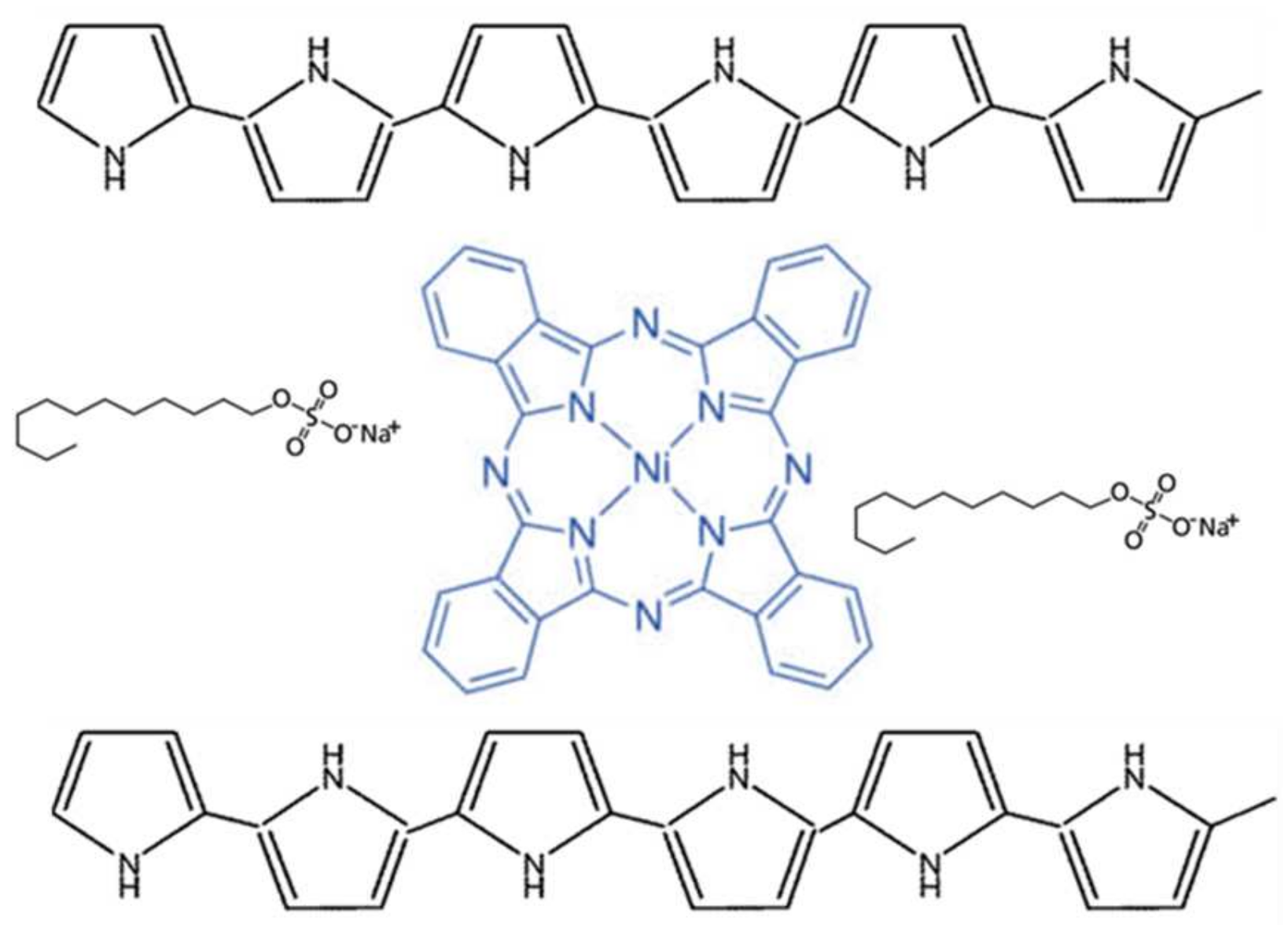
Microwave Gas Sensors Based on Electrodeposited Polypyrrole–Nickel Phthalocyanine Hybrid Films
Abstract
:1. Introduction
2. Experimental Section
- Materials
- Electrochemistry
- Mechanical profilometry
- Scanning electron microscopy (SEM)
- Energy-dispersive X-ray spectroscopy (EDS)
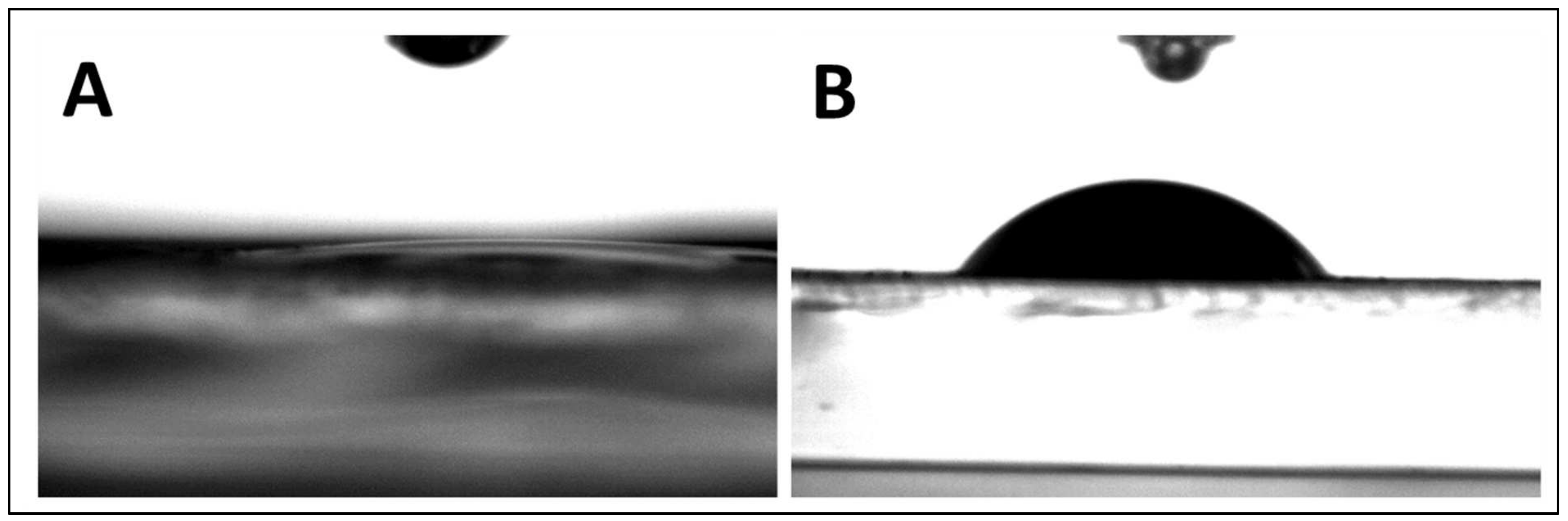
- Water contact angle measurements
- Infrared spectroscopy
3. Results and Discussion
3.1. Electrodeposition and Characterization of PPy-SDS and PPy/NiPc-SDS Films
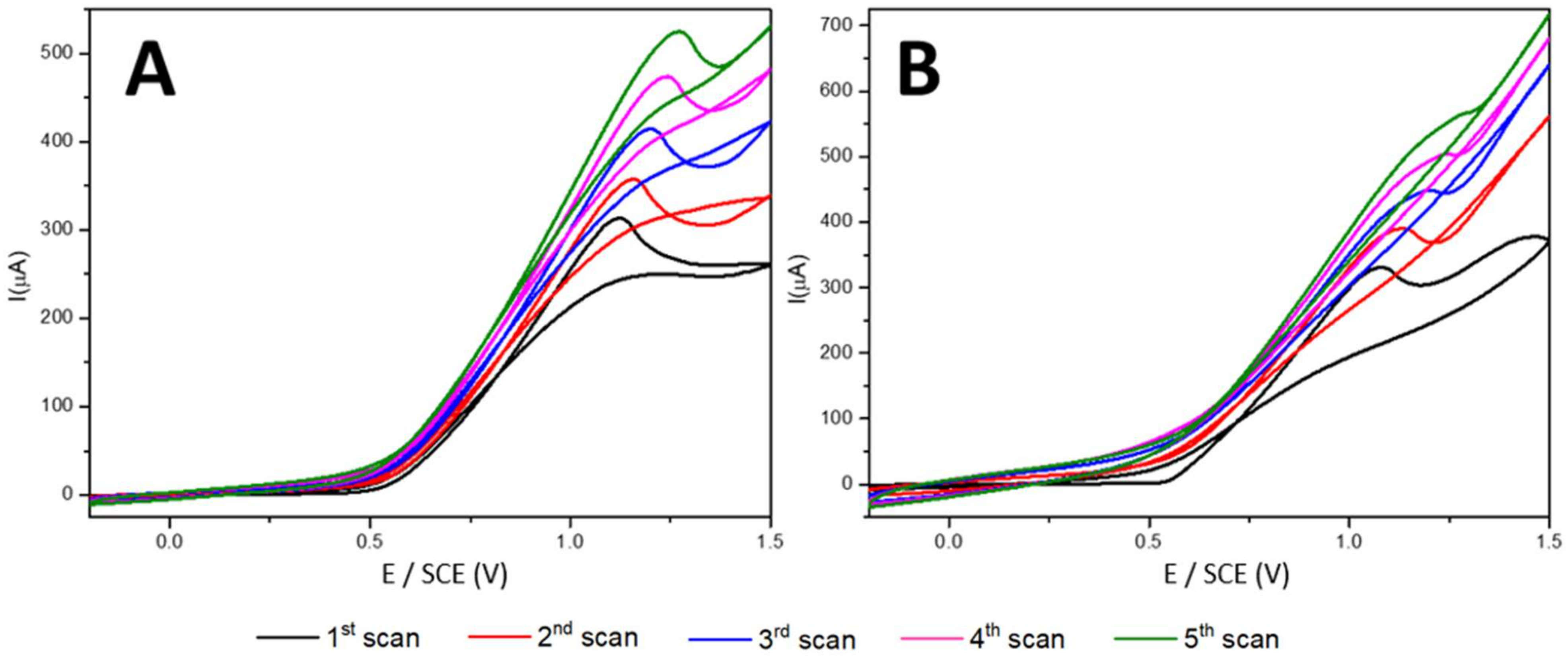
- Electrochemical deposition of PPy-SDS and PPy/NiPc-SDS films
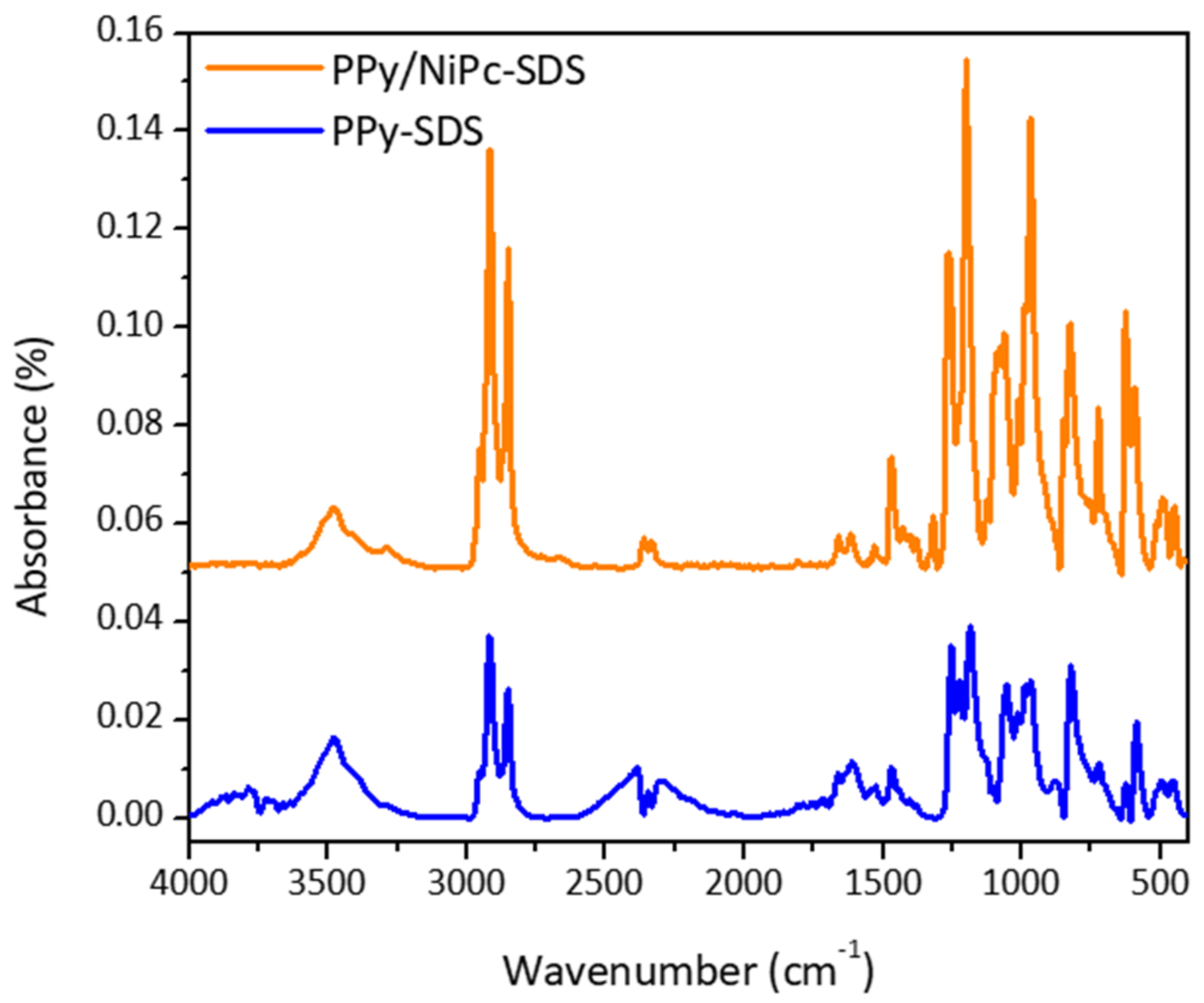
- Characterization of PPy-SDS and Ppy/NiPc-SDS films
3.2. Development of Polymer-Based Microwave Sensors
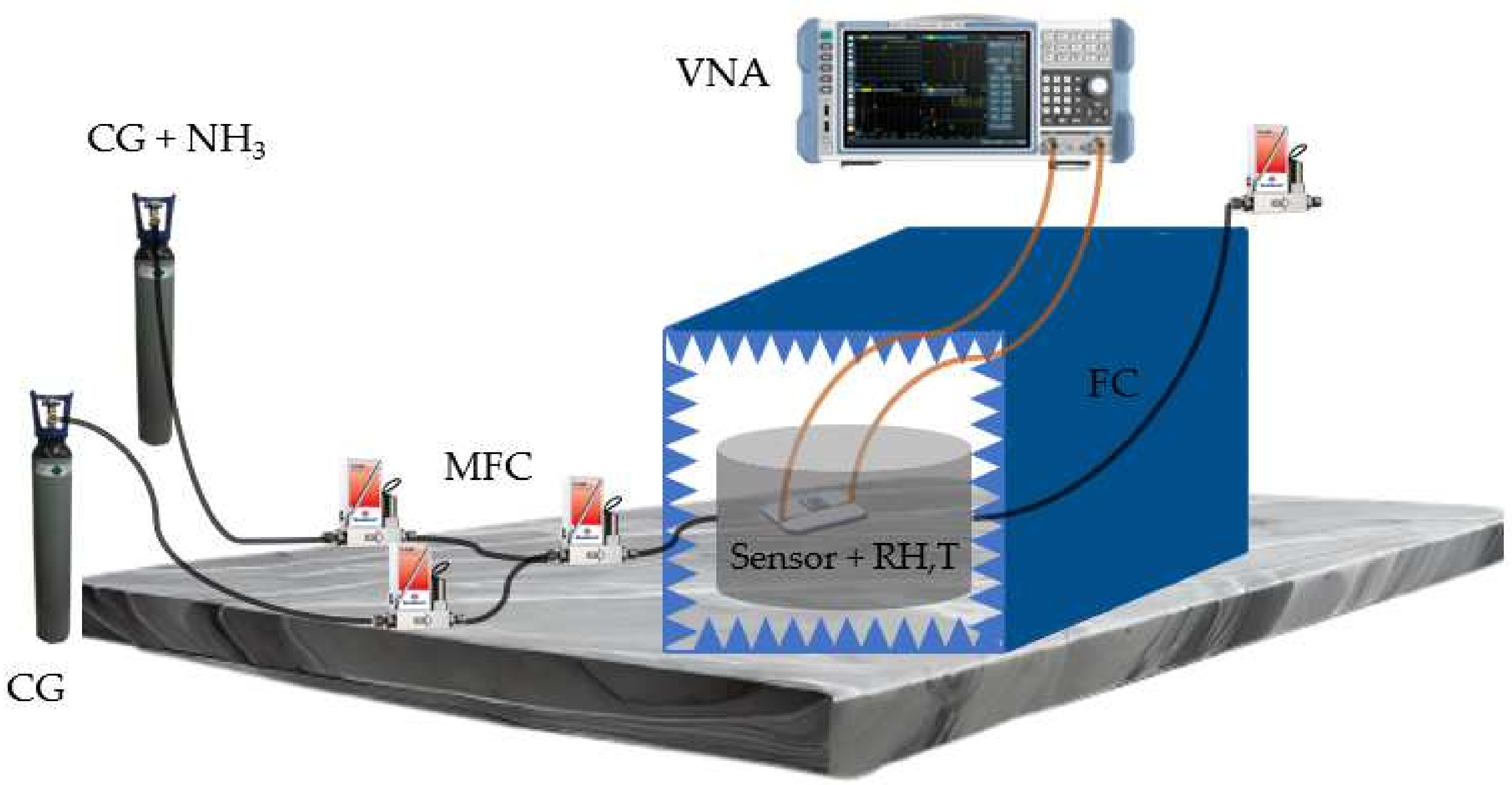
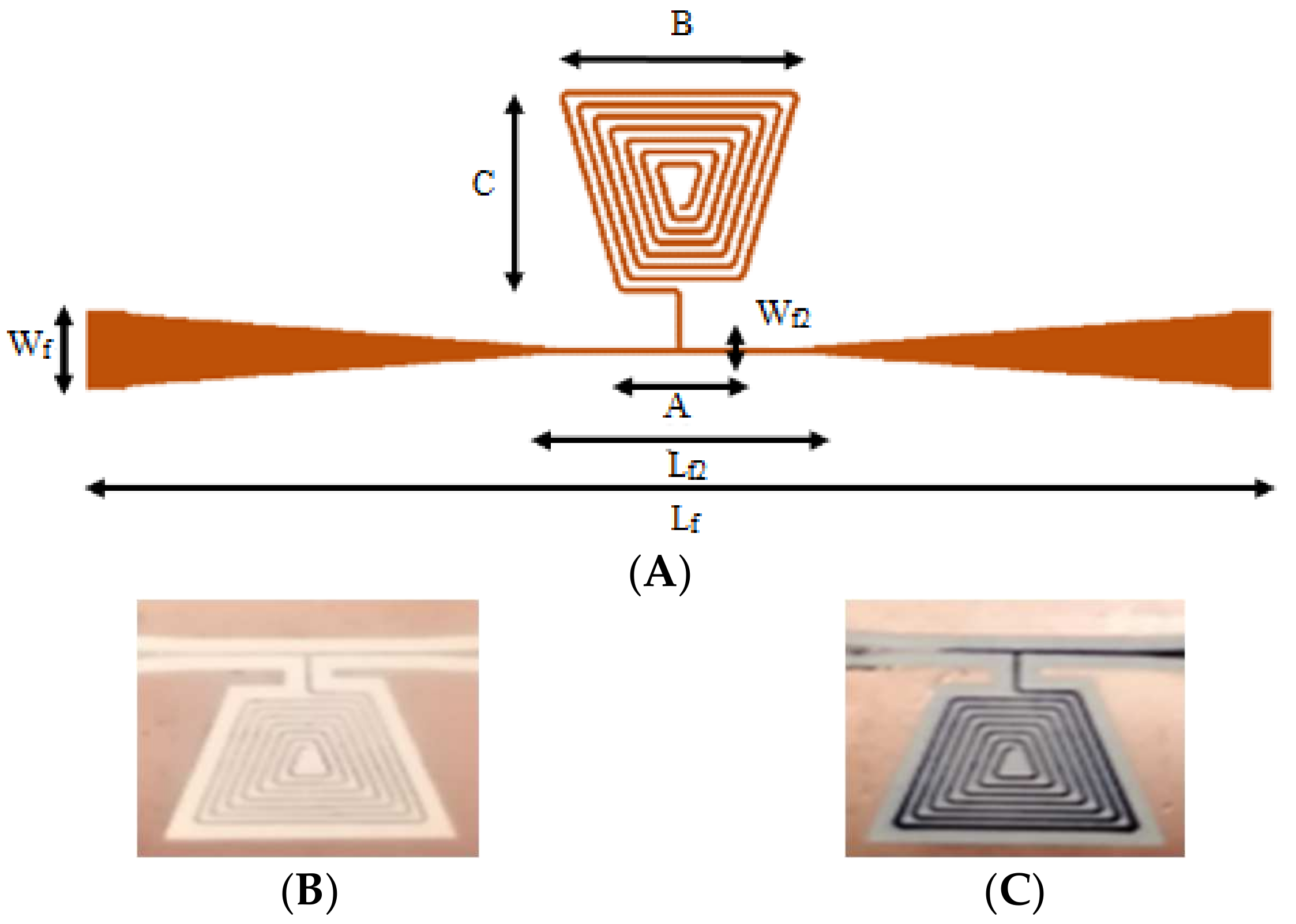
- Fabrication and chemical modification of the microwave gas sensors
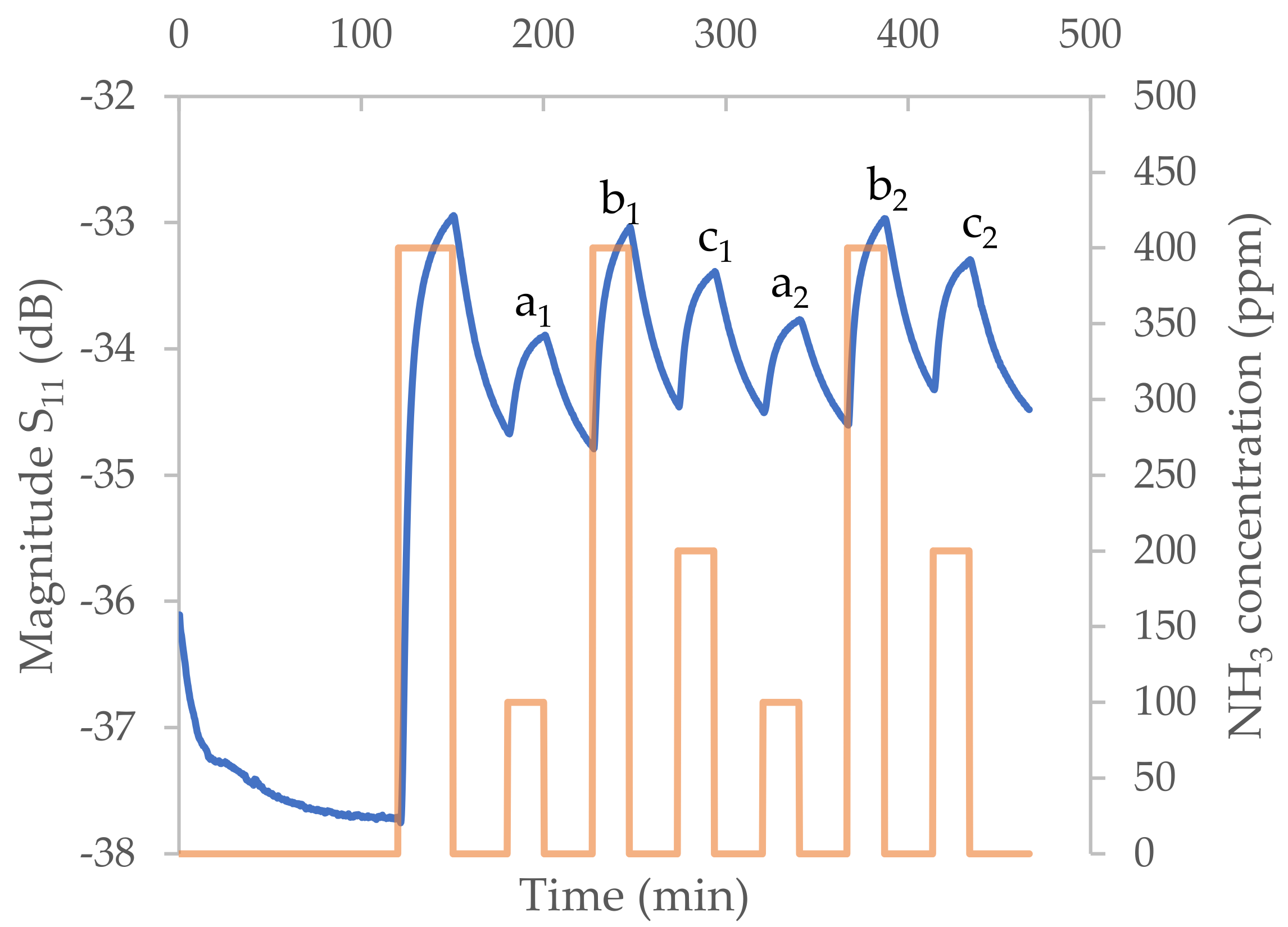
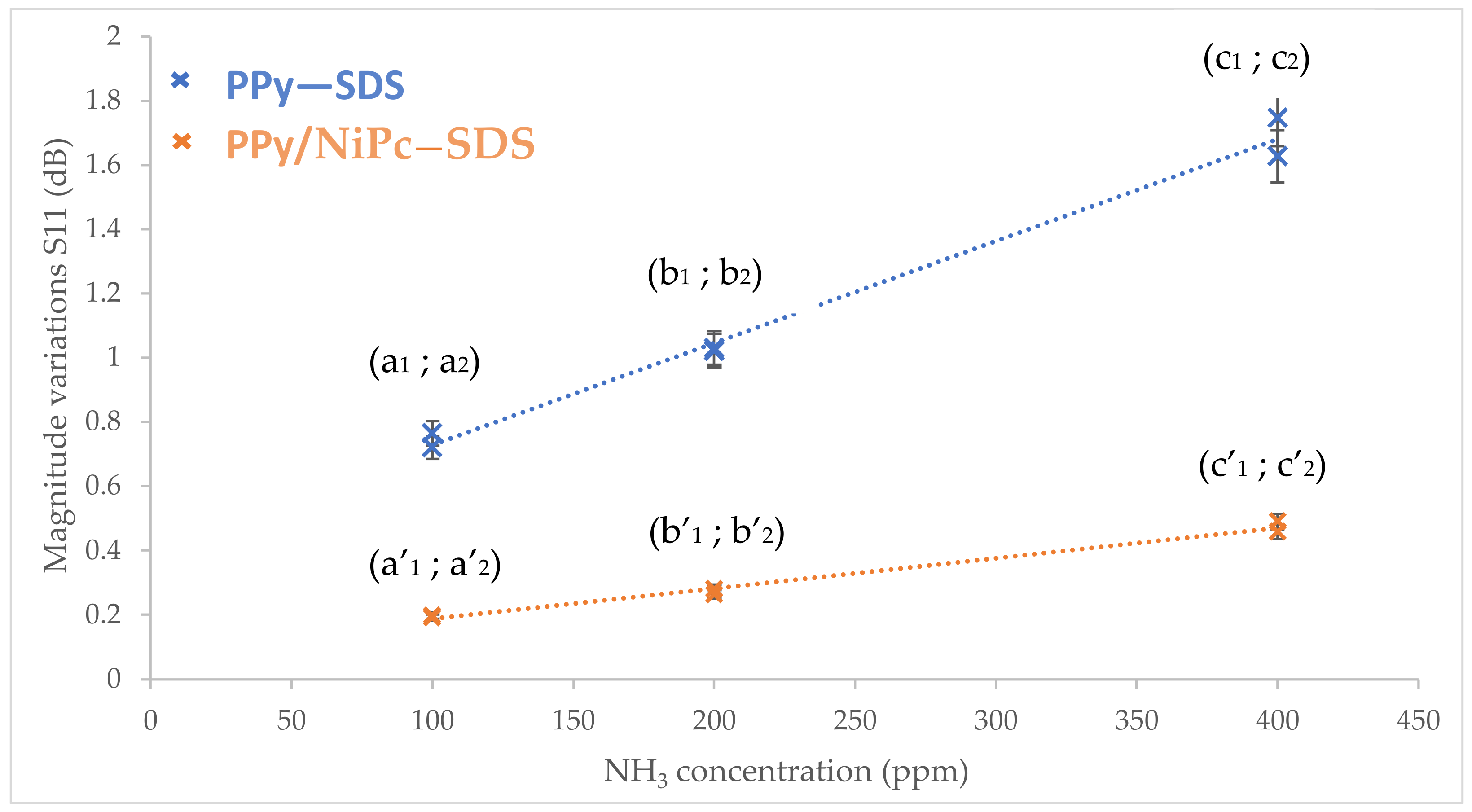
- Gas sensing results
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eduard, W.; Pearce, N.; Douwes, J. Chronic Bronchitis, COPD, and Lung Function in Farmers: The Role of Biological Agents. Chest 2009, 136, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Freeman, L.B. Evaluation of Agricultural Exposures: The Agricultural Health Study and the Agricultural Cohort Consortium. Rev. Environ. Health 2009, 24, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Lockwood, A.H. Blood Ammonia Levels and Hepatic Encephalopathy. Metab. Brain Dis. 2004, 19, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.P.; Aggarwal, A.; Krieger, D.; Easley, K.A.; Karafa, M.T.; Van Lente, F.; Arroliga, A.C.; Mullen, K.D. Correlation between ammonia levels and the severity of hepatic encephalopathy. Am. J. Med. 2003, 114, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.