Using Sparfloxacin-Capped Gold Nanoparticles to Modify a Screen-Printed Carbon Electrode Sensor for Ethanol Determination
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals and Materials
2.2. Characterizations
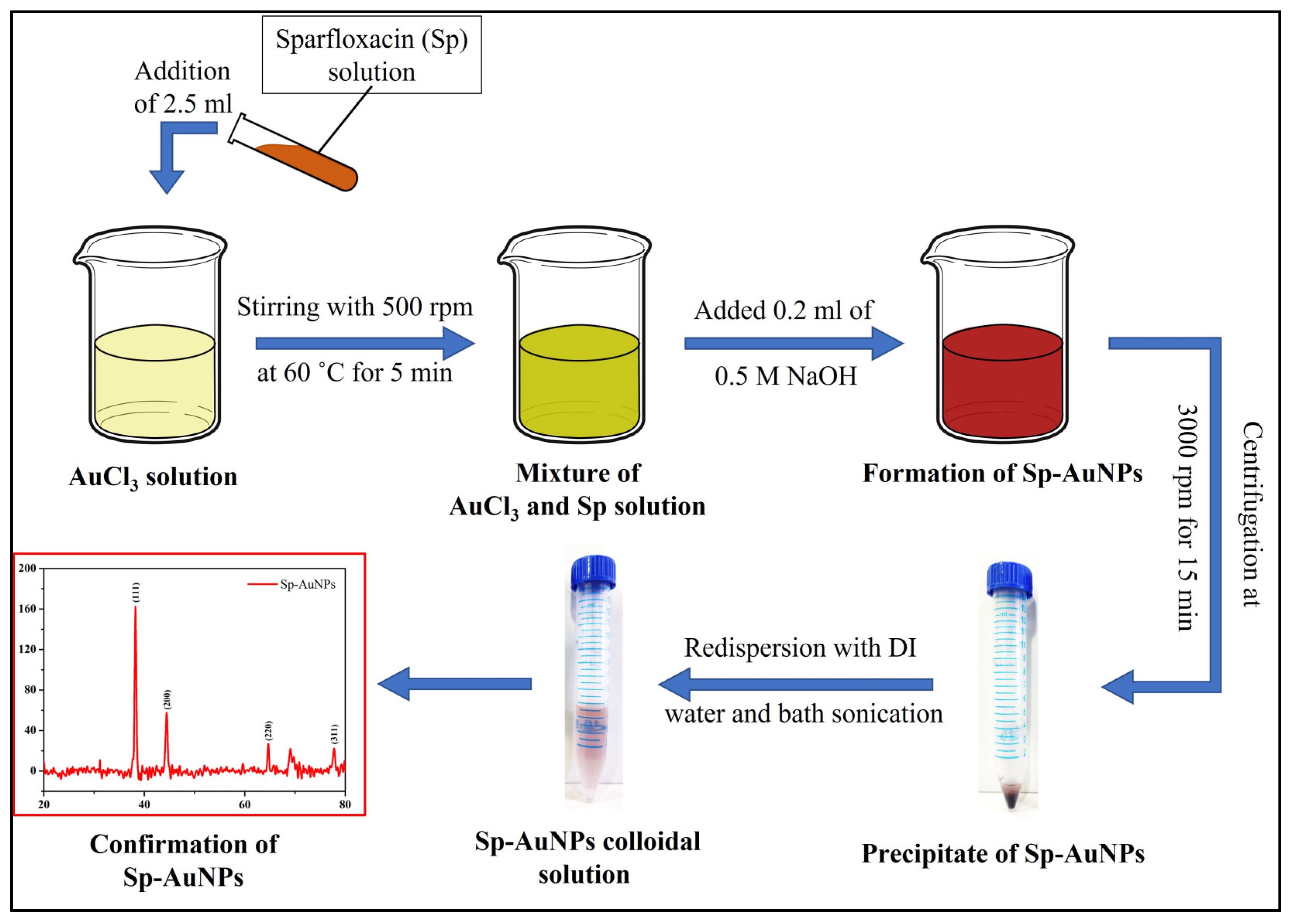
2.3. Preparation of Gold Nanoparticles (Sp-AuNPs)
2.4. Fabrication of Ethanol Sensor
2.5. Electrochemical Measurements
2.6. Preparation of Real-World Sample
3. Results and Discussion
3.1. Material Characterizations
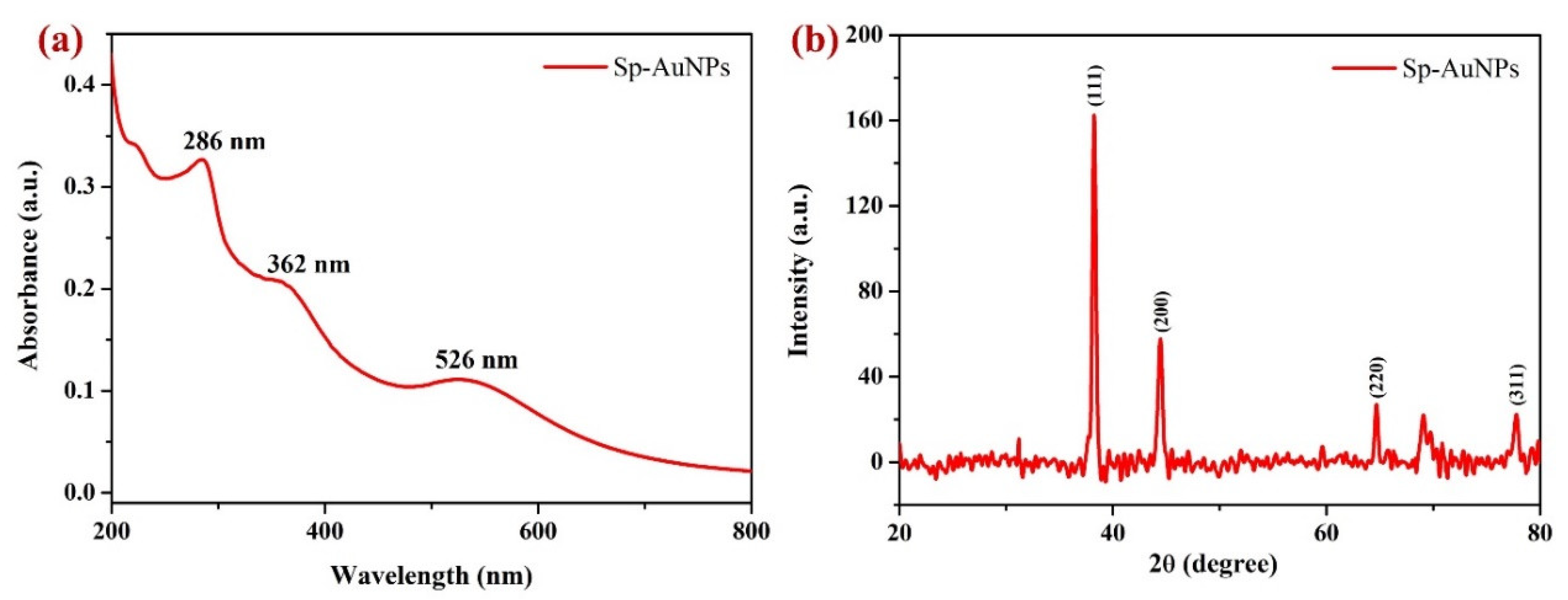
3.1.1. UV-Vis and X-ray Diffraction (XRD) Studies
3.1.2. Surface Morphology, Composition and Elemental Analysis
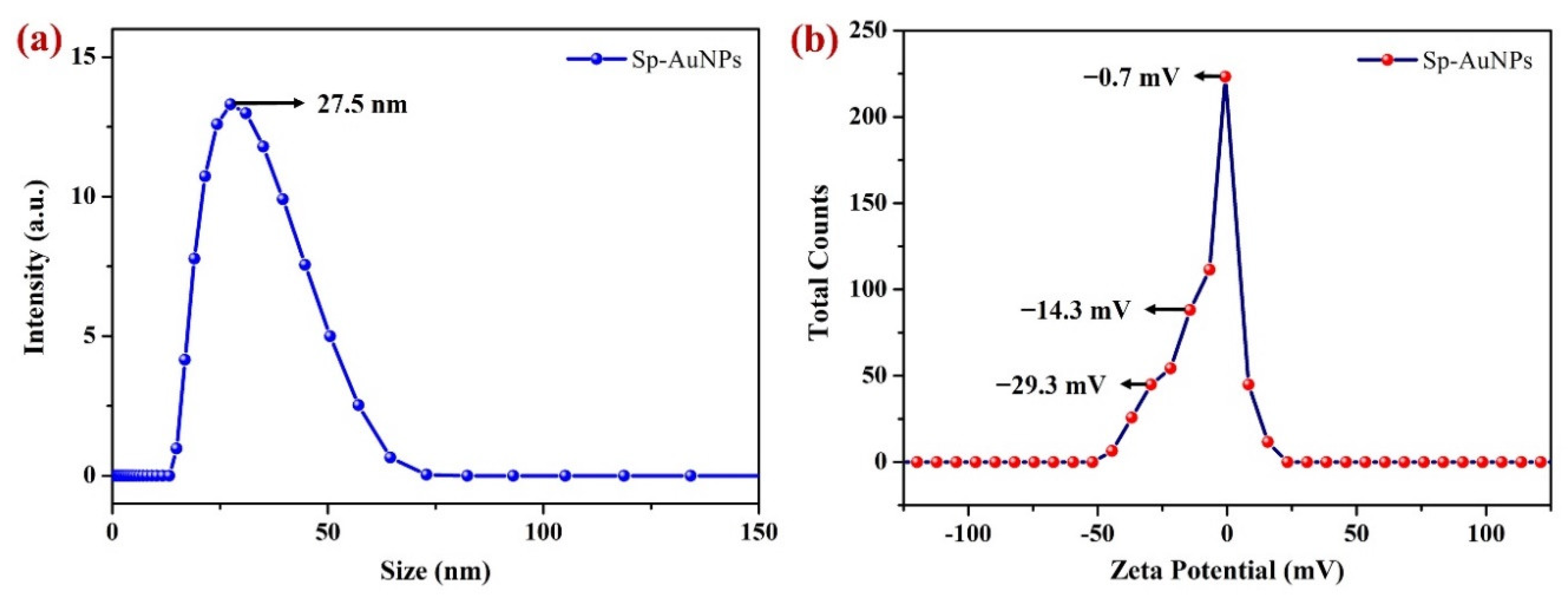
3.1.3. DLS and Zeta Potential Analysis
3.2. Electrochemical Analysis of Sp-AuNPs
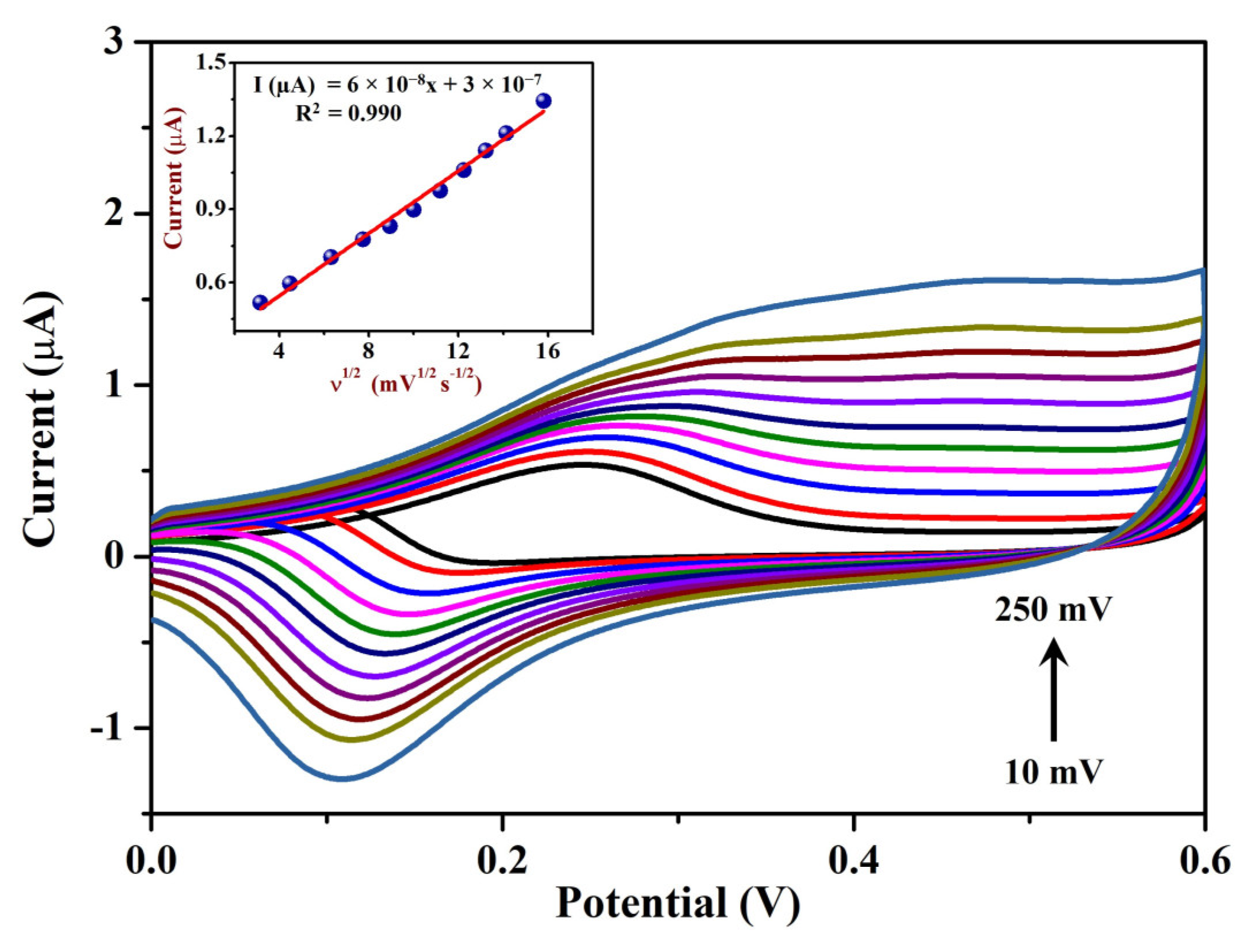
3.2.1. Cyclic Voltammetry (CV) and EIS
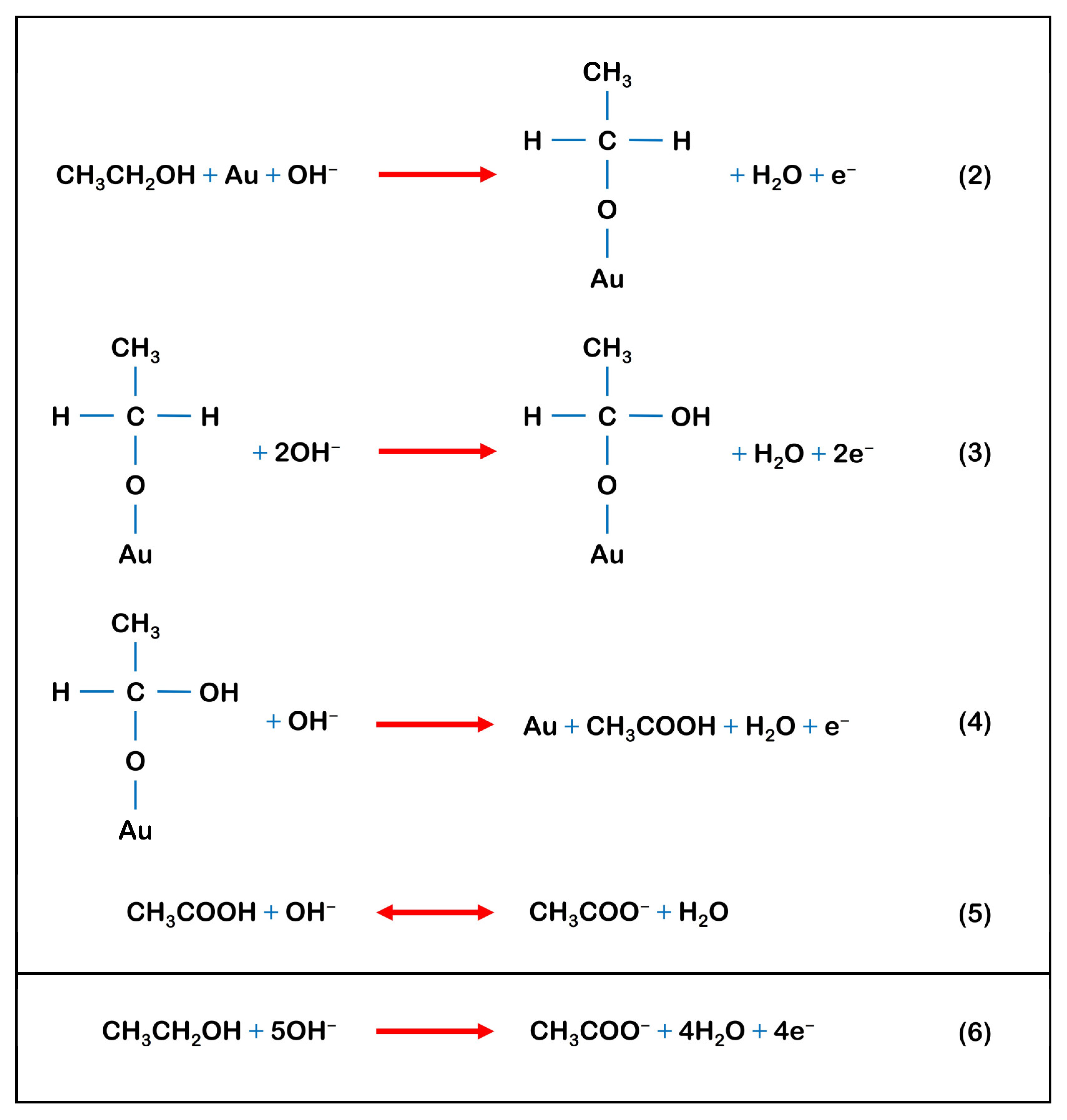
3.2.2. Electrochemical Oxidation of Ethanol (EtOH)
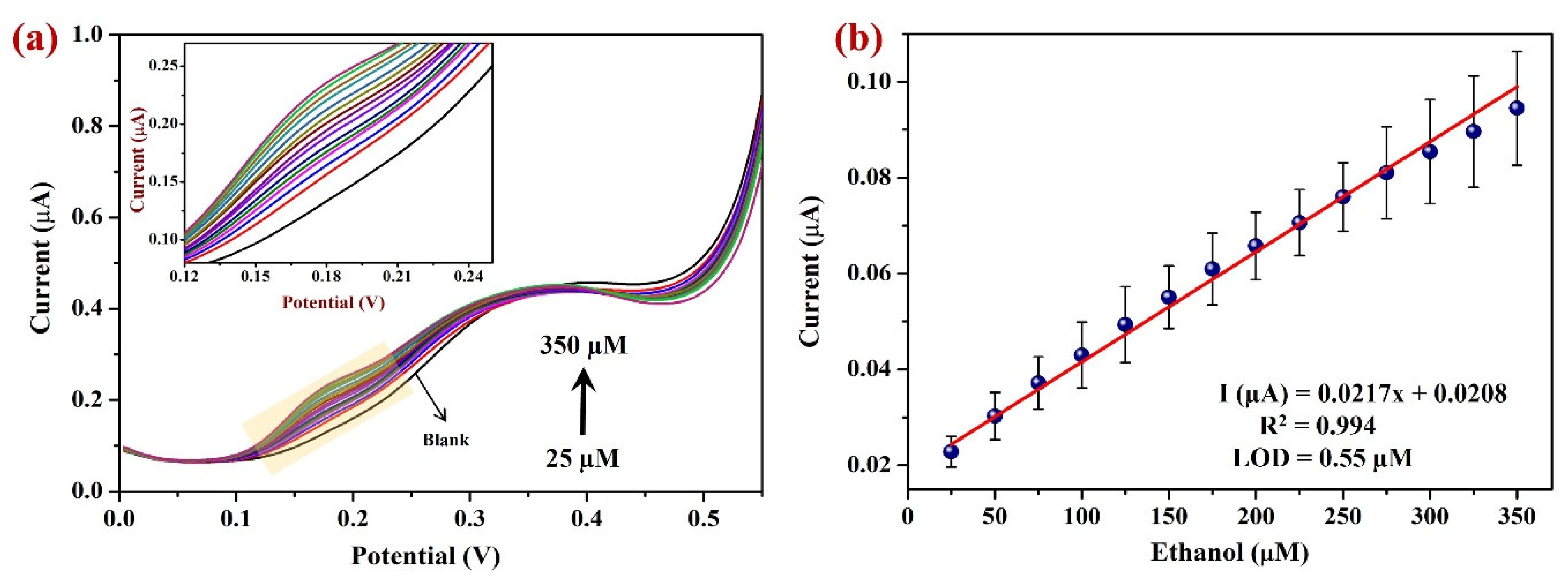
3.2.3. Determination of EtOH
3.2.4. Differential Pulse Voltammetry (DPV) Studies
3.2.5. Repeatability, Reproducibility, and Stability Studies
3.2.6. Interference and Real Sample Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Joudeh, N.; Linke, D. Nanoparticle Classification, Physicochemical Properties, Characterization, and Applications: A Comprehensive Review for Biologists. J. Nanobiotechnol. 2022, 20, 262. [Google Scholar] [CrossRef] [PubMed]
- Njoki, P.N.; Lim, I.-I.S.; Mott, D.; Park, H.-Y.; Khan, B.; Mishra, S.; Sujakumar, R.; Luo, J.; Zhong, C.-J. Size Correlation of Optical and Spectroscopic Properties for Gold Nanoparticles. J. Phys. Chem. C 2007, 111, 14664–14669. [Google Scholar] [CrossRef]
- Ahmad, T.; Wani, I.A.; Ahmed, J.; Al-Hartomy, O.A. Effect of Gold Ion Concentration on Size and Properties of Gold Nanoparticles in TritonX-100 Based Inverse Microemulsions. Appl. Nanosci. 2014, 4, 491–498. [Google Scholar] [CrossRef]
- Dheyab, M.A.; Aziz, A.A.; Oladzadabbasabadi, N.; Alsaedi, A.; Braim, F.S.; Jameel, M.S.; Ramizy, A.; Alrosan, M.; Almajwal, A.M. Comparative Analysis of Stable Gold Nanoparticles Synthesized Using Sonochemical and Reduction Methods for Antibacterial Activity. Molecules 2023, 28, 3931. [Google Scholar] [CrossRef]
- Philip, D. Synthesis and Spectroscopic Characterization of Gold Nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2008, 71, 80–85. [Google Scholar] [CrossRef]
- Kadhim, R.J.; Karsh, E.H.; Taqi, Z.J.; Jabir, M.S. Biocompatibility of Gold Nanoparticles: In-Vitro and In-Vivo Study. Mater. Today Proc. 2021, 42, 3041–3045. [Google Scholar] [CrossRef]
- Shalkevich, N.; Escher, W.; Bürgi, T.; Michel, B.; Si-Ahmed, L.; Poulikakos, D. On the Thermal Conductivity of Gold Nanoparticle Colloids. Langmuir 2010, 26, 663–670. [Google Scholar] [CrossRef]
- Alba-Molina, D.; Puente Santiago, A.R.; Giner-Casares, J.J.; Martín-Romero, M.T.; Camacho, L.; Luque, R.; Cano, M. Citrate-Stabilized Gold Nanoparticles as High-Performance Electrocatalysts: The Role of Size in the Electroreduction of Oxygen. J. Phys. Chem. C 2019, 123, 9807–9812. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Y.; Ding, T.; Liu, J.; Zhao, H. Multifunctional Gold Nanoparticles: A Novel Nanomaterial for Various Medical Applications and Biological Activities. Front. Bioeng. Biotechnol. 2020, 8, 990. [Google Scholar] [CrossRef]
- Yeh, Y.-C.; Creran, B.; Rotello, V.M. Gold Nanoparticles: Preparation, Properties, and Applications in Bionanotechnology. Nanoscale 2012, 4, 1871–1880. [Google Scholar] [CrossRef] [PubMed]
- Anjana, P.M.; Bindhu, M.R.; Rakhi, R.B. Green Synthesized Gold Nanoparticle Dispersed Porous Carbon Composites for Electrochemical Energy Storage. Mater. Sci. Energy Technol. 2019, 2, 389–395. [Google Scholar] [CrossRef]
- Gad, G.M.A.; Hegazy, M.A. Optoelectronic Properties of Gold Nanoparticles Synthesized by Using Wet Chemical Method. Mater. Res. Express 2019, 6, 85024. [Google Scholar] [CrossRef]
