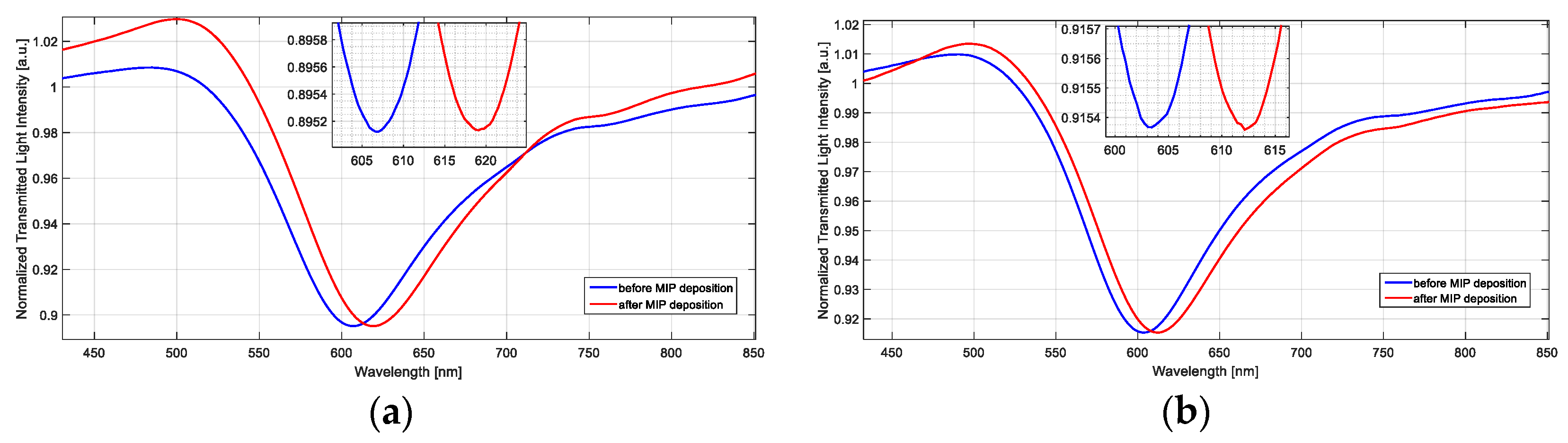
4.2. Optical Characterization of the Doped SPR-POF Probes
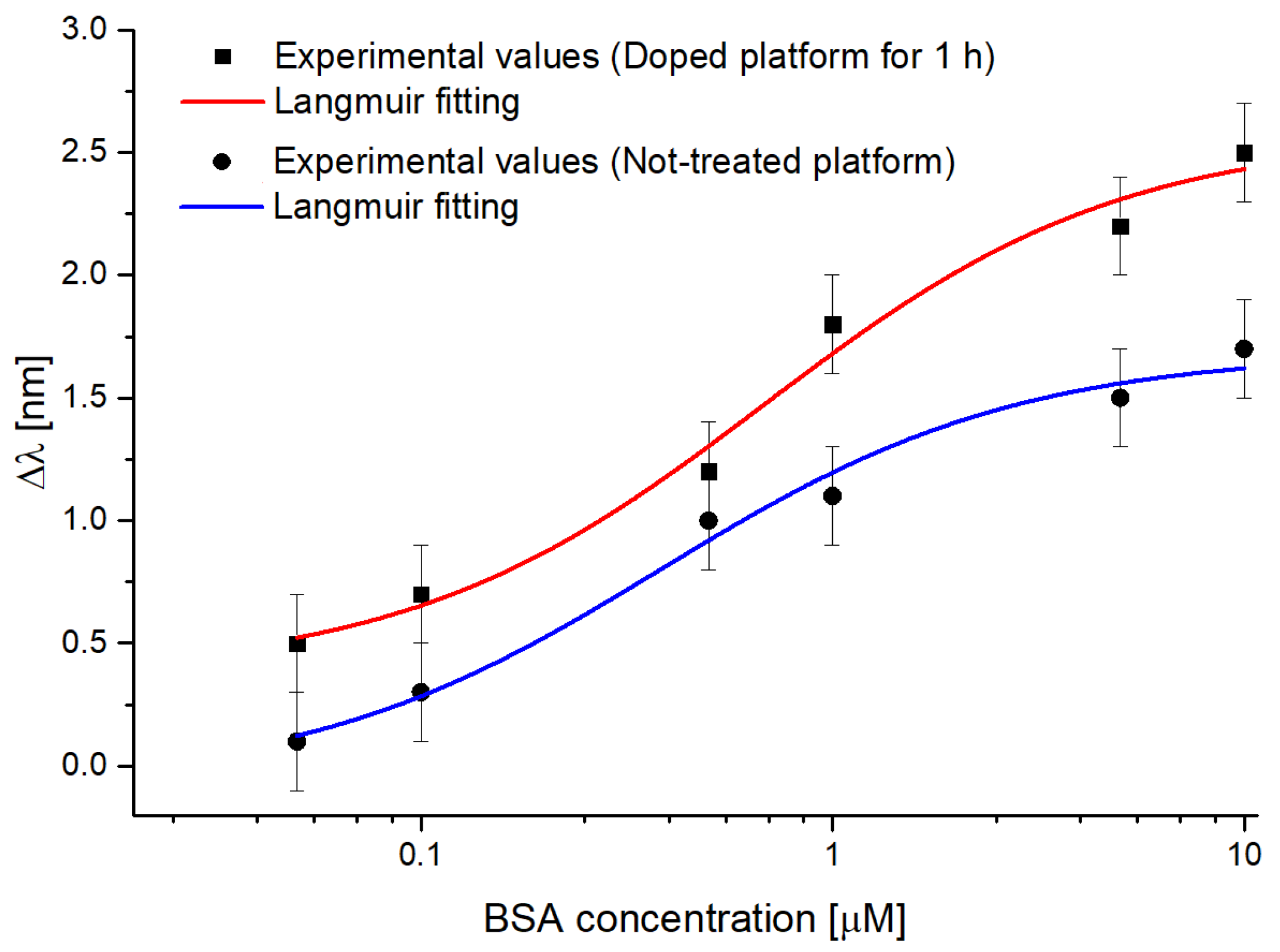
4.3. Binding Tests Exploiting Different SPR-POF Probes Combined with MIP
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schasfoort, R.B.M. Introduction to Surface Plasmon Resonance. In Handbook of Surface Plasmon Resonance; The Royal Society of Chemistry: London, UK, 2017; pp. 1–26. [Google Scholar]
- De Mol, N.J.; Fischer, M.J.E. Surface Plasmon Resonance: A General Introduction. In Surface Plasmon Resonance. Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2010; Volume 627, pp. 1–14. [Google Scholar]
- Pandey, P.S.; Raghuwanshi, S.K.; Kumar, S. Recent advances in two-dimensional materials-based Kretschmann configuration for SPR sensors: A review. IEEE Sens. J. 2021, 22, 1069–1080. [Google Scholar] [CrossRef]
- Uniyal, S.; Choudhary, K.; Sachdev, S.; Kumar, S. Recent advances in K-SPR sensors for the detection of biomolecules and microorganisms: A review. IEEE Sens. J. 2022, 22, 11415–11426. [Google Scholar] [CrossRef]
- Vahed, H.; Nadri, C. Sensitivity enhancement of SPR optical biosensor based on Graphene–MoS2 structure with nanocomposite layer. Opt. Mater. 2019, 88, 161–166. [Google Scholar] [CrossRef]
- Klantsataya, E.; Jia, P.; Ebendorff-Heidepriem, H.; Monro, T.M.; François, A. Plasmonic fiber optic refractometric sensors: From conventional architectures to recent design trends. Sensors 2016, 17, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, B.D.; Verma, R.K. Surface Plasmon Resonance-Based Fiber Optic Sensors: Principle, Probe Designs, and Some Applications. J. Sens. 2009, 2009, 979761. [Google Scholar] [CrossRef] [Green Version]
- Cennamo, N.; Massarotti, D.; Conte, L.; Zeni, L. Low cost sensors based on SPR in a plastic optical fiber for biosensor implementation. Sensors 2011, 11, 11752–11760. [Google Scholar] [CrossRef] [PubMed]
- Cennamo, N.; Pesavento, M.; De Maria, L.; Galatus, R.; Mattiello, F.; Zeni, L. Comparison of different photoresist buffer layers in SPR sensors based on D-shaped POF and gold film. In Proceedings of the 2017 IEEE 25th Optical Fiber Sensors Conference (OFS), Jeju, Republic of Korea, 24–28 April 2017. [Google Scholar]
