Fabrication and Performance of a Ta2O5 Thin Film pH Sensor Manufactured Using MEMS Processes
Abstract
:1. Introduction
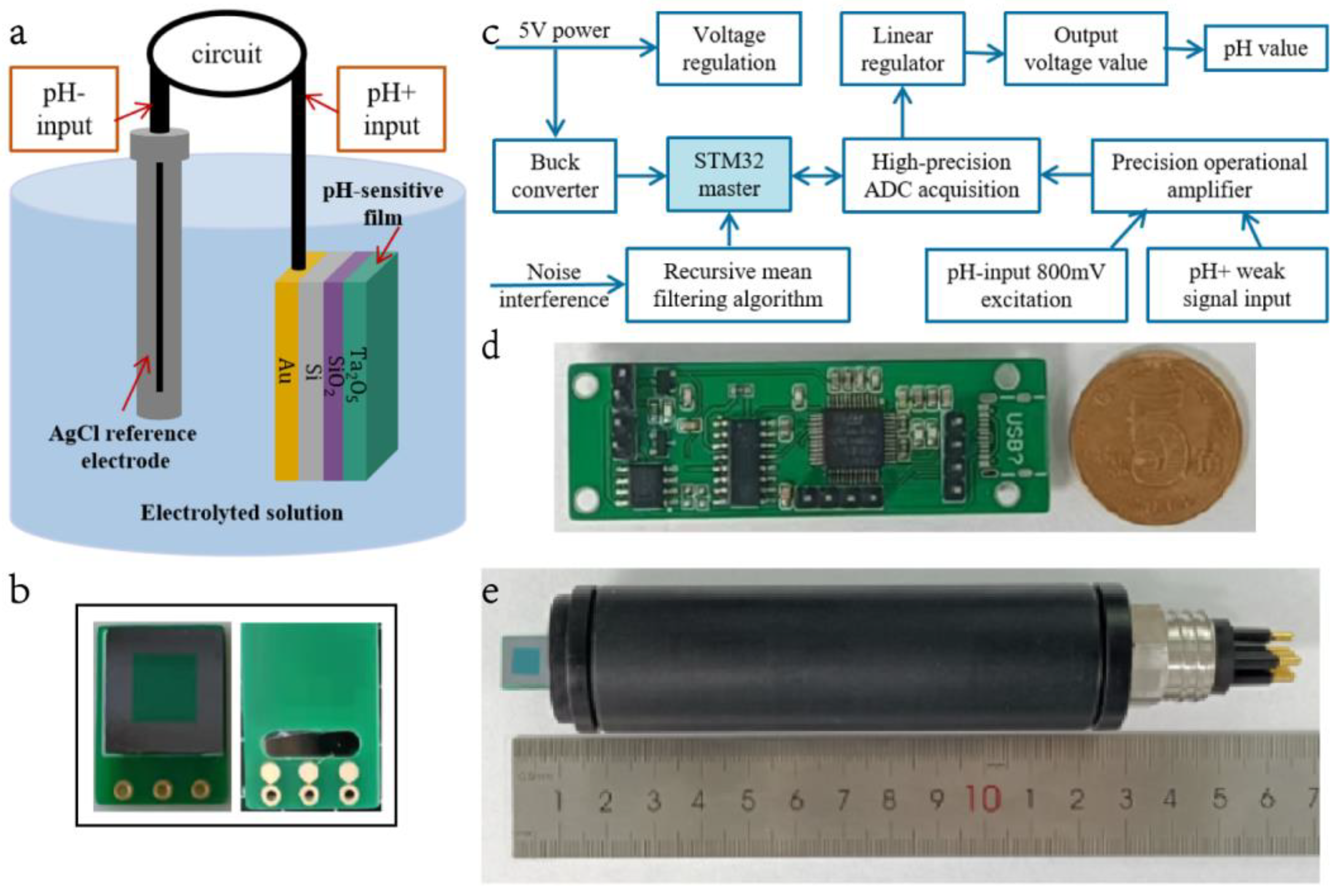
2. Working Principle of Sensors
3. Fabrication and Measurement
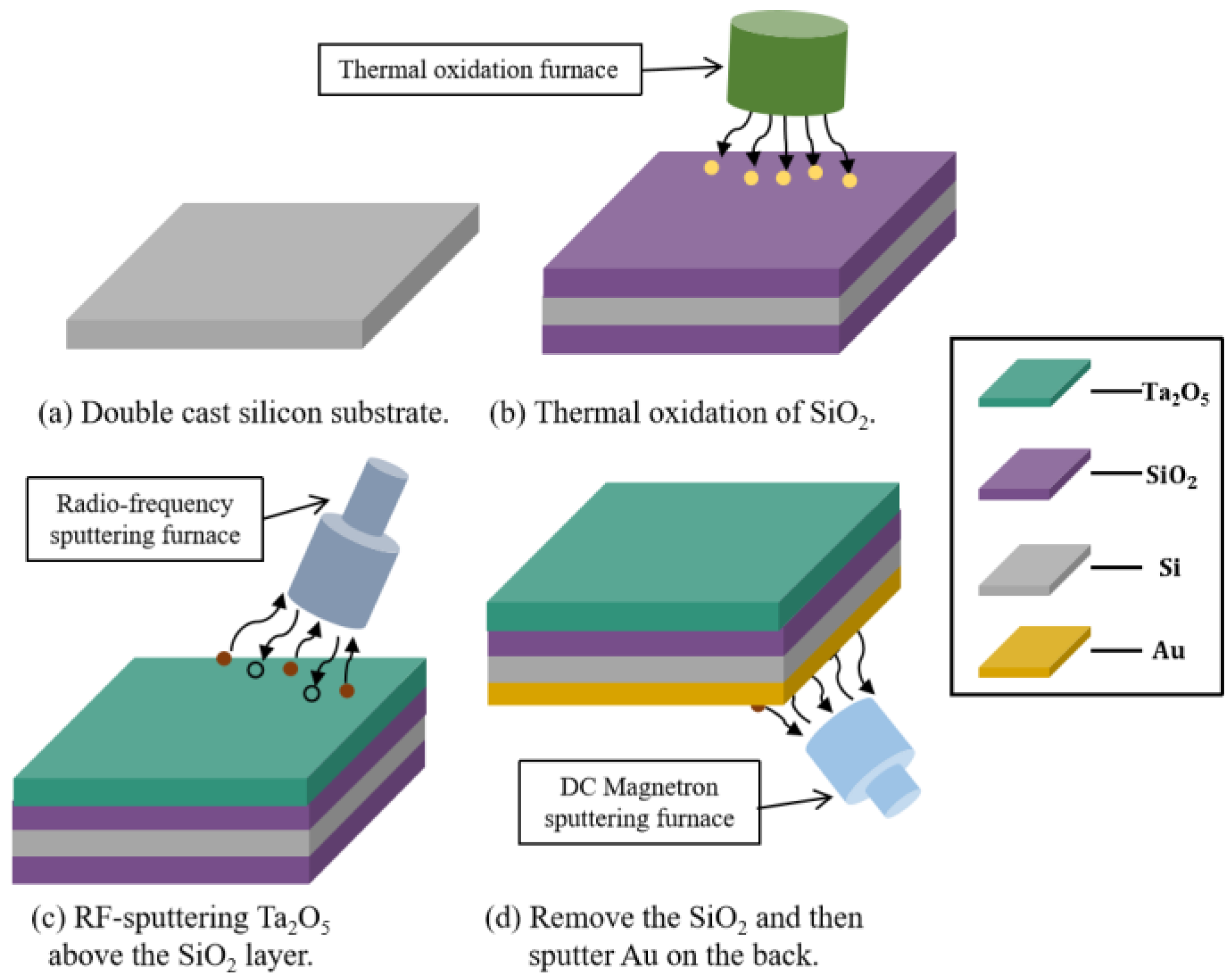
3.1. Fabrication Process and Package Design
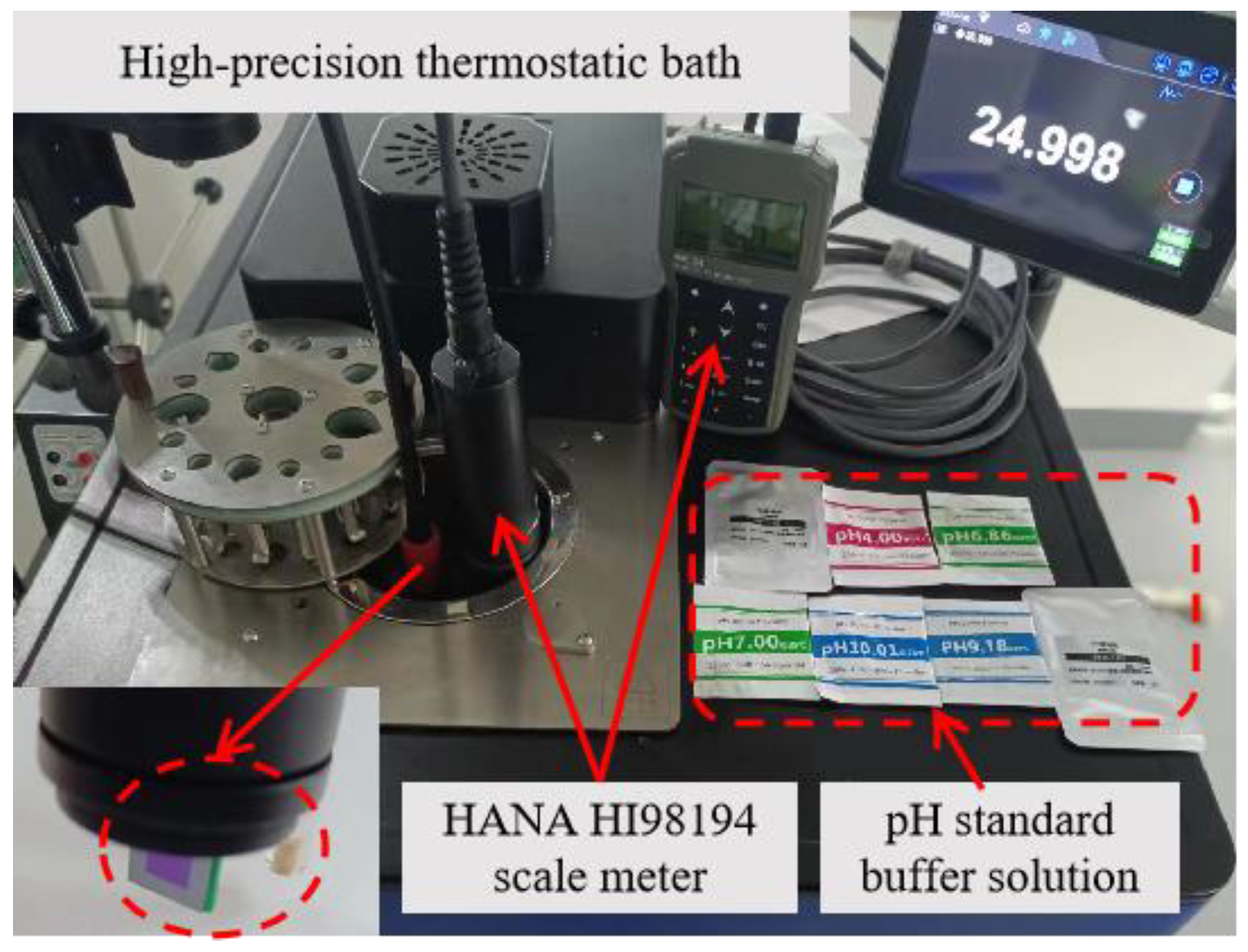
3.2. Measurement
4. Results and Discussion
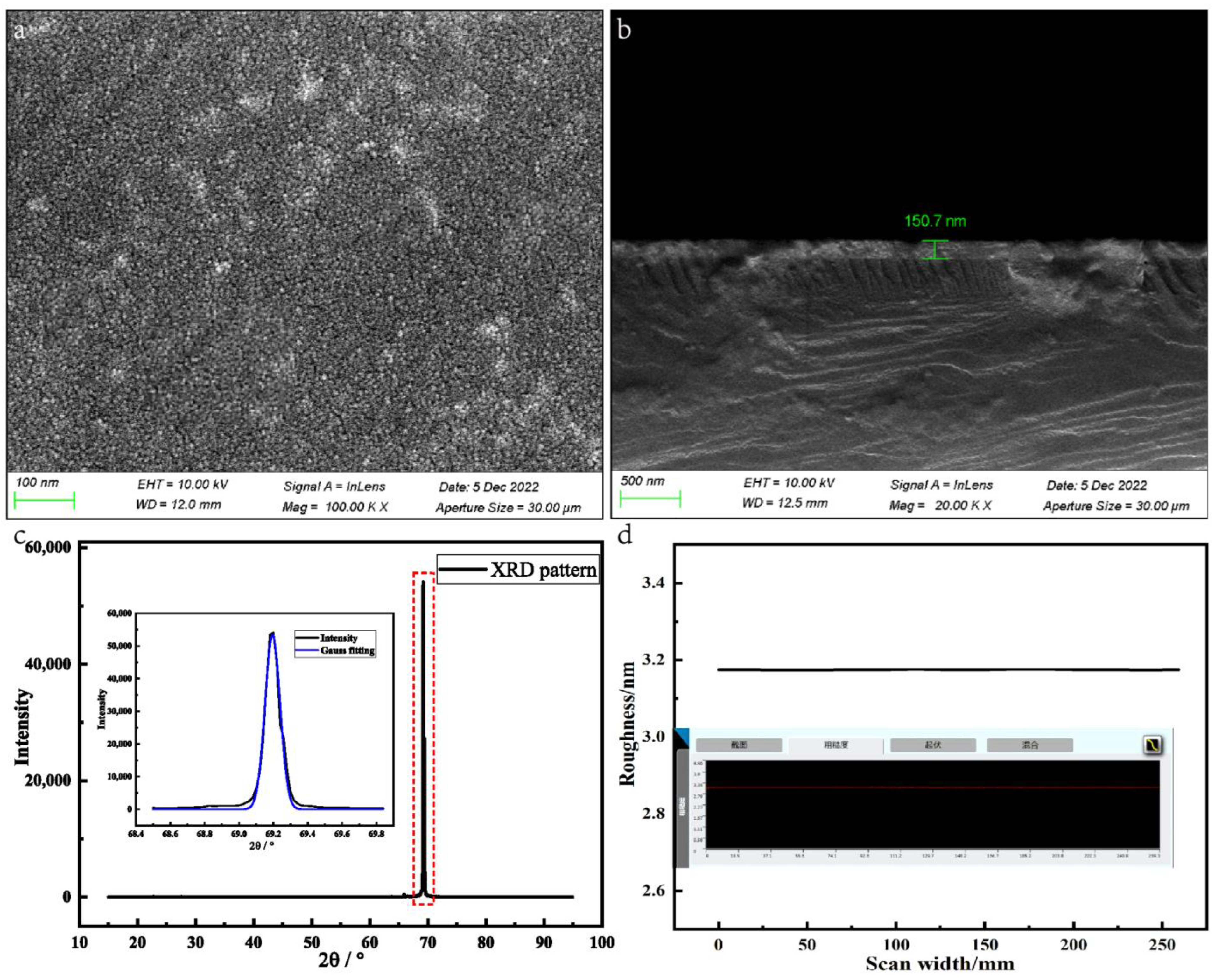
4.1. Microstructural Analysis
4.2. Testing
4.2.1. Response Time and Sensitivity
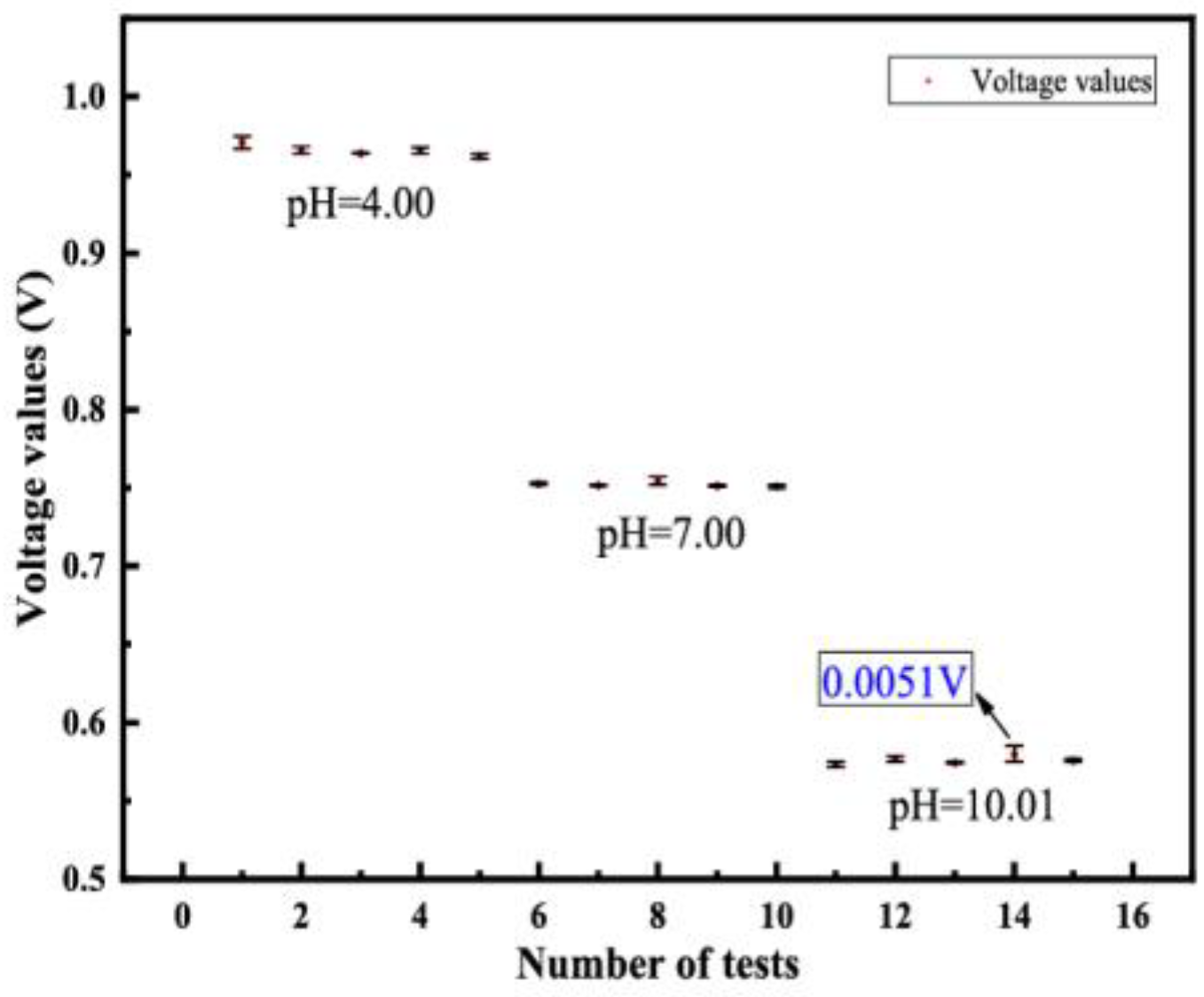
4.2.2. Repeatability and Drift Value Measurement
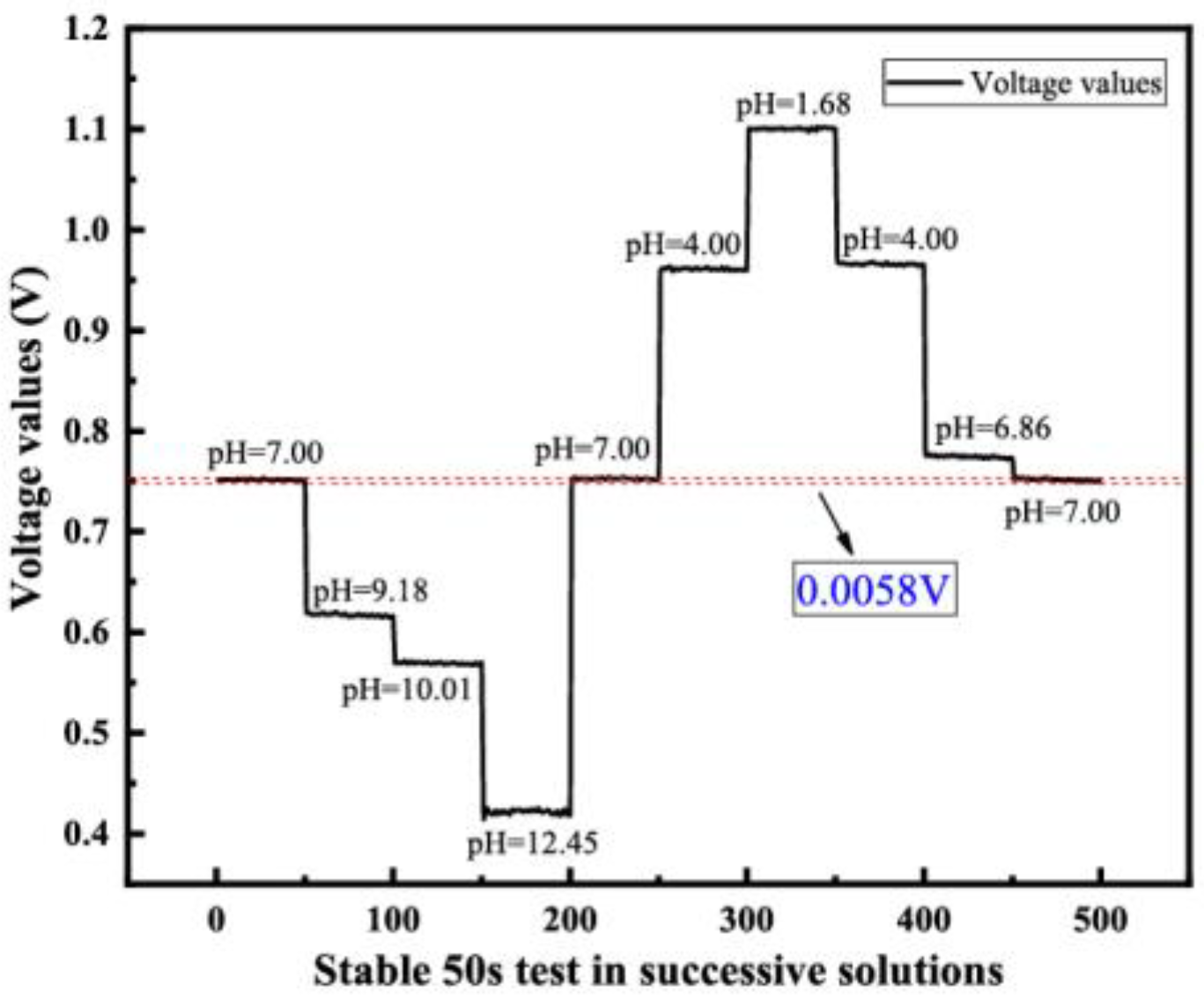
4.2.3. Hysteresis Effect
4.2.4. Performance Summary and Comparison
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, N.; Huo, C.; Xu, X.; Zang, K.; Mu, J.; Zhao, H.; Wang, J. Progress of pH Determination in the Context of Ocean Acidification. Ocean. Dev. Manag. 2018, 24–29. [Google Scholar] [CrossRef]
- Manjakkal, L.; Szwagierczak, D.; Dahiya, R. Metal oxides based electrochemical pH sensors: Current progress and future perspectives. Prog. Mater. Sci. 2020, 109, 100635. [Google Scholar] [CrossRef]