- Kumar, S.A.; Chang, Y.-T.; Wang, S.-F.; Lu, H.-C. Synthetic Antibacterial Agent Assisted Synthesis of Gold Nanoparticles: Characterization and Application Studies. J. Phys. Chem. Solids 2010, 71, 1484–1490. [Google Scholar] [CrossRef]
- Tyagi, H.; Kushwaha, A.; Kumar, A.; Aslam, M. A Facile PH Controlled Citrate-Based Reduction Method for Gold Nanoparticle Synthesis at Room Temperature. Nanoscale Res. Lett. 2016, 11, 362. [Google Scholar] [CrossRef]
- Zou, C.; Yang, B.; Bin, D.; Wang, J.; Li, S.; Yang, P.; Wang, C.; Shiraishi, Y.; Du, Y. Electrochemical Synthesis of Gold Nanoparticles Decorated Flower-like Graphene for High Sensitivity Detection of Nitrite. J. Colloid Interface Sci. 2017, 488, 135–141. [Google Scholar] [CrossRef]
- Hu, Y.; Song, Y.; Wang, Y.; Di, J. Electrochemical Synthesis of Gold Nanoparticles onto Indium Tin Oxide Glass and Application in Biosensors. Thin Solid Films 2011, 519, 6605–6609. [Google Scholar] [CrossRef]
- Lomelí-Rosales, D.A.; Zamudio-Ojeda, A.; Reyes-Maldonado, O.K.; López-Reyes, M.E.; Basulto-Padilla, G.C.; Lopez-Naranjo, E.J.; Zuñiga-Mayo, V.M.; Velázquez-Juárez, G. Green Synthesis of Gold and Silver Nanoparticles Using Leaf Extract of Capsicum Chinense Plant. Molecules 2022, 27, 1692. [Google Scholar] [CrossRef]
- Yu, J.; Xu, D.; Guan, H.N.; Wang, C.; Huang, L.K.; Chi, D.F. Facile One-Step Green Synthesis of Gold Nanoparticles Using Citrus Maxima Aqueous Extracts and Its Catalytic Activity. Mater. Lett. 2016, 166, 110–112. [Google Scholar] [CrossRef]
- Noruzi, M.; Zare, D.; Khoshnevisan, K.; Davoodi, D. Rapid Green Synthesis of Gold Nanoparticles Using Rosa Hybrida Petal Extract at Room Temperature. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 79, 1461–1465. [Google Scholar] [CrossRef]
- Dong, S.; Tang, C.; Zhou, H.; Zhao, H. Photochemical Synthesis of Gold Nanoparticles by the Sunlight Radiation Using a Seeding Approach. Gold Bull. 2004, 37, 187–195. [Google Scholar] [CrossRef]
- Pal, A. Photochemical Synthesis of Gold Nanoparticles via Controlled Nucleation Using a Bioactive Molecule. Mater. Lett. 2004, 58, 529–534. [Google Scholar] [CrossRef]
- Chauhan, A.; Zubair, S.; Tufail, S.; Sherwani, A.; Sajid, M.; Raman, S.C.; Azam, A.; Owais, M. Fungus-Mediated Biological Synthesis of Gold Nanoparticles: Potential in Detection of Liver Cancer. Int. J. Nanomed. 2011, 6, 2305–2319. [Google Scholar] [CrossRef]
- Liu, W.; Wang, L.; Wang, J.; Du, J.; Jing, C. New Insights into Microbial-Mediated Synthesis of Au@biolayer Nanoparticles. Environ. Sci. Nano 2018, 5, 1757–1763. [Google Scholar] [CrossRef]
- Gupta, R.; Padmanabhan, P. Biogenic Synthesis and Characterization of Gold Nanoparticles by a Novel Marine Bacteria Marinobacter Algicola: Progression from Nanospheres to Various Geometrical Shapes. J. Microbiol. Biotechnol. Food Sci. 2018, 8, 732. [Google Scholar] [CrossRef]
- Hammami, I.; Alabdallah, N.M.; Jomaa, A.A.; Kamoun, M. Gold Nanoparticles: Synthesis Properties and Applications. J. King Saud Univ. Sci. 2021, 33, 101560. [Google Scholar] [CrossRef]
- Deraedt, C.; Salmon, L.; Gatard, S.; Ciganda, R.; Hernandez, R.; Ruiz, J.; Astruc, D. Sodium Borohydride Stabilizes Very Active Gold Nanoparticle Catalysts. Chem. Commun. 2014, 50, 14194–14196. [Google Scholar] [CrossRef]
- Luty-Błocho, M.; Fitzner, K.; Hessel, V.; Löb, P.; Maskos, M.; Metzke, D.; Pacławski, K.; Wojnicki, M. Synthesis of Gold Nanoparticles in an Interdigital Micromixer Using Ascorbic Acid and Sodium Borohydride as Reducers. Chem. Eng. J. 2011, 171, 279–290. [Google Scholar] [CrossRef]
- Agunloye, E.; Panariello, L.; Gavriilidis, A.; Mazzei, L. A Model for the Formation of Gold Nanoparticles in the Citrate Synthesis Method. Chem. Eng. Sci. 2018, 191, 318–331. [Google Scholar] [CrossRef]
- Simeonova, S.; Georgiev, P.; Exner, K.S.; Mihaylov, L.; Nihtianova, D.; Koynov, K.; Balashev, K. Kinetic Study of Gold Nanoparticles Synthesized in the Presence of Chitosan and Citric Acid. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 557, 106–115. [Google Scholar] [CrossRef]