- Cennamo, N.; D’Agostino, G.; Arcadio, F.; Perri, C.; Porto, G.; Biasiolo, A.; Zeni, L. Measurement of MIPs responses deposited on Two SPR-POF sensors realized by different photoresist buffer layers. IEEE Trans. Instrum. Meas. 2020, 69, 1464–1473. [Google Scholar] [CrossRef]
- Teng, C.; Wang, Y.; Yuan, L. Polymer optical fibers-based surface plasmon resonance sensors and their applications: A review. Opt. Fiber Technol. 2023, 77, 103256. [Google Scholar] [CrossRef]
- Zhao, Y.; Tong, R.; Xia, F.; Peng, Y. Current Status of Optical Fiber Biosensor Based on Surface Plasmon Resonance. Biosens. Bioelectron. 2019, 142, 111505. [Google Scholar] [CrossRef]
- Allsop, T.; Neal, R. A review: Evolution and diversity of optical fibre plasmonic sensors. Sensors 2019, 19, 4874. [Google Scholar] [CrossRef] [Green Version]
- Luo, W.; Li, X.; Meng, J.; Wang, Y.; Hong, X. Surface Plasmon Resonance Sensor Based on Side-Polished D-Shaped Photonic Crystal Fiber with Split Cladding Air Holes. IEEE Trans. Instrum. Meas. 2021, 70, 7002411. [Google Scholar] [CrossRef]
- De-Jun, F.; Mao-Sen, Z.; Liu, G.; Xi-Lu, L.; Dong-Fang, J. D-Shaped Plastic Optical Fiber Sensor for Testing Refractive Index. IEEE Sens. J. 2014, 14, 1673–1676. [Google Scholar] [CrossRef]
- Kumar, S.; Kaushik, B.K.; Singh, R.; Chen, N.-K.; Yang, Q.S.; Zhang, X.; Wang, W.; Zhang, B. LSPR-based cholesterol biosensor using a tapered optical fiber structure. Biomed. Opt. Express 2019, 10, 2150–2160. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; He, Y.; Yang, Y.; Zhang, H.; Xu, Y. Highly sensitive surface plasmon resonance sensor based on graphene-coated U-shaped fiber. Plasmonics 2020, 1, 205–213. [Google Scholar] [CrossRef]
- Takagi, K.; Sasaki, H.; Seki, A.; Watanabe, K. Surface Plasmon Resonances of a Curved Hetero-Core Optical Fiber Sensor. Sens. Actuator A Phys. 2010, 161, 1–5. [Google Scholar] [CrossRef]
- Kim, J.A.; Hwang, T.; Dugasani, S.R.; Amin, R.; Kulkarni, A.; Park, S.H.; Kim, T. Graphene Based Fiber Optic Surface Plasmon Resonance for Bio-Chemical Sensor Applications. Sens. Actuators B Chem. 2013, 187, 426–433. [Google Scholar] [CrossRef] [Green Version]