- Qu, B.; Song, J.; Li, X. Advances in ocean acidification time-series studies. Mar. Sci. Bull. 2020, 281–290. [Google Scholar] [CrossRef]
- Orr, J.C.; Fabry, V.J.; Aumont, O.; Bopp, L.; Doney, S.C.; Feely, R.A.; Gnanadesikan, A.; Gruber, N.; Ishida, A.; Joos, F.; et al. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 2005, 437, 681–686. [Google Scholar] [CrossRef] [Green Version]
- Seelmann, K.; Steinhoff, T.; Aßmann, S.; Körtzinger, A. Enhance Ocean Carbon Observations: Successful Implementation of a Novel Autonomous Total Alkalinity Analyzer on a Ship of Opportunity. Front. Mar. Sci. 2020, 7, 571301. [Google Scholar] [CrossRef]
- Kraikaew, P.; Jeanneret, S.; Soda, Y.; Cherubini, T.; Bakker, E. Ultrasensitive Seawater pH Measurement by Capacitive Readout of Potentiometric Sensors. ACS Sens. 2020, 5, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Manjakkal, L.; Cvejin, K.; Kulawik, J.; Zaraska, K.; Socha, R.P.; Szwagierczak, D. X-ray photoelectron spectroscopic and electrochemical impedance spectroscopic analysis of RuO2-Ta2O5 thick film pH sensors. Anal. Chim. Acta 2016, 931, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Schöning, M.J.; Brinkmann, D.; Rolka, D.; Demuth, C.; Poghossian, A. CIP (cleaning-in-place) suitable “non-glass” pH sensor based on a Ta2O5-gate EIS structure. Sens. Actuators B Chem. 2005, 111–112, 423–429. [Google Scholar] [CrossRef]