- Cabrera, G.F.S.; Balbin, M.M.; Eugenio, P.J.G.; Zapanta, C.S.; Monserate, J.J.; Salazar, J.R.; Mingala, C.N. Green Synthesis of Gold Nanoparticles Reduced and Stabilized by Sodium Glutamate and Sodium Dodecyl Sulfate. Biochem. Biophys. Res. Commun. 2017, 484, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Song, X.; Li, J.; Bai, Y.; Yang, W.; Peng, X. Size Control of Gold Nanocrystals in Citrate Reduction: The Third Role of Citrate. J. Am. Chem. Soc. 2007, 129, 13939–13948. [Google Scholar] [CrossRef] [PubMed]
- Dozol, H.; Mériguet, G.; Ancian, B.; Cabuil, V.; Xu, H.; Wang, D.; Abou-Hassan, A. On the Synthesis of Au Nanoparticles Using EDTA as a Reducing Agent. J. Phys. Chem. C 2013, 117, 20958–20966. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A Study of the Nucleation and Growth Processes in the Synthesis of Colloidal Gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Frens, G. Controlled Nucleation for the Regulation of the Particle Size in Monodisperse Gold Suspensions. Nat. Phys. Sci. 1973, 241, 20–22. [Google Scholar] [CrossRef]
- Brust, M.; Walker, M.; Bethell, D.; Schiffrin, D.J.; Whyman, R. Synthesis of Thiol-Derivatised Gold Nanoparticles in a Two-Phase Liquid–Liquid System. J. Chem. Soc. Chem. Commun. 1994, 7, 801–802. [Google Scholar] [CrossRef]
- Javed, R.; Zia, M.; Naz, S.; Aisida, S.O.; Ain, N.U.; Ao, Q. Role of Capping Agents in the Application of Nanoparticles in Biomedicine and Environmental Remediation: Recent Trends and Future Prospects. J. Nanobiotechnol. 2020, 18, 172. [Google Scholar] [CrossRef]
- Xaba, T.; Moloto, M.J.; Al-Shakban, M.; Malik, M.A.; Moloto, N.; O’Brien, P. The Influences of the Concentrations of “Green Capping Agents” as Stabilizers and of Ammonia as an Activator in the Synthesis of ZnS Nanoparticles and Their Polymer Nanocomposites. J. Green Process. Synth. 2017, 6, 173–182. [Google Scholar] [CrossRef]
- Pedroso-Santana, S.; Fleitas-Salazar, N. The Use of Capping Agents in the Stabilization and Functionalization of Metallic Nanoparticles for Biomedical Applications. Part. Part. Syst. Charact. 2023, 40, 2200146. [Google Scholar] [CrossRef]
- Wang, R.; Lang, J.; Liu, Y.; Lin, Z.; Yan, X. Ultra-Small, Size-Controlled Ni(OH)2 Nanoparticles: Elucidating the Relationship between Particle Size and Electrochemical Performance for Advanced Energy Storage Devices. NPG Asia Mater. 2015, 7, e183. [Google Scholar] [CrossRef]
- He, Y.T.; Wan, J.; Tokunaga, T. Kinetic Stability of Hematite Nanoparticles: The Effect of Particle Sizes. J. Nanopart. Res. 2008, 10, 321–332. [Google Scholar] [CrossRef]
- Bélteky, P.; Rónavári, A.; Zakupszky, D.; Boka, E.; Igaz, N.; Szerencsés, B.; Pfeiffer, I.; Vágvölgyi, C.; Kiricsi, M.; Kónya, Z. Are Smaller Nanoparticles Always Better? Understanding the Biological Effect of Size-Dependent Silver Nanoparticle Aggregation Under Biorelevant Conditions. Int. J. Nanomed. 2021, 16, 3021–3040. [Google Scholar] [CrossRef] [PubMed]
- Matei, I.; Buta, C.M.; Turcu, I.M.; Culita, D.; Munteanu, C.; Ionita, G. Formation and Stabilization of Gold Nanoparticles in Bovine Serum Albumin Solution. Molecules 2019, 24, 3395. [Google Scholar] [CrossRef] [PubMed]
- Nurakhmetova, Z.A.; Azhkeyeva, A.N.; Klassen, I.A.; Tatykhanova, G.S. Synthesis and Stabilization of Gold Nanoparticles Using Water-Soluble Synthetic and Natural Polymers. Polymers 2020, 12, 2625. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari, M.; Schwarzenberger, K.; Javadi, A.; Eckert, K. The Influence of Negatively Charged Silica Nanoparticles on the Surface Properties of Anionic Surfactants: Electrostatic Repulsion or the Effect of Ionic Strength? Phys. Chem. Chem. Phys. 2020, 22, 2238–2248. [Google Scholar] [CrossRef] [PubMed]