- Tabassum, R.; Gupta, B.D. Influence of Oxide Overlayer on the Performance of a Fiber Optic SPR Sensor with Al/Cu Layers. IEEE J. Sel. Top Quantum. Electron. 2017, 23, 81–88. [Google Scholar] [CrossRef]
- Saad, Y.; Selmi, M.; Gazzah, M.H.; Bajahzar, A.; Belmabrouk, H. Performance Enhancement of a Copper-Based Optical Fiber SPR Sensor by the Addition of an Oxide Layer. Optik 2019, 190, 1–9. [Google Scholar] [CrossRef]
- Usha, S.; Mishra, S.; Gupta, B. Fabrication and Characterization of a SPR Based Fiber Optic Sensor for the Detection of Chlorine Gas Using Silver and Zinc Oxide. Materials 2015, 8, 2204–2216. [Google Scholar] [CrossRef] [Green Version]
- Singh, Y.; Raghuwanshi, S.K. Titanium Dioxide (TiO2) Coated Optical Fiber-Based SPR Sensor in near-Infrared Region with Bimetallic Structure for Enhanced Sensitivity. Optik 2021, 226, 165842. [Google Scholar] [CrossRef]
- Cennamo, N.; Arcadio, F.; Noel, L.; Zeni, L.; Soppera, O. Flexible and ultrathin metal-oxide films for multiresonance-based sensors in plastic optical fibers. ACS Appl. Nano Mater. 2021, 4, 10902–10910. [Google Scholar] [CrossRef]
- Rai, A.; Bhaskar, S.; Ganesh, K.M.; Ramarthury, S.S. Hottest Hotspots from the Coldest Cold: Welcome to Nano 4.0. ACS Appl. Nano Mater. 2022, 5, 12245–12264. [Google Scholar] [CrossRef]
- Xiong, Y.; Shepherd, S.; Tibbs, J.; Bacon, A.; Liu, W.; Akin, L.D.; Ayupova, T.; Bhaskar, S.; Cunningham, B.T. Photonic Crystal Enhanced Fluorescence: A Review on Design Strategies and Applications. Micromachines 2023, 14, 668. [Google Scholar] [CrossRef] [PubMed]
- Cunha, N.H.O.; Da Silva, J.P. High Sensitivity Surface Plasmon Resonance Sensor Based on a Ge-Doped Defect and D-Shaped Microstructured Optical Fiber. Sensors 2022, 22, 3220. [Google Scholar] [CrossRef] [PubMed]
- Elsheikh, A. Bistable Morphing Composites for Energy-Harvesting Applications. Polymers 2022, 14, 1893. [Google Scholar] [CrossRef]
- Moustafa, E.B.; Elsheikh, A. Predicting Characteristics of Dissimilar Laser Welded Polymeric Joints Using a Multi-Layer Perceptrons Model Coupled with Archimedes Optimizer. Polymers 2023, 15, 233. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.; Showaib, E.; Elsheik, A. Effect of single period overload parameters on fatigue crack retardation for high-density polyethylene. Theor. Appl. Fract. Mech. 2022, 118, 103249. [Google Scholar] [CrossRef]