- Vonau, W.; Guth, U. pH Monitoring: A review. J. Solid State Electrochem. 2006, 10, 746–752. [Google Scholar] [CrossRef]
- Chen, M.; Jin, Y.; Qu, X.; Jin, Q.; Zhao, J. Electrochemical impedance spectroscopy study of Ta2O5 based EIOS pH sensors in acid environment. Sens. Actuators B Chem. 2014, 192, 399–405. [Google Scholar] [CrossRef]
- Manjakkal, L.; Zaraska, K.; Cvejin, K.; Kulawik, J.; Szwagierczak, D. Potentiometric RuO2-Ta2O5 pH sensors fabricated using thick film and LTCC technologies. Talanta 2016, 147, 233–240. [Google Scholar] [CrossRef]
- Kurzweil, P. Metal Oxides and Ion-Exchanging Surfaces as pH Sensors in Liquids: State-of-the-Art and Outlook. Sensors 2009, 9, 4955–4985. [Google Scholar] [CrossRef] [Green Version]
- Glab, S.; Hulanicki, A.; Edwall, G.; Ingman, F. Metal-Metal Oxide and Metal Oxide Electrodes as pH Sensors. Crit. Rev. Anal. Chem. 1989, 21, 29–47. [Google Scholar] [CrossRef] [PubMed]
- Bousse, L.; Mostar-shed, S.; Schoot, B.v.d.; Rooij, N.F.d. Comparison of the hysteresis of Ta2O5 and Si3N4, pH-sensing insulators. Sens. Actuators B 1994, 17, 157–164. [Google Scholar] [CrossRef]