- Aggrawal, R.; Halder, S.; Dyagala, S.; Saha, S.K. Refolding of Denatured Gold Nanoparticles-Conjugated Bovine Serum Albumin through Formation of Catanions between Gemini Surfactant and Sodium Dodecyl Sulphate. RSC Adv. 2022, 12, 16014–16028. [Google Scholar] [CrossRef] [PubMed]
- Din, A.T.U.; Alam, F.; Tameez-ud-din, A.; Chaudhary, F.M.D. Auto-Brewery Syndrome: A Clinical Dilemma. Cureus 2020, 12, e10983. [Google Scholar] [CrossRef]
- Hafez, E.M.; Hamad, M.A.; Fouad, M.; Abdel-Lateff, A. Auto-Brewery Syndrome: Ethanol Pseudo-Toxicity in Diabetic and Hepatic Patients. Hum. Exp. Toxicol. 2016, 36, 445–450. [Google Scholar] [CrossRef]
- Welch, B.T.; Coelho Prabhu, N.; Walkoff, L.; Trenkner, S.W. Auto-Brewery Syndrome in the Setting of Long-Standing Crohn’s Disease: A Case Report and Review of the Literature. J. Crohn’s Colitis 2016, 10, 1448–1450. [Google Scholar] [CrossRef]
- Cordell, B.J.; Kanodia, A.; Miller, G.K. Case–Control Research Study of Auto-Brewery Syndrome. Glob. Adv. Heal. Med. 2019, 8, 2164956119837566. [Google Scholar] [CrossRef]
- O’Neal, C.L.; Poklis, A. Postmortem Production of Ethanol and Factors That Influence Interpretation: A Critical Review. Am. J. Forensic Med. Pathol. 1996, 17, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Kugelberg, F.C.; Jones, A.W. Interpreting Results of Ethanol Analysis in Postmortem Specimens: A Review of the Literature. Forensic Sci. Int. 2007, 165, 10–29. [Google Scholar] [CrossRef] [PubMed]
- Harper, D.R. A Comparative Study of the Microbiological Contamination of Postmortem Blood and Vitreous Humour Samples Taken for Ethanol Determination. Forensic Sci. Int. 1989, 43, 37–44. [Google Scholar] [CrossRef] [PubMed]
- National Collaborating Centre for Mental Health. Diagnoses Assessment and Management of Harmful Drinking and Alcohol Dependence. NICE Clinical Guidelines, No. 115; The British Psychological Society: Leicester, UK; The Royal College of Psychiatrists: London, UK, 2011; ISBN 9781904671268. [Google Scholar]
- Scholar, E. Sparfloxacin. In xPharm: The Comprehensive Pharmacology Reference; Elsevier: Amsterdam, The Netherlands, 2007; pp. 1–8. [Google Scholar] [CrossRef]
- Goa, K.L.; Bryson, H.M.; Markham, A. Sparfloxacin. Drugs 1997, 53, 700–725. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Starostin, S.A.; Li, S.; Hessel, V. Synthesis of Metallic Nanoparticles by Microplasma. Phys. Sci. Rev. 2018, 3, 20170121. [Google Scholar] [CrossRef]
- Khalil, I.; Julkapli, N.M.; Yehye, W.A.; Basirun, W.J.; Bhargava, S.K. Graphene–Gold Nanoparticles Hybrid—Synthesis, Functionalization, and Application in a Electrochemical and Surface-Enhanced Raman Scattering Biosensor. Materials 2016, 9, 406. [Google Scholar] [CrossRef]
- Kamalasekaran, K.; Magesh, V.; Atchudan, R.; Arya, S.; Sundramoorthy, A.K. Development of Electrochemical Sensor Using Iron (III) Phthalocyanine/Gold Nanoparticle/Graphene Hybrid Film for Highly Selective Determination of Nicotine in Human Salivary Samples. Biosensors 2023, 13, 839. [Google Scholar] [CrossRef]
- Wan, H.; Sun, Q.; Li, H.; Sun, F.; Hu, N.; Wang, P. Screen-Printed Gold Electrode with Gold Nanoparticles Modification for Simultaneous Electrochemical Determination of Lead and Copper. Sensors Actuators B Chem. 2015, 209, 336–342. [Google Scholar] [CrossRef]
- Kumar, A.S.; Zen, J.-M. Electrochemical Investigation of Glucose Sensor Fabricated at Copper-Plated Screen-Printed Carbon Electrodes. Electroanalysis 2002, 14, 671–678. [Google Scholar] [CrossRef]
- Karikalan, N.; Sundaresan, P.; Chen, S.-M.; Karthik, R. Exploring the Electrocatalytic Edge Plane Activity of Screen Printed Carbon Electrode and Various Carbonaceous Materials towards the Catecholic Derivatives. J. Electrochem. Soc. 2018, 165, H969. [Google Scholar] [CrossRef]
- Hernández, H.H.; Reynoso, A.M.R.; González, J.C.T.; Morán, C.O.G.; Hernández, J.G.M.; Ruiz, A.M.; Hernández, J.M.; Cruz, R.O. Electrochemical Impedance Spectroscopy (EIS): A Review Study of Basic Aspects of the Corrosion Mechanism Applied to Steels. In Electrochemical Impedance Spectroscopy; El-Azazy, M., Min, M., Annus, P., Eds.; IntechOpen: Rijeka, Croatia, 2020; pp. 137–144. ISBN 978-1-78985-216-5. [Google Scholar]