- Zidan, H.M.; El-ÄêKhodary, A.; El-ÄêSayed, I.A.; El-ÄêBohy, H.I. Optical parameters and absorption studies of UV-irradiated azo dye-doped PMMA films. J. Appl. Polym. Sci. 2010, 117, 1416–1423. [Google Scholar]
- Deshmukh, S.H.; Burghate, D.K.; Shilaskar, S.N.; Chaudhari, G.N.; Deshmukh, P.T. Optical properties of polyaniline doped PVC-PMMA thin films. Indian J. Pure Appl. Phys. 2008, 46, 344–348. [Google Scholar]
- Gavade, C.; Singh, N.L.; Singh, D.; Shah, S.; Tripathi, A.; Avasthi, D.K. Study of Dielectrical Properties of Swift Heavy Ion Induced Modifications in MetalOxide/PMMA Nanocomposites. Integr. Ferroelectr. 2010, 117, 76–84. [Google Scholar] [CrossRef]
- Horinouchi, S.; Wada, M.; Ishihara, K.; Eguchi, M.; Zhang, G.; Ishikawa, T.; Watanabe, A.; Wang, L.; Ogata, N. Fabrication and characterization of rare-earth metal chelates-doped plastic film and fiber materials: Eu(3+)-chelates doped PMMA. In Proceedings of the 2002 SPIE 4905 Materials and Devices for Optical and Wireless Communications, Shanghai, China, 29 August 2002. [Google Scholar]
- Zhang, H.J.; Fan, R.Q.; Wang, X.M.; Wang, P.; Wang, Y.L.; Yang, Y.L. Preparation, characterization, and properties of PMMA-doped polymer film materials: A study on the effect of terbium ions on luminescence and lifetime enhancement. Dalton. Trans. 2015, 44, 2871–2879. [Google Scholar] [CrossRef]
- Soman, V.V.; Kelkar, D.S. FTIR studies of doped PMMA-PVC blend system. Macromol. Symp. 2009, 277, 152–161. [Google Scholar] [CrossRef]
- Velasco, D.S.; De Moura, A.P.; Medina, A.N.; Baesso, M.L.; Rubira, A.F.; Cremona, M.; Bento, A.C. Preparation, characterization, and spectroscopic properties of PC/PMMA doped blends: Study of the effect of rare-earth doping on luminescence, quenching rate, and lifetime enhancement. J. Phys. Chem. B. 2010, 114, 5657–5660. [Google Scholar] [CrossRef] [PubMed]
- Zidan, H.M.; Abu-Elnader, M. Structural and optical properties of pure PMMA and metal chloride-doped PMMA films. Physica B Condens. Matter 2005, 355, 308–317. [Google Scholar] [CrossRef]
- Płocharski, J.; Wyciślik, H. Mixed conductivity in poly (p-phenylene) doped with iron chloride. Solid State Ion. 2000, 127, 337–344. [Google Scholar] [CrossRef]