- Becker, T.; Arshak, K.; Cané, C.; Gill, E.; Korostynska, O.; Barker, N.S.; Arshak, A. Mixed metal oxide films as pH sensing materials. In Proceedings of the Smart Sensors, Actuators, and MEMS III, Gran Canaria, Spain, 2–4 May 2007. [Google Scholar] [CrossRef]
- Maeng, S.; Axe, L.; Tyson, T.; Jiang, A. An Investigation of Structures of Thermal and Anodic Tantalum Oxide Films. J. Electrochem. Soc. 2005, 152, B60–B64. [Google Scholar] [CrossRef]
- Manjakkal, L.; Cvejin, K.; Bajac, B.; Kulawik, J.; Zaraska, K.; Szwagierczak, D. Microstructural, Impedance Spectroscopic and Potentiometric Analysis of Ta2O5 Electrochemical Thick Film pH Sensors. Electroanalysis 2015, 27, 770–781. [Google Scholar] [CrossRef]
- Ren, W.; Yang, G.-D.; Feng, A.-L.; Miao, R.-X.; Xia, J.-B.; Wang, Y.-G. Annealing effects on the optical and electrochemical properties of tantalum pentoxide films. J. Adv. Ceram. 2021, 10, 704–713. [Google Scholar] [CrossRef]
- Yoon, S.G.; Kim, Y.T.; Kim, H.K.; Kim, M.J.; Lee, H.M.; Yoon, D.H. Comparision of residual stress and optical properties in Ta2O5 thin films deposited by single and dual ion beam sputtering. Mater. Sci. Eng. B 2005, 118, 234–237. [Google Scholar] [CrossRef]