-J.; Li, Y.-J.; Wu, C.-C.; Lee, Y.-C.; Zan, H.-W.; Meng, H.-F.; Hsieh, M.-H.; Lai, C.-S.; Tian, Y.-C. Breath Ammonia Is a Useful Biomarker Predicting Kidney Function in Chronic Kidney Disease Patients. Biomedicines 2020, 8, 468. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-C.; Hsieh, J.-C.; Chao, C.-H.; Yang, W.-S.; Cheng, H.-T.; Chan, C.-K.; Lu, C.-J.; Meng, H.-F.; Zan, H.-W. Correlation between breath ammonia and blood urea nitrogen levels in chronic kidney disease and dialysis patients. J. Breath Res. 2020, 14, 036002. [Google Scholar] [CrossRef] [PubMed]
- Amano, A.; Yoshida, Y.; Oho, T.; Koga, T. Monitoring ammonia to assess halitosis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2002, 94, 692–696. [Google Scholar] [CrossRef]
- Kearney, D.J.; Hubbard, T.; Putnam, D. Breath Ammonia Measurement in Helicobacter pylori Infection. Dig. Dis. Sci. 2002, 47, 2523–2530. [Google Scholar] [CrossRef]
- Pavel, I.-A.; Lakard, S.; Lakard, B. Flexible Sensors Based on Conductive Polymers. Chemosensors 2022, 10, 97. [Google Scholar] [CrossRef]
- Wong, Y.C.; Ang, B.C.; Haseeb, A.S.M.A.; Baharuddin, A.A.; Wong, Y.H. Review—Conducting Polymers as Chemiresistive Gas Sensing Materials: A Review. J. Electrochem. Soc. 2020, 167, 037503. [Google Scholar] [CrossRef]
- Direksilp, C.; Sirivat, A. Tunable size and shape of conductive poly(N-methylaniline) based on surfactant template and doping. Polym. Int. 2019, 68, 1042–1053. [Google Scholar] [CrossRef]
- Kaur, A.; Ishpal; Dhawan, S. Tuning of EMI shielding properties of polypyrrole nanoparticles with surfactant concentration. Synth. Met. 2012, 162, 1471–1477. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, F.K.; Pan, Z.; Zhang, J.; Zhao, B. Bio-Inspired Dopamine Functionalization of Polypyrrole for Improved Adhesion and Conductivity. Macromol. Rapid Commun. 2014, 35, 350–354. [Google Scholar] [CrossRef]