- Tawansi, A.; El-Khodary, A.; Zidan, H.M.; Badr, S.I. The effect of MnCl2 filler on the optical window and the physical properties of PMMA films. Polym. Test. 2002, 21, 381–387. [Google Scholar] [CrossRef]
- Cennamo, N.; D’Agostino, G.; Perri, C.; Arcadio, F.; Chiaretti, G.; Parisio, E.M.; Camarlinghi, G.; Vettori, C.; Di Marzo, F.; Cennamo, R.; et al. Proof of Concept for a Quick and Highly Sensitive On-Site Detection of SARS-CoV-2 by Plasmonic Optical Fibers and Molecularly Imprinted Polymers. Sensors 2021, 21, 1681. [Google Scholar] [CrossRef]
- Speranza, G.; Canteri, R. RxpsG a new open project for Photoelectron and Electron Spectroscopy data processing. SoftwareX 2019, 10, 100282. [Google Scholar] [CrossRef]
- Piccarolo, S.; Titomanlio, G. Synergism in the Swelling and Solubility of Poly(methy1 methacrylate) in Presence of Ethanol/Water Mixtures. Makrom. Chem. Rapid Comm. 1982, 3, 383–387. [Google Scholar] [CrossRef]
- Papanu, J.S.; Hess, D.W.; Soane, D.S.; Bells, A.T. Swelling of Poly(methyl methacrylate) thin films in low molecular weights alcohols. J. Appl. Polym. Sci. 1990, 39, 803–823. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, C.C.; Huang, W.M.; Purnawali, H. Ethanol Induced Shape Recovery and Swelling in Poly(methyl methacrylate) and Applications in Fabrication of Microlens Array. Adv. Sci. Technol. 2013, 77, 354–358. [Google Scholar]
- Beamson, G.; Bunn, A.; Briggs, D. High-resolution monochromated XPS of poly(methyl methacrylate) thin films on a conducting substrate. Surf. Interface Anal. 1991, 17, 105–115. [Google Scholar] [CrossRef]
- Girardeaux, C.; Pireaux, J.J. Analysis of Poly (methyl methacrylate) (PMMA) by XPS. Surf. Sci. Spectra 1996, 4, 134–137. [Google Scholar] [CrossRef]
- Aronniem, M.; Sainio, J.; Lahtinen, J. XPS study on the correlation between chemical state and oxygen-sensing properties of an iron oxide thin film. Appl. Surf. Sci. 2007, 253, 9476–9482. [Google Scholar] [CrossRef]
- Pyeon, M.; Ruoko, T.P.; Leduc, J.; Gönüllü, Y.; Deo, M.; Tkachenko, N.V.; Mathur, S. Critical role and modification of surface states in hematite films for enhancing oxygen evolution activity. J. Mater. Res. 2018, 33, 455–466. [Google Scholar] [CrossRef]