- Charoenkitamorn, K.; Siangproh, W.; Chailapakul, O.; Oyama, M.; Chaneam, S. Simple Portable Voltammetric Sensor Using Anodized Screen-Printed Graphene Electrode for the Quantitative Analysis of p-Hydroxybenzoic Acid in Cosmetics. ACS Omega 2022, 7, 16116–16126. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Wang, Y.; Song, Z.; Feng, Y.; Chen, Y.; Zhang, D.; Feng, L. The Basic Properties of Gold Nanoparticles and Their Applications in Tumor Diagnosis and Treatment. Int. J. Mol. Sci. 2020, 21, 2480. [Google Scholar] [CrossRef] [PubMed]
- Guglielmelli, A.; Pierini, F.; Tabiryan, N.; Umeton, C.; Bunning, T.J.; De Sio, L. Thermoplasmonics with Gold Nanoparticles: A New Weapon in Modern Optics and Biomedicine. Adv. Photonics Res. 2021, 2, 2000198. [Google Scholar] [CrossRef]
- López-Muñoz, G.A.; Pescador-Rojas, J.A.; Ortega-Lopez, J.; Salazar, J.S.; Balderas-López, J.A. Thermal Diffusivity Measurement of Spherical Gold Nanofluids of Different Sizes/Concentrations. Nanoscale Res. Lett. 2012, 7, 423. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Astruc, D. Nanogold Plasmonic Photocatalysis for Organic Synthesis and Clean Energy Conversion. Chem. Soc. Rev. 2014, 43, 7188–7216. [Google Scholar] [CrossRef] [PubMed]
- Saleh, N.M.; Aziz, A.A. Simulation of Surface Plasmon Resonance on Different Size of a Single Gold Nanoparticle. J. Phys. Conf. Ser. 2018, 1083, 12041. [Google Scholar] [CrossRef]
- Trivedi, M.K.; Dahryn Trivedi, A.B.; Khemraj Bairwa, H.S. Fourier Transform Infrared and Ultraviolet-Visible Spectroscopic Characterization of Biofield Treated Salicylic Acid and Sparfloxacin. Nat. Prod. Chem. Res. 2015, 3, 1000186. [Google Scholar] [CrossRef]
- Rajakumar, G.; Gomathi, T.; Abdul Rahuman, A.; Thiruvengadam, M.; Mydhili, G.; Kim, S.-H.; Lee, T.-J.; Chung, I.-M. Biosynthesis and Biomedical Applications of Gold Nanoparticles Using Eclipta Prostrata Leaf Extract. Appl. Sci. 2016, 6, 222. [Google Scholar] [CrossRef]
- Ren, X.; Song, Y.; Liu, A.; Zhang, J.; Yang, P.; Zhang, J.; An, M. Experimental and Theoretical Studies of DMH as a Complexing Agent for a Cyanide-Free Gold Electroplating Electrolyte. RSC Adv. 2015, 5, 64997–65004. [Google Scholar] [CrossRef]
- Krishnamurthy, S.; Esterle, A.; Sharma, N.C.; Sahi, S. V Yucca-Derived Synthesis of Gold Nanomaterial and Their Catalytic Potential. Nanoscale Res. Lett. 2014, 9, 627. [Google Scholar] [CrossRef]
- Holzwarth, U.; Gibson, N. The Scherrer Equation versus the “Debye-Scherrer Equation”. Nat. Nanotechnol. 2011, 6, 534. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Rahman, A.K.; Ghann, W.; Uddin, J.; Kang, H.G. Terahertz Multispectral Imaging for the Analysis of Gold Nanoparticles’ Size and the Number of Unit Cells in Comparison with Other Techniques. Int. J. Biosens. Bioelectron. 2018, 4, 169. [Google Scholar] [CrossRef]
- Weisbecker, C.S.; Merritt, M.V.; Whitesides, G.M. Molecular Self-Assembly of Aliphatic Thiols on Gold Colloids. Langmuir 1996, 12, 3763–3772. [Google Scholar] [CrossRef]
- Jayalakshmi, A.; Kim, I.-C.; Kwon, Y.-N. Suppression of Gold Nanoparticle Agglomeration and Its Separation via Nylon Membranes. Chin. J. Chem. Eng. 2017, 25, 931–937. [Google Scholar] [CrossRef]
- Ivanov, M.R.; Bednar, H.R.; Haes, A.J. Investigations of the Mechanism of Gold Nanoparticle Stability and Surface Functionalization in Capillary Electrophoresis. ACS Nano 2009, 3, 386–394. [Google Scholar] [CrossRef]
- Pardo-Yissar, V.; Katz, E.; Lioubashevski, O.; Willner, I. Layered Polyelectrolyte Films on Au Electrodes: Characterization of Electron-Transfer Features at the Charged Polymer Interface and Application for Selective Redox Reactions. Langmuir 2001, 17, 1110–1118. [Google Scholar] [CrossRef]