- Arulraj, A.D.; Vijayan, M.; Vasantha, V.S. Highly selective and sensitive simple sensor based on electrochemically treated nano polypyrrole-sodium dodecyl sulphate film for the detection of para-nitrophenol. Anal. Chim. Acta 2015, 899, 66–74. [Google Scholar] [CrossRef]
- Gholami, M.; Rezayi, M.; Nia, P.M.; Yusoff, I.; Alias, Y. A novel method for fabricating Fe2+ ion selective sensor using polypyrrole and sodium dodecyl sulfate based on carbon screen-printed electrode. Measurement 2015, 69, 115–125. [Google Scholar] [CrossRef]
- Musa, I.; Raffin, G.; Hangouet, M.; Martin, M.; Alcacer, A.; Zine, N.; Bellagambi, F.; Jaffrezic-Renault, N.; Errachid, A. Development of a Chitosan/Nickel Phthalocyanine Composite Based Conductometric Micro-sensor for Methanol Detection. Electroanalysis 2022, 34, 1338–1347. [Google Scholar] [CrossRef]
- Musa, I.; Raffin, G.; Hangouet, M.; Martin, M.; Bausells, J.; Zine, N.; Bellagambi, F.; Jaffrezic-Renault, N.; Errachid, A. Electrospun PVC-nickel phthalocyanine composite nanofiber based conductometric methanol microsensor. Microchem. J. 2022, 182, 107899. [Google Scholar] [CrossRef]
- Sizun, T.; Bouvet, M.; Suisse, J.-M. Humidity effect on ammonia sensing properties of substituted and unsubstituted cobalt phthalocyanines. Talanta 2012, 97, 318–324. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, A.; Debnath, A.; Samanta, S.; Aswal, D.; Gupta, S.; Yakhmi, J.V. Room temperature ppb level Cl2 sensing using sulphonated copper phthalocyanine films. Talanta 2010, 82, 1485–1489. [Google Scholar] [CrossRef]
- Gulppi, M.; Bedioui, F.; Zagal, J.H. Overoxidized Polypyrrole/Cobalt Tetrasulfonated Phthalocyanine Modified Ultramicro-Carbon-Fiber Electrodes for the Electrooxidation of 2-Mercaptoethanol. Electroanalysis 2001, 13, 1136–1139. [Google Scholar] [CrossRef]