- Wei, A.X.; Ge, Z.X.; Zhao, X.H.; Liu, J.; Zhao, Y. Electrical and optical properties of tantalum oxide thin films prepared by reactive magnetron sputtering. J. Alloys Compd. 2011, 509, 9758–9763. [Google Scholar] [CrossRef]
- Maurya, D.; Sardarinejad, A.; Alameh, K. Recent Developments in R.F. Magnetron Sputtered Thin Films for pH Sensing Applications—An Overview. Coatings 2014, 4, 756–771. [Google Scholar] [CrossRef] [Green Version]
- Gu, M.; Hu, S.; Peng, R.; Li, Y.; Chen, J.; Yuan, X.; Qiu, M. A review of developments in the preparation methods of tantalum pentoxide film. In Proceedings of the International Symposium on Photoelectronic Detection and Imaging 2013: Micro/Nano Optical Imaging Technologies and Applications, Beijing, China, 25–27 June 2013. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Sinha, S.; Sahu, N.; Majumder, S.; Narang, P.; Mukhiya, R. Modeling and simulation of temperature drift for ISFET-based pH sensor and its compensation through machine learning techniques. Int. J. Circuit Theory Appl. 2019, 47, 954–970. [Google Scholar] [CrossRef]
- Chang, K.M.; Chang, C.T.; Chao, K.Y.; Lin, C.H. A novel pH-dependent drift improvement method for zirconium dioxide gated pH-ion sensitive field effect transistors. Sensors 2010, 10, 4643–4654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nograles, A.H.; Cruz, J.C.D.; Chung, W.-Y. pH ISFET Stabilization Time Measurement Analysis and Its Readout and Control Circuit. In Proceedings of the 2021 IEEE International Conference on Automatic Control & Intelligent Systems (I2CACIS), Shah Alam, Malaysia, 26 June 2021; pp. 208–212. [Google Scholar] [CrossRef]
- Chen, H.J.H.; Huang, Y.-C.; Lee, T.N.; Chen, S.-Z. Characterizations of Electrolyte–Insulator–Semiconductor Sensors With Array Wells and a Stack-Sensing Membrane. IEEE Trans. Electron Devices 2020, 67, 3761–3766. [Google Scholar] [CrossRef]
- Siu, W.M.; Cobbold, R.S.C. Basic Properties of the Electrolyte-SiO2-Si System: Physical and Theoretical Aspects. IEEE Trans. Electron Devices 1979, 26, 1805–1815. [Google Scholar] [CrossRef]
- Yates, D.E.; Levine, S.; Healy, T.W. Faraday Transactions. Site-binding model of the electrical double layer at the oxide/water interface. J. Chem. Soc. Faraday Trans. Phys. Chem. Condens. Phases 1974, 70, 1807–1818. [Google Scholar] [CrossRef]