- Magar, H.S.; Hassan, R.Y.A.; Mulchandani, A. Electrochemical Impedance Spectroscopy (EIS): Principles, Construction, and Biosensing Applications. Sensors 2021, 21, 6578. [Google Scholar] [CrossRef]
- Burke, L.D.; Nugent, P.F. The Electrochemistry of Gold: II the Electrocatalytic Behaviour of the Metal in Aqueous Media. Gold Bull. 1998, 31, 39–50. [Google Scholar] [CrossRef]
- De Lima, R.B.; Varela, H. Catalytic Oxidation of Ethanol on Gold Electrode in Alkaline Media. Gold Bull. 2008, 41, 15–22. [Google Scholar] [CrossRef]
- Weinberg, W.H. Eley−Rideal Surface Chemistry: Direct Reactivity of Gas Phase Atomic Hydrogen with Adsorbed Species. Acc. Chem. Res. 1996, 29, 479–487. [Google Scholar] [CrossRef]
- Bełtowska-Brzezinska, M. Untersuchungen Zum Mechanismus Der Elektrochemischen Oxidation von Alkoholen an Gold. Electrochim. Acta 1980, 25, 267–271. [Google Scholar] [CrossRef]
- Holze, R.; Beŧowska-Brzezinska, M. On the Adsorption of Aliphatic Alcohols on Gold. Electrochim. Acta 1985, 30, 937–939. [Google Scholar] [CrossRef]
- Bełtowska-Brzezinska, M.; Łuczak, T.; Holze, R. Electrocatalytic Oxidation of Mono- and Polyhydric Alcohols on Gold and Platinum. J. Appl. Electrochem. 1997, 27, 999–1011. [Google Scholar] [CrossRef]
- Tremiliosi-Filho, G.; Gonzalez, E.R.; Motheo, A.J.; Belgsir, E.M.; Léger, J.-M.; Lamy, C. Electro-Oxidation of Ethanol on Gold: Analysis of the Reaction Products and Mechanism. J. Electroanal. Chem. 1998, 444, 31–39. [Google Scholar] [CrossRef]
- Jena, B.K.; Raj, C.R. Synthesis of Flower-like Gold Nanoparticles and Their Electrocatalytic Activity Towards the Oxidation of Methanol and the Reduction of Oxygen. Langmuir 2007, 23, 4064–4070. [Google Scholar] [CrossRef]
- Lou, Y.; Maye, M.M.; Han, L.; Luo, J.; Zhong, C.-J. Gold–Platinum Alloy Nanoparticle Assembly as Catalyst for Methanol Electrooxidation. Chem. Commun. 2001, 5, 473–474. [Google Scholar] [CrossRef]
- Janda, D.C.; Barma, K.; Kurapati, N.; Klymenko, O.V.; Oleinick, A.; Svir, I.; Amatore, C.; Amemiya, S. Systematic Assessment of Adsorption-Coupled Electron Transfer toward Voltammetric Discrimination between Concerted and Non-Concerted Mechanisms. Electrochim. Acta 2022, 428, 140912. [Google Scholar] [CrossRef]
- Magesh, V.; Sundramoorthy, A.K.; Ganapathy, D.; Atchudan, R.; Arya, S.; Alshgari, R.A.; Aljuwayid, A.M. Palladium Hydroxide (Pearlman’s Catalyst) Doped MXene (Ti3C2Tx) Composite Modified Electrode for Selective Detection of Nicotine in Human Sweat. Biosensors 2023, 13, 54. [Google Scholar] [CrossRef]
- Hatamie, A.; Rezvani, E.; Rasouli, A.S.; Simchi, A. Electrocatalytic Oxidation of Ethanol on Flexible Three-Dimensional Interconnected Nickel/Gold Composite Foams in Alkaline Media. Electroanalysis 2019, 31, 504–511. [Google Scholar] [CrossRef]
- Benchettara, A. Electrochemical Determination of Ethanol, 2-Propanol and 1-Butanol on Glassy Carbon Electrode Modified with Nickel Oxide Film. J. Fundam. Appl. Sci. 2014, 6, 187–196. [Google Scholar] [CrossRef]
- Yu, Y.; Zhai, M.; Hu, J. Electrocatalytic Oxidation of Ethanol and Ethylene Glycol on Bimetallic Ni and Ti Nanoparticle-Modified Indium Tin Oxide Electrode in Alkaline Solution. Prog. Nat. Sci. Mater. Int. 2019, 29, 511–516. [Google Scholar] [CrossRef]
- Simões, F.R.; Codognoto, L.; Lima, T.d.S.; Fernandes-Filho, C. Pulsed Voltammetry for Monitoring of Emerging Contaminants. In Encyclopedia of Solid-Liquid Interfaces, 1st ed.; Wandelt, K., Bussetti, G., Eds.; Elsevier: Oxford, UK, 2023; pp. 450–460. ISBN 978-0-323-85670-6. [Google Scholar]
- Prete, M.C.; Rocha, L.R.; Tarley, C.R.T. Chapter 4—Development of New Electroanalytical Method Based on Graphene Oxide-Modified Glassy Carbon Electrode for Mephedrone Illicit Drug Determination. In Carbon Nanomaterials-Based Sensors Emerging Research Trends in Devices and Applications; Manjunatha, J.G., Hussain, C.M., Eds.; Elsevier: New York, NY, USA, 2022; pp. 43–56. ISBN 978-0-323-91174-0. [Google Scholar]