- Milczarek, G. Self-doped polyaniline films prepared by electropolymerization in the presence of sulfonated nickel phthalocyanine. Thin Solid Films 2009, 517, 6100–6104. [Google Scholar] [CrossRef]
- Gaudillat, P.; Jurin, F.; Lakard, B.; Buron, C.; Suisse, J.-M.; Bouvet, M. From the Solution Processing of Hydrophilic Molecules to Polymer-Phthalocyanine Hybrid Materials for Ammonia Sensing in High Humidity Atmospheres. Sensors 2014, 14, 13476–13495. [Google Scholar] [CrossRef]
- Zhihua, L.; Xucheng, Z.; Jiyong, S.; Xiaobo, Z.; Xiaowei, H.; Tahir, H.E.; Holmes, M. Fast response ammonia sensor based on porous thin film of polyaniline/sulfonated nickel phthalocyanine composites. Sens. Actuators B Chem. 2016, 226, 553–562. [Google Scholar] [CrossRef]
- Sizun, T.; Patois, T.; Bouvet, M.; Lakard, B. Microstructured electrodeposited polypyrrole–phthalocyanine hybrid material, from morphology to ammonia sensing. J. Mater. Chem. 2012, 22, 25246–25253. [Google Scholar] [CrossRef]
- Paul, S.; Joseph, M. Polypyrrole functionalized with FePcTSA for NO2 sensor application. Sens. Actuators B Chem. 2009, 140, 439–444. [Google Scholar] [CrossRef]
- Muthuraman, G.; Shim, Y.-B.; Yoon, J.-H.; Won, M.-S. Simultaneous immobilization of cobalt tetrasulfonated phthalocyanine during electropolymerization of pyrrole in presence of surfactants: A study of film morphology and its conductivity. Synth. Met. 2005, 150, 165–173. [Google Scholar] [CrossRef]
- Tiwari, D.; Sharma, R.; Vyas, K.; Boopathi, M.; Singh, V.V.; Pandey, P. Electrochemical incorporation of copper phthalocyanine in conducting polypyrrole for the sensing of DMMP. Sens. Actuators B Chem. 2010, 151, 256–264. [Google Scholar] [CrossRef]