- Cooper, W.J.; Krasicky, P.D.; Rodriguez, F. Dissolution Rates of Poly(Methy1 Methacrylate) Films in Mixed Solvents. J. Appl. Polym. Sci. 1986, 31, 65–73. [Google Scholar] [CrossRef]
- González-Benito, J.; Koenig, J.K. Nature of PMMA dissolution process by mixtures of acetonitrile/alcohol (poor solvent/nonsolvent) monitored by FTIR-imaging. Polymer 2006, 47, 3065–3072. [Google Scholar] [CrossRef]
- Tran, H.H.; Wu, W.; Lee, N.Y. Ethanol and UV-assisted instantaneous bonding of PMMA assemblies and tuning in bonding reversibility. Sens. Actuators B 2013, 181, 955–962. [Google Scholar] [CrossRef]
- Ocola, L.E.; Costales, M.; Gosztola, D.J. Development characteristics of polymethyl methacrylate in alcohol/water mixtures: A lithography and Raman spectroscopy study. Nanotechnology 2016, 27, 035032. [Google Scholar] [CrossRef]
- Kanso, M.; Cuenot, S.; Louarn, G. Sensitivity of optical fiber sensors based on surface plasmon resonance: Modelling and experiments. Plasmonics 2008, 3, 49–57. [Google Scholar] [CrossRef]
- Dwivedi, Y.S.; Sharma, A.K.; Gupta, B.D. Influence of design parameters on the performance of a surface plasmon sensor-based fiber optic sensor. Plasmonics 2008, 3, 79–86. [Google Scholar] [CrossRef]
- Thompson, M.; Ellison, S.L.; Wood, R. Harmonized guidelines for single-laboratory validation of methods of analysis (IUPAC Technical Report). Pure Appl. Chem. 2002, 74, 835–855. [Google Scholar] [CrossRef]
- Indyk, H.E.; Gill, B.D.; Woollard, D.C. An optical biosensor-based immunoassay for the determination of bovine serum albumin in milk and milk products. Int. Dairy J. 2015, 47, 72–78. [Google Scholar] [CrossRef]
- Arcadio, F.; Zeni, L.; Perri, C.; D’Agostino, G.; Chiaretti, G.; Porto, G.; Cennamo, N. Bovine serum albumin protein detection by a removable SPR chip combined with a specific MIP receptor. Chemosensors 2021, 9, 218. [Google Scholar] [CrossRef]
- Cui, M.; Xin, Y.; Song, R.; Sun, Q.; Wang, X.; Lu, D. Fluorescence sensor for bovine serum albumin detection based on the aggregation and release of CdS QDs within CMC. Cellulose 2020, 27, 1621–1633. [Google Scholar] [CrossRef]