- Bousse, L.; Bergveld, P. The role of buried OH sites in the response mechanism of inorganic-gate pH-sensitive ISFETs. Sens. Actuators 1984, 1, 65–78. [Google Scholar] [CrossRef] [Green Version]
- Nakata, S.; Shiomi, M.; Fujita, Y.; Arie, T.; Akita, S.; Takei, K. A wearable pH sensor with high sensitivity based on a flexible charge-coupled device. Nat. Electron. 2018, 1, 596–603. [Google Scholar] [CrossRef]
- Poghossian, A.A. Determination of the pHpzc of insulators surface from capacitance–voltage characteristics of MIS and EIS structures. Sens. Actuators B Chem. 1997, 44, 551–553. [Google Scholar] [CrossRef]
- Jarolimova, Z.; Han, T.; Mattinen, U.; Bobacka, J.; Bakker, E. Capacitive Model for Coulometric Readout of Ion-Selective Electrodes. Anal. Chem. 2018, 90, 8700–8707. [Google Scholar] [CrossRef]
- Sophocleous, M.; Atkinson, J.K. A review of screen-printed silver/silver chloride (Ag/AgCl) reference electrodes potentially suitable for environmental potentiometric sensors. Sens. Actuators A Phys. 2017, 267, 106–120. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Liang, Z.; Shao, G.; Liu, Q.; Chen, D.; Yang, W. Enhanced Capacitive Performance of Mesoporous Vanadium Nitride Nanobelts. J. Electrochem. Soc. 2021, 168, 070529. [Google Scholar] [CrossRef]