- Bobkov, A.; Luchinin, V.; Moshnikov, V.; Nalimova, S.; Spivak, Y. Impedance Spectroscopy of Hierarchical Porous Nanomaterials Based on por-Si, por-Si Incorporated by Ni and Metal Oxides for Gas Sensors. Sensors 2022, 22, 1530. [Google Scholar] [CrossRef]
- Ponce, M.; Castro, M.; Aldao, C. Resistance and capacitance analysis of Pd-doped and undoped SnO2 thick films sensors exposed to CO atmospheres. Ceram. Int. 2006, 32, 733–737. [Google Scholar] [CrossRef]
- Gugliandolo, G.; Naishadham, K.; Crupi, G.; Donato, N. Design and Characterization of a Microwave Transducer for Gas Sensing Applications. Chemosensors 2022, 10, 127. [Google Scholar] [CrossRef]
- de Fonseca, B.; Rossignol, J.; Stuerga, D.; Pribetich, P. Microwave signature for gas sensing: 2005 to present. Urban Clim. 2015, 14, 502–515. [Google Scholar] [CrossRef]
- Mirzaei, A.; Neri, G. Microwave-assisted synthesis of metal oxide nanostructures for gas sensing application: A review. Sens. Actuators B Chem. 2016, 237, 749–775. [Google Scholar] [CrossRef]
- de Fonseca, B.; Rossignol, J.; Bezverkhyy, I.; Bellat, J.; Stuerga, D.; Pribetich, P. Detection of VOCs by microwave transduction using dealuminated faujasite DAY zeolites as gas sensitive materials. Sens. Actuators B Chem. 2015, 213, 558–565. [Google Scholar] [CrossRef]
- Jang, C.; Park, J.-K.; Yun, G.-H.; Choi, H.H.; Lee, H.-J.; Yook, J.-G. Radio-Frequency/Microwave Gas Sensors Using Conducting Polymer. Materials 2020, 13, 2859. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, J.; Barochi, G.; de Fonseca, B.; Brunet, J.; Bouvet, M.; Pauly, A.; Markey, L. Development of Gas Sensors by Microwave Transduction with Phthalocyanine Film. Procedia Eng. 2012, 47, 1191–1194. [Google Scholar] [CrossRef]
- Sinha, M.; Verma, P.; Panda, S. Metal-phthalocyanine modified doped polyaniline for VOC sensing applications. Flex. Print. Electron. 2020, 5, 014014. [Google Scholar] [CrossRef]
