Enhancing the Longevity and Functionality of Ti-Ag Dry Electrodes for Remote Biomedical Applications: A Comprehensive Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Ti-Ag Dry Electrodes
2.2. Ti-Ag Thin Film Characterization
2.3. Ti-Ag Electrode Lifespan
3. Results
3.1. Composition and Microstructural Analysis
3.2. Ti-Ag Electrode Lifespan
3.2.1. Optical Evaluation
3.2.2. Chemical Analysis
3.2.3. Electrical Behavior
3.2.4. Voltammetry Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Papa, A.; Mital, M.; Pisano, P.; Del Giudice, M. E-health and wellbeing monitoring using smart healthcare devices: An empirical investigation. Technol. Forecast. Soc. Change 2020, 153, 119226. [Google Scholar] [CrossRef]
- Garcia-Moreno, F.M.; Bermudez-Edo, M.; Garrido, J.L.; Rodríguez-García, E.; Pérez-Mármol, J.M.; Rodríguez-Fórtiz, M.J. A Microservices e-Health System for Ecological Frailty Assessment Using Wearables. Sensors 2020, 20, 3427. [Google Scholar] [CrossRef]
- Ha, S.; Kim, C.; Chi, Y.M.; Akinin, A.; Maier, C.; Ueno, A.; Cauwenberghs, G. Integrated Circuits and Electrode Interfaces for Noninvasive Physiological Monitoring. IEEE Trans. Biomed. Eng. 2014, 61, 1522–1537. [Google Scholar] [CrossRef]
- Oldroyd, P.; Malliaras, G.G. Achieving long-term stability of thin-film electrodes for neurostimulation. Acta Biomater. 2022, 139, 65–81. [Google Scholar] [CrossRef]
- Popović, D.B. Advances in functional electrical stimulation (FES). J. Electromyogr. Kinesiol. 2014, 24, 795–802. [Google Scholar] [CrossRef]
- Frigo, C.; Ferrarin, M.; Frasson, W.; Pavan, E.; Thorsen, R. EMG signals detection and processing for on-line control of functional electrical stimulation. J. Electromyogr. Kinesiol. 2000, 10, 351–360. [Google Scholar] [CrossRef]
- Zheng, X.S.; Tan, C.; Castagnola, E.; Cui, X.T. Electrode Materials for Chronic Electrical Microstimulation. Adv. Healthc. Mater. 2021, 10, 2100119. [Google Scholar] [CrossRef]
- Lee, S.-M.; Lee, J.-E.; Lee, Y.-K.; Yoo, D.-A.; Seon, D.-B.; Lee, D.-W.; Kim, C.-B.; Choi, H.; Lee, K.-H. Thermal-Corrosion-Free Electrode-Integrated Cell Chip for Promotion of Electrically Stimulated Neurite Outgrowth. BioChip J. 2022, 16, 99–110. [Google Scholar] [CrossRef]
- Meziane, N.; Webster, J.G.; Attari, M.; Nimunkar, A.J. Dry electrodes for electrocardiography. Physiol. Meas. 2013, 34, R47–R69. [Google Scholar] [CrossRef]
- Rodrigues, M.S.; Fiedler, P.; Küchler, N.; Domingues, R.P.; Lopes, C.; Borges, J.; Haueisen, J.; Vaz, F. Dry Electrodes for Surface Electromyography Based on Architectured Titanium Thin Films. Materials 2020, 13, 2135. [Google Scholar] [CrossRef]
- Ruvalcaba, J.A.; Gutiérrez, M.I.; Vera, A.; Leija, L. Wearable Active Electrode for sEMG Monitoring Using Two-Channel Brass Dry Electrodes with Reduced Electronics. J. Healthc. Eng. 2020, 2020, 5950218. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.M.; Jung, T.-P.; Cauwenberghs, G. Dry-Contact and Noncontact Biopotential Electrodes: Methodological Review. IEEE Rev. Biomed. Eng. 2010, 3, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Dai, M.; Xu, C.; Chen, C.; Tang, M.; Shi, X.; Dong, X. Performance Evaluation of Five Types of Ag/AgCl Bio-Electrodes for Cerebral Electrical Impedance Tomography. Ann. Biomed. Eng. 2011, 39, 2059–2067. [Google Scholar] [CrossRef]
- Mota, A.; Duarte, L.; Rodrigues, D.; Martins, A.; Machado, A.; Vaz, F.; Fiedler, P.; Haueisen, J.; Nóbrega, J.; Fonseca, C. Development of a quasi-dry electrode for EEG recording. Sens. Actuators A Phys. 2013, 199, 310–317. [Google Scholar] [CrossRef]
- Formica, D.; Schena, E.; Nunes, T.; Da Silva, H.P. Characterization and Validation of Flexible Dry Electrodes for Wearable Integration. Sensors 2023, 23, 1468. [Google Scholar] [CrossRef]