- Xu, X.J.; Huang, J.; Li, J.J.; Yan, J.W.; Qin, J.G.; Li, Z. A graphene oxide-based AIE biosensor with high selectivity toward bovine serum albumin. Chem. Commun. 2011, 47, 12385–12387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaushik, S.; Tiwari, U.K.; Deep, A.; Sinha, R.K. Two-dimensional transition metal dichalcogenides assisted biofunctionalized optical fiber SPR biosensor for efficient and rapid detection of bovine serum albumin. Sci. Rep. 2019, 9, 6987. [Google Scholar] [CrossRef] [Green Version]
- Jia, K.; Khaywah, M.Y.; Li, Y.; Bijeon, J.L.; Adam, P.M.; Déturche, R.; Guelorget, B.; François, M.; Louarn, G.; Ionescu, R.E. Strong Improvements of Localized Surface Plasmon Resonance Sensitivity by Using Au/Ag Bimetallic Nanostructures Modified with Polydopamine Films. ACS Appl. Mater. Interfaces 2014, 6, 219–227. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, T. Surface plasmon resonance sensor chips for the recognition of bovine serum albumin via electropolymerized molecularly imprinted polymers. Chin. Chem. Lett. 2013, 24, 813–816. [Google Scholar] [CrossRef]


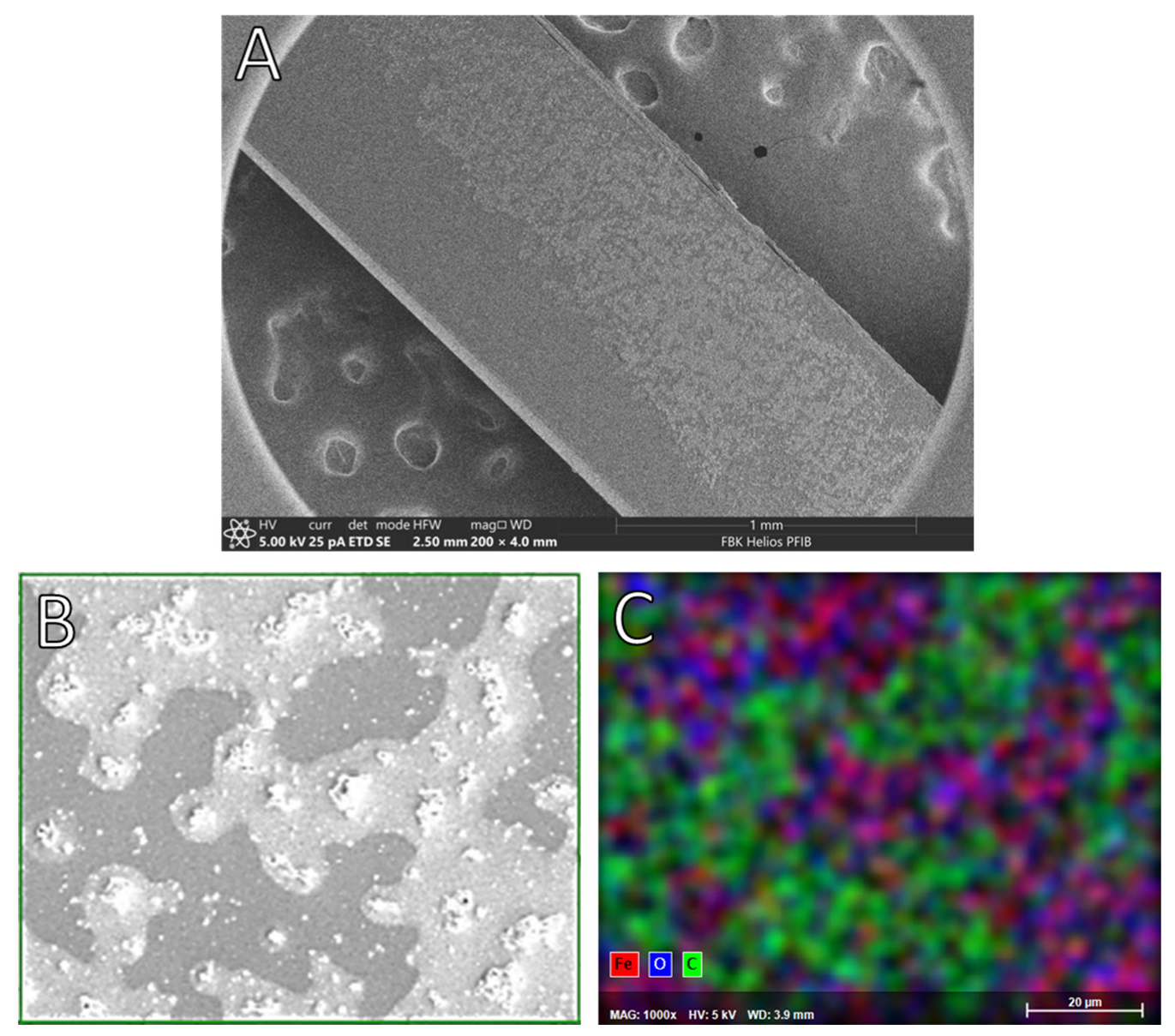



| Sample | O (%) | O Components (B.E. (eV)—%) | C (%) | Fe (%) |
|---|---|---|---|---|
| A_0° | 22.3 | O1: - O2: 532.1–11.9 O3: 533.5–10.4 | 77.7 | - |
| A_45° | 20.7 | O1: - O2: 532.1–10.5 O3: 533.5–10.2 | 79.3 | - |
| A_60° | 20.6 | O1: - O2: 532.2–11.0 O3: 533.6–9.6 | 79.4 | - |
| B_0° | 23.6 | O1: 530.5–0.2 O2: 532.1–13.2 O3: 533.5–10.2 | 75.1 | 1.3 |
| B_45° | 20.2 | O1: 530.4–0.3 O2: 532.1 -11.2 O3: 533.5–8.7 | 78.6 | 1.2 |
| B_60° | 17.9 | O1: 530.5–0.7 O2: 532.2–11.4 O3: 533.6–5.8 | 80.5 | 1.6 |
| C_0° | 28.8 | O1: 530.2–3.2 O2: 531.7–13.8 O3: 533.4–11.8 | 64.6 | 6.6 |
| C_45° | 27.0 | O1: 530.1–5.9 O2: 531.8–15.5 O3: 533.5–5.6 | 65.0 | 8.0 |
| C_60° | 37.4 | O1: 530.0–7.9 O2: 531.7–21.9 O3: 533.4–7.6 | 49.9 | 12.7 |
| Configuration | A | B | C | Adj. R-Square |
|---|---|---|---|---|
| Not-treated Platform | 8697.8 | −22,303.1 | 14,275.1 | 0.999 |
| Doped Platform for 1 h | 7783.2 | −19,684.2 | 12,409.5 | 0.998 |
| Doped Platform for 3 h | 6766.7 | −16,981.6 | 10,612.2 | 0.998 |
| Photoresist-coated Platform | 6904.3 | −17,358.8 | 10,871.4 | 0.997 |
| Sensor | Δλ [nm] |
|---|---|
| Doped sensor | 12 |
| Not-treated sensor | 9 |
| Configuration | Δλ0 [nm] | Δλmax [nm] | K [µM] | Statistics | ||||