- Zucolotto, V.; Ferreira, M.; Cordeiro, M.R.; Constantino, C.J.; Moreira, W.C.; Oliveira, O.N. Nanoscale processing of polyaniline and phthalocyanines for sensing applications. Sens. Actuators B Chem. 2006, 113, 809–815. [Google Scholar] [CrossRef]
- Olgac, R.; Soganci, T.; Baygu, Y.; Gök, Y.; Ak, M. Zinc(II) phthalocyanine fused in peripheral positions octa-substituted with alkyl linked carbazole: Synthesis, electropolymerization and its electro-optic and biosensor applications. Biosens. Bioelectron. 2017, 98, 202–209. [Google Scholar] [CrossRef]
- Kulkarni, G.; Kandesar, P.; Velhal, N.N.; Kim, H.; Puri, V. Facile synthesis of coral cauliflower-like polypyrrole hemispheres toward screening electromagnetic interference pollution. J. Appl. Polym. Sci. 2021, 138, 50447. [Google Scholar] [CrossRef]
- Bailly, G.; Harrabi, A.; Rossignol, J.; Michel, M.; Stuerga, D.; Pribetich, P. Microstrip Spiral Resonator for Microwave-Based Gas Sensing. IEEE Sens. Lett. 2017, 1, 4500404. [Google Scholar] [CrossRef]
- Fu, Y.; Su, Y.-S.; Manthiram, A. Sulfur-Polypyrrole Composite Cathodes for Lithium-Sulfur Batteries. J. Electrochem. Soc. 2012, 159, A1420. [Google Scholar] [CrossRef]
- Liang, X.; Liu, Y.; Wen, Z.; Huang, L.; Wang, X.; Zhang, H. A nano-structured and highly ordered polypyrrole-sulfur cathode for lithium-sulfur batteries. J. Power Sources 2011, 196, 6951–6956. [Google Scholar] [CrossRef]
- Kato, H.; Nishikawa, O.; Matsui, T.; Honma, S.; Kokado, H. Fourier transform infrared spectroscopy study of conducting polymer polypyrrole: Higher order structure of electrochemically-synthesized film. J. Phys. Chem. 1991, 95, 6014–6016. [Google Scholar] [CrossRef]
- Periodic Table for EDS Analysis. Available online: https://www.jeolusa.com/RESOURCES/Posters (accessed on 3 May 2023).