- Faisal, M.; Khan, S.B.; Rahman, M.M.; Jamal, A.; Akhtar, K.; Abdullah, M.M. Role of ZnO-CeO2 Nanostructures as a Photo-Catalyst and Chemi-Sensor. J. Mater. Sci. Technol. 2011, 27, 594–600. [Google Scholar] [CrossRef]
- Ismail, A.A.; Harraz, F.A.; Faisal, M.; El-Toni, A.M.; Al-Hajry, A.; Al-Assiri, M.S. A Facile Synthesis of Mesoporous PdZnO Nanocomposites as Efficient Chemical Sensor. Superlattices Microstruct. 2016, 95, 128–139. [Google Scholar] [CrossRef]
- Abdullah, M.M.; Rahman, M.M.; Bouzid, H.; Faisal, M.; Khan, S.B.; Al-Sayari, S.A.; Ismail, A.A. Sensitive and Fast Response Ethanol Chemical Sensor Based on As-Grown Gd2O3 Nanostructures. J. Rare Earths 2015, 33, 214–220. [Google Scholar] [CrossRef]
- Umar, A.; Al-Hazmi, F.; Dar, G.N.; Zaidi, S.A.; Al-Tuwirqi, R.M.; Alnowaiserb, F.; Al-Ghamdi, A.A.; Hwang, S.W. Ultra-Sensitive Ethanol Sensor Based on Rapidly Synthesized Mg(OH)2 Hexagonal Nanodisks. Sens. Actuators B Chem. 2012, 166–167, 97–102. [Google Scholar] [CrossRef]
- Faisal, M.; Khan, S.B.; Rahman, M.M.; Jamal, A.; Umar, A. Ethanol Chemi-Sensor: Evaluation of Structural, Optical and Sensing Properties of CuO Nanosheets. Mater. Lett. 2011, 65, 1400–1403. [Google Scholar] [CrossRef]
- Al-Hazmi, F.; Umar, A.; Dar, G.N.; Al-Ghamdi, A.A.; Al-Sayari, S.A.; Al-Hajry, A.; Kim, S.H.; Al-Tuwirqi, R.M.; Alnowaiserb, F.; El-Tantawy, F. Microwave Assisted Rapid Growth of Mg(OH)2 Nanosheet Networks for Ethanol Chemical Sensor Application. J. Alloys Compd. 2012, 519, 4–8. [Google Scholar] [CrossRef]
- Alqahtani, M.M.; Ali, A.M.; Harraz, F.A.; Faisal, M.; Ismail, A.A.; Sayed, M.A.; Al-Assiri, M.S. Highly Sensitive Ethanol Chemical Sensor Based on Novel Ag-Doped Mesoporous α–Fe2O3 Prepared by Modified Sol-Gel Process. Nanoscale Res. Lett. 2018, 13, 157. [Google Scholar] [CrossRef]
- Harraz, F.A.; Ismail, A.A.; Ibrahim, A.A.; Al-Sayari, S.A.; Al-Assiri, M.S. Highly Sensitive Ethanol Chemical Sensor Based on Nanostructured SnO2 Doped ZnO Modified Glassy Carbon Electrode. Chem. Phys. Lett. 2015, 639, 238–242. [Google Scholar] [CrossRef]
- Boroujerdi, R.; Paul, R. Introducing Graphene–Indium Oxide Electrochemical Sensor for Detecting Ethanol in Aqueous Samples with CCD-RSM Optimization. Chemosensors 2022, 10, 42. [Google Scholar] [CrossRef]
- Guo, K.; Qian, K.; Zhang, S.; Kong, J.; Yu, C.; Liu, B. Bio-Electrocatalysis of NADH and Ethanol Based on Graphene Sheets Modified Electrodes. Talanta 2011, 85, 1174–1179. [Google Scholar] [CrossRef] [PubMed]
- Boroujerdi, R.; Abdelkader, A.; Paul, R. Highly Selective Detection of Ethanol in Biological Fluids and Alcoholic Drinks Using Indium Ethylenediamine Functionalized Graphene. Sens. Diagn. 2022, 1, 566–578. [Google Scholar] [CrossRef]
- Aydoğdu Tığ, G. Highly Sensitive Amperometric Biosensor for Determination of NADH and Ethanol Based on Au-Ag Nanoparticles/Poly(L-Cysteine)/Reduced Graphene Oxide Nanocomposite. Talanta 2017, 175, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Shan, C.; Yang, H.; Han, D.; Zhang, Q.; Ivaska, A.; Niu, L. Electrochemical Determination of NADH and Ethanol Based on Ionic Liquid-Functionalized Graphene. Biosens. Bioelectron. 2010, 25, 1504–1508. [Google Scholar] [CrossRef]
- Xu, C.; Sun, F.; Gao, H.; Wang, J. Nanoporous Platinum–Cobalt Alloy for Electrochemical Sensing for Ethanol, Hydrogen Peroxide, and Glucose. Anal. Chim. Acta 2013, 780, 20–27. [Google Scholar] [CrossRef]
- Xu, C.; Wang, J.; Zhou, J. Nanoporous PtNi Alloy as an Electrochemical Sensor for Ethanol and H2O2. Sens. Actuators B Chem. 2013, 182, 408–415. [Google Scholar] [CrossRef]
- Zhu, J.; Wu, X.-Y.; Shan, D.; Yuan, P.-X.; Zhang, X.-J. Sensitive Electrochemical Detection of NADH and Ethanol at Low Potential Based on Pyrocatechol Violet Electrodeposited on Single Walled Carbon Nanotubes-Modified Pencil Graphite Electrode. Talanta 2014, 130, 96–102. [Google Scholar] [CrossRef]