|---|---|---|---|---|---|---|---|---|
| Value | St. Error | Value | St. Error | Value | St. Error | Χ2 | R2 | |
| Doped sensor | 0.3719 | 0.0852 | 2.5924 | 0.1241 | 0.4416 | 0.1997 | 0.3663 | 0.9779 |
| Not-treated sensor | −0.0816 | 0.1267 | 1.6847 | 0.0841 | 0.3828 | 0.1192 | 0.2206 | 0.9783 |
| Configuration | Sensitivity at Low Concentrations (Δλmax/K) [nm/μM] | LOD (3 × Standard Deviation of Blank (St. Error of Δλ0)/Sensitivity at Low Concentration) [μM] | Kaff (1/K) [μM−1] |
|---|---|---|---|
| Doped sensor | 5.87 | 0.04 | 2.26 |
| Not-treated sensor | 4.40 | 0.09 | 2.61 |
| Configuration | LOD [μM] | BSA Detection Range [μM] | Reference |
|---|---|---|---|
| Doped SPR D-shaped POF | 0.04 | 0.05–10 | This work |
| SPR-immunoassay | 0.3 | 0.00015–0.15 | [57] |
| SPR in a PMMA slab waveguide | 0.0085 | 0.0085–1 | [58] |
| SPR-D-shaped POF | 0.37 | 0.37–6.5 | [41] |
| Fluorescence sensor | 0.01 | 0.01–2 | [59] |
| Aggregation-induced emission biosensor coupled with graphene-oxide | 0.4 | 0.4–1.5 | [60] |
| SPR-MoS2 optical fiber | 0.00436 | 0.00436–0.75 | [61] |
| LSPR based on bimetallic nanoparticles | 0.15 × 10−6 | 0.15–15 × 10−6 | [62] |
| SPR-Kretschmann configuration | 0.3 | 0.3–120 | [63] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pitruzzella, R.; Rovida, R.; Perri, C.; Chiodi, A.; Arcadio, F.; Cennamo, N.; Pasquardini, L.; Vanzetti, L.; Fedrizzi, M.; Zeni, L.; et al. Polymer Doping as a Novel Approach to Improve the Performance of Plasmonic Plastic Optical Fibers Sensors. Sensors 2023, 23, 5548. https://doi.org/10.3390/s23125548
Pitruzzella R, Rovida R, Perri C, Chiodi A, Arcadio F, Cennamo N, Pasquardini L, Vanzetti L, Fedrizzi M, Zeni L, et al. Polymer Doping as a Novel Approach to Improve the Performance of Plasmonic Plastic Optical Fibers Sensors. Sensors. 2023; 23(12):5548. https://doi.org/10.3390/s23125548
Chicago/Turabian StylePitruzzella, Rosalba, Riccardo Rovida, Chiara Perri, Alessandro Chiodi, Francesco Arcadio, Nunzio Cennamo, Laura Pasquardini, Lia Vanzetti, Michele Fedrizzi, Luigi Zeni, and et al. 2023. "Polymer Doping as a Novel Approach to Improve the Performance of Plasmonic Plastic Optical Fibers Sensors" Sensors 23, no. 12: 5548. https://doi.org/10.3390/s23125548