- Lasserre, A.; Grzelak, L.; Rossignol, J.; Brousse, O.; Stuerga, D.; Paindavoine, M. From microwave gas sensor conditioning to ammonia concentration prediction by machine learning. Sens. Actuators B Chem. 2022, 367, 132138. [Google Scholar] [CrossRef]
- Rossignol, J.; Harrabi, A.; Stuerga, D.; Pribetich, P.; Bailly, G.; Leblois, T. Critical Influence of Dielectric Sensitive Material and Manufactured Process in Microwave Gas-Sensing: Application of Ammonia Detection with an Interdigital Sensor. ACS Omega 2020, 5, 11507–11514. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhang, N.; Wang, T.; Liu, F.; Wang, X.; Yan, X.; Wang, C.; Liu, X.; Sun, P.; Lu, G. Microwave gas sensor for detection of ammonia at room-temperature. Sens. Actuators B Chem. 2022, 350, 130854. [Google Scholar] [CrossRef]
- Ivanova, V.; Klyamer, D.; Krasnov, P.; Kaya, E.N.; Kulu, I.; Kostakoğlu, S.T.; Durmuş, M.; Basova, T. Hybrid materials based on pyrene-substituted metallo phthalocyanines as sensing layers for ammonia detection: Effect of the number of pyrene substituents. Sens. Actuators B Chem. 2023, 375, 132843. [Google Scholar] [CrossRef]
- Gai, S.; Wang, B.; Wang, X.; Zhang, R.; Miao, S.; Wu, Y. Ultrafast NH3 gas sensor based on phthalocyanine-optimized non-covalent hybrid of carbon nanotubes with pyrrole. Sens. Actuators B Chem. 2022, 357, 131352. [Google Scholar] [CrossRef]
- Ridhi, R.; Neeru; Gautam, S.; Saini, G.; Tripathi, S.; Rawat, J.; Jha, P. Amendment in sensing response of Single Walled Carbon nanotube (SWCNT) towards ammonia gas with copper phthalocyanine functionalization. Mater. Today Proc. 2020, 28, 1759–1763. [Google Scholar] [CrossRef]
- Kang, D.; Wang, B.; Wang, X.; Li, Y.; Chen, Z.; He, C.; Wu, Y. Stably dispersed metallophthalocyanine noncovalently bonded to multiwalled carbon nanotubes for ammonia sensing at room temperature. Sens. Actuators B Chem. 2017, 246, 262–270. [Google Scholar] [CrossRef]