- Tao, B.; Zhang, J.; Hui, S.; Wan, L. An Amperometric Ethanol Sensor Based on a Pd–Ni/SiNWs Electrode. Sens. Actuators B Chem. 2009, 142, 298–303. [Google Scholar] [CrossRef]
- Maddu, N. Functions of Saliva. In Saliva and Salivary Diagnostics; Gokul, S., Ed.; IntechOpen: Rijeka, Croatia, 2019; Chapter 2; ISBN 978-1-78984-310-1. [Google Scholar]
- Mäkilä, E.; Kirveskari, P. A Study of Ascorbic Acid in Human Saliva. Arch. Oral Biol. 1969, 14, 1285–1292. [Google Scholar] [CrossRef]
- Jaiswal, A.; Madaan, S.; Acharya, N.; Kumar, S.; Talwar, D.; Dewani, D. Salivary Uric Acid: A Noninvasive Wonder for Clinicians? Cureus 2021, 13, e19649. [Google Scholar] [CrossRef]
- Ferrag, C.; Kerman, K. Grand Challenges in Nanomaterial-Based Electrochemical Sensors. Front. Sens. 2020, 1, 583822. [Google Scholar] [CrossRef]
- Oliveira, B.B.; Ferreira, D.; Fernandes, A.R.; Baptista, P.V. Engineering Gold Nanoparticles for Molecular Diagnostics and Biosensing. WIREs Nanomed. Nanobiotechnol. 2023, 15, e1836. [Google Scholar] [CrossRef] [PubMed]







| Hkl Value | 2θ | FWHM | β = π/180 × FWHM | Cos θ | Crystalline Size–D (nm) |
|---|---|---|---|---|---|
| 111 | 38.23 | 0.3919 | 0.0068399 | 0.9448 | 23.3 |
| 200 | 44.42 | 0.5082 | 0.0088697 | 0.9258 | 18.3 |
| 220 | 64.67 | 0.3576 | 0.0062412 | 0.8449 | 28.6 |
| 311 | 77.75 | 0.4562 | 0.0079621 | 0.7785 | 24.3 |
| Average crystalline size (nm) | 23.6 | ||||
| Sensing Material | Method | Sensitivity (μA mM−1 cm−2) | Linear Range (mM) | LOD (µM) | References |
|---|---|---|---|---|---|
| ZnO-CeO2 | I-V characteristics | 0.833 | 0.17–17.04 | 160 | [97] |
| Mesoporous Pd–ZnO | I-V characteristics | 33.08 | 0.05–0.8 | 19.2 | [98] |
| Gd2O3 | I-V characteristics | 0.266 | 0.17–850 | 52.2 | [99] |
| Mg (OH)2 hexagonal nano disks | I-V characteristics | 6.89 | 0.0001–10 | 0.073 | [100] |
| CuO nanosheets | I-V characteristics | 0.972 | 0.17–1700 | 143 | [101] |
| Mg (OH)2 nanosheet | I-V characteristics | 3.99 | 0.01–1000 | 5 | [102] |
| Mesoporous Ag/α–Fe2O3 | I-V characteristics | 2.93 | 0.8–15 | 15.4 | [103] |
| SnO2 doped ZnO | I-V characteristics | 62.56 | 0.195–25 | 137 | [104] |
| Gr/In2O3 | CV | – | 100–1200 | 68 mM | [105] |
| ADH/Gr/GCE | AMP | – | 0.2–21 | 25 | [106] |
| rGO/In-en | CV | – | 0.1–3000 | 100 | [107] |
| Au-AgNPs/P(L-Cys)-rGO | C-AMP | 0.644 | 0.017–658 | 5 | [108] |
| IL-graphene/chitosan/ADH | C-AMP | 6.91 | 0.025–0.2 | 5 | [109] |
| NP–Pt/Co alloy | AMP | - | 0.2–12 | 8 | [110] |
| NP–Pt/Ni | AMP | - | 0.2–11 | 10 | [111] |
| PGE/SWCNTs/PCV/ADH | AMP | 1.94 | 0.009–0.32 | - | [112] |
| Pd–Ni/Si-NWs | AMP | 0.76 mA | 0–20.4 | 10 | [113] |
| Sp-AuNPs | DPV | 0.31 µA µM−1 cm2 | 0.025–0.35 | 0.55 | Current work |
| S. No. | Samples | Added (mM) | Found (mM) | Recovery (%) | SD | RSD% |
|---|---|---|---|---|---|---|
| 1 | Human saliva | - | 1.56 | - | 0.421 | 26.956 |
| 2 | Spiked EtOH | 8.56 | 10.09 | 99.68 | 0.456 | 5.347 |
| 3 | Spiked EtOH | 17.1 | 17.08 | 99.87 | 0.291 | 1.703 |
| 4 | Spiked EtOH | 34.1 | 35.19 | 103.18 | 0.324 | 0.922 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magesh, V.; Kothari, V.S.; Ganapathy, D.; Atchudan, R.; Arya, S.; Nallaswamy, D.; Sundramoorthy, A.K. Using Sparfloxacin-Capped Gold Nanoparticles to Modify a Screen-Printed Carbon Electrode Sensor for Ethanol Determination. Sensors 2023, 23, 8201. https://doi.org/10.3390/s23198201
Magesh V, Kothari VS, Ganapathy D, Atchudan R, Arya S, Nallaswamy D, Sundramoorthy AK. Using Sparfloxacin-Capped Gold Nanoparticles to Modify a Screen-Printed Carbon Electrode Sensor for Ethanol Determination. Sensors. 2023; 23(19):8201. https://doi.org/10.3390/s23198201
Chicago/Turabian StyleMagesh, Vasanth, Vishaka S. Kothari, Dhanraj Ganapathy, Raji Atchudan, Sandeep Arya, Deepak Nallaswamy, and Ashok K. Sundramoorthy. 2023. "Using Sparfloxacin-Capped Gold Nanoparticles to Modify a Screen-Printed Carbon Electrode Sensor for Ethanol Determination" Sensors 23, no. 19: 8201. https://doi.org/10.3390/s23198201