| pH Sensor | HANNA HI98194 | Seema pH838 | Chen M. et al. [10] | Self-Developed |
|---|---|---|---|---|
| Electrode type | Glass | Glass | Ta2O5-EIOS | Ta2O5-EIOS |
| Range/(pH) | 0–14 | 0–14 | 1–10 | 2–12 |
| Sensitivity/(mV/pH) | 85.71 | - | 56.19 | 63.12 |
| Response time/(s) | 5 | 60 | - | 11.25 |
| Drift value/(mV) | 2 | 30 | 3 | 5.1 |
| Hysteresis/(mV) | 1 | - | 5 | 5.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Zhang, Z.; Yao, B.; Chai, J.; Zhang, S.; Liu, J.; Zhao, Z.; Xue, C. Fabrication and Performance of a Ta2O5 Thin Film pH Sensor Manufactured Using MEMS Processes. Sensors 2023, 23, 6061. https://doi.org/10.3390/s23136061
Guo Y, Zhang Z, Yao B, Chai J, Zhang S, Liu J, Zhao Z, Xue C. Fabrication and Performance of a Ta2O5 Thin Film pH Sensor Manufactured Using MEMS Processes. Sensors. 2023; 23(13):6061. https://doi.org/10.3390/s23136061
Chicago/Turabian StyleGuo, Yuzhen, Zengxing Zhang, Bin Yao, Jin Chai, Shiqiang Zhang, Jianwei Liu, Zhou Zhao, and Chenyang Xue. 2023. "Fabrication and Performance of a Ta2O5 Thin Film pH Sensor Manufactured Using MEMS Processes" Sensors 23, no. 13: 6061. https://doi.org/10.3390/s23136061