- Li, Y.; Wang, B.; Yu, Z.; Zhou, X.; Kang, D.; Wu, Y.; Chen, Z.; He, C.; Zhou, X. The effects of central metals on ammonia sensing of metallophthalocyanines covalently bonded to graphene oxide hybrids. RSC Adv. 2017, 7, 34215–34225. [Google Scholar] [CrossRef]
- Hallil, H.; Dejous, C.; Hage-Ali, S.; Elmazria, O.; Rossignol, J.; Stuerga, D.; Talbi, A.; Mazzamurro, A.; Joubert, P.-Y.; Lefeuvre, E. Passive Resonant Sensors: Trends and Future Prospects. IEEE Sens. J. 2021, 21, 12618–12632. [Google Scholar] [CrossRef]
- Zhang, N.; Jiang, B.; Xue, S.; Wang, X.; Wang, T.; Sun, P.; Lu, G. General analysis method for the signal enhancement of microwave gas sensor though variation of energy loss. Sens. Actuators B Chem. 2022, 367, 132117. [Google Scholar] [CrossRef]

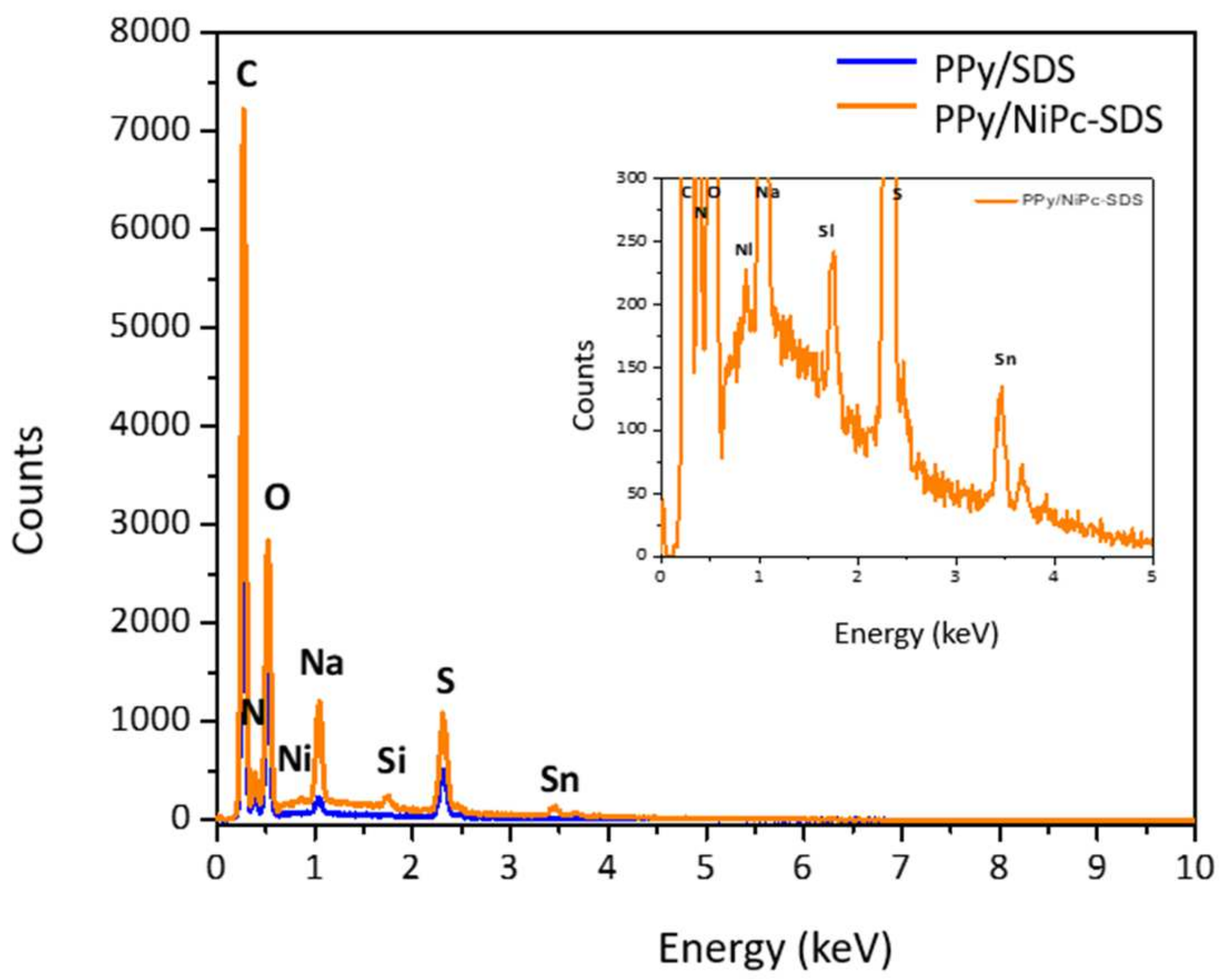


| W% (C) | W% (N) | W% (O) | W% (Na) | W% (S) | W% (Ni) | |
|---|---|---|---|---|---|---|
| PPy-SDS | 47.44 | 9.92 | 28.43 | 1.62 | 12.57 | 0 |
| PPy/NiPc-SDS | 45.24 | 8.13 | 26.56 | 6.11 | 13.50 | 0.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavel, I.-A.; Lasserre, A.; Simon, L.; Rossignol, J.; Lakard, S.; Stuerga, D.; Lakard, B. Microwave Gas Sensors Based on Electrodeposited Polypyrrole–Nickel Phthalocyanine Hybrid Films. Sensors 2023, 23, 5550. https://doi.org/10.3390/s23125550
Pavel I-A, Lasserre A, Simon L, Rossignol J, Lakard S, Stuerga D, Lakard B. Microwave Gas Sensors Based on Electrodeposited Polypyrrole–Nickel Phthalocyanine Hybrid Films. Sensors. 2023; 23(12):5550. https://doi.org/10.3390/s23125550
Chicago/Turabian StylePavel, Ileana-Alexandra, Alexis Lasserre, Léo Simon, Jérôme Rossignol, Sophie Lakard, Didier Stuerga, and Boris Lakard. 2023. "Microwave Gas Sensors Based on Electrodeposited Polypyrrole–Nickel Phthalocyanine Hybrid Films" Sensors 23, no. 12: 5550. https://doi.org/10.3390/s23125550