- Salvo, P.; Raedt, R.; Carrette, E.; Schaubroeck, D.; Vanfleteren, J.; Cardon, L. A 3D printed dry electrode for ECG/EEG recording. Sens. Actuators A Phys. 2012, 174, 96–102. [Google Scholar] [CrossRef]
- Lopes, C.; Veloso, H.; Hayes, M.; Cullinan, M.A.; Vaz, F. Nanostructured (Ti,Cu)N Dry Electrodes for Advanced Control of the Neuromuscular Activity. IEEE Sens. J. 2023, 23, 3629–3639. [Google Scholar] [CrossRef]
- Hinrichs, H.; Scholz, M.; Baum, A.K.; Kam, J.W.Y.; Knight, R.T.; Heinze, H.-J. Comparison between a wireless dry electrode EEG system with a conventional wired wet electrode EEG system for clinical applications. Sci. Rep. 2020, 10, 5218. [Google Scholar] [CrossRef]
- Poon, C.C.; Liu, Q.; Gao, H.; Lin, W.-H.; Zhang, Y.-T. Wearable Intelligent Systems for E-Health. J. Comput. Sci. Eng. 2011, 5, 246–256. [Google Scholar] [CrossRef]
- Kim, H.; Kim, E.; Choi, C.; Yeo, W.-H. Advances in Soft and Dry Electrodes for Wearable Health Monitoring Devices. Micromachines 2022, 13, 629. [Google Scholar] [CrossRef]
- Fu, Y.; Zhao, J.; Dong, Y.; Wang, X. Dry Electrodes for Human Bioelectrical Signal Monitoring. Sensors 2020, 20, 3651. [Google Scholar] [CrossRef]
- Abdoli-Eramaki, M.; Damecour, C.; Christenson, J.; Stevenson, J. The effect of perspiration on the sEMG amplitude and power spectrum. J. Electromyogr. Kinesiol. 2012, 22, 908–913. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, T.; Kim, D.S.; Chung, W.K. Curved Microneedle Array-Based sEMG Electrode for Robust Long-Term Measurements and High Selectivity. Sensors 2015, 15, 16265–16280. [Google Scholar] [CrossRef] [PubMed]
- Alcan, V.; Ozkendir, O.M. Evaluating electronic and structural properties of Au and Cu substituted AgCl electrode for application in surface electromyography. Materialia 2021, 18, 101170. [Google Scholar] [CrossRef]
- Ansuini, F.J.; Dimond, J.R. Factors Affecting the Accuracy of Reference Electrodes. Mater. Perform. 1994, 33, 14–17. [Google Scholar]
- Lopes, C.; Fiedler, P.; Rodrigues, M.S.; Borges, J.; Bertollo, M.; Alves, E.; Barradas, N.P.; Comani, S.; Haueisen, J.; Vaz, F. Me-Doped Ti–Me Intermetallic Thin Films Used for Dry Biopotential Electrodes: A Comparative Case Study. Sensors 2021, 21, 8143. [Google Scholar] [CrossRef]
- Lopes, C.; Gabor, C.; Cristea, D.; Costa, R.; Domingues, R.; Rodrigues, M.; Borges, J.; Alves, E.; Barradas, N.; Munteanu, D.; et al. Evolution of the mechanical properties of Ti-based intermetallic thin films doped with different metals to be used as biomedical devices. Appl. Surf. Sci. 2019, 505, 144617. [Google Scholar] [CrossRef]
- Etiemble, A.; Lopes, C.; Bouala, G.I.N.; Borges, J.; Malchère, A.; Langlois, C.; Vaz, F.; Steyer, P. Fracture resistance of Ti-Ag thin films deposited on polymeric substrates for biosignal acquisition applications. Surf. Coat. Technol. 2019, 358, 646–653. [Google Scholar] [CrossRef]
- Peng, H.-L.; Liu, J.-Q.; Tian, H.-C.; Dong, Y.-Z.; Yang, B.; Chen, X.; Yang, C.-S. A novel passive electrode based on porous Ti for EEG recording. Sens. Actuators B Chem. 2016, 226, 349–356. [Google Scholar] [CrossRef]
- Lopes, C.; Gonçalves, C.; Pedrosa, P.; Macedo, F.; Alves, E.; Barradas, N.; Martin, N.; Fonseca, C.; Vaz, F. TiAgx thin films for lower limb prosthesis pressure sensors: Effect of composition and structural changes on the electrical and thermal response of the films. Appl. Surf. Sci. 2013, 285, 10–18. [Google Scholar] [CrossRef]
- Song, Y.; Shan, D.; Han, E. Corrosion behaviors of electroless plating Ni–P coatings deposited on magnesium alloys in artificial sweat solution. Electrochim. Acta 2007, 53, 2009–2015. [Google Scholar] [CrossRef]
- Łos, M.J.; Hudecki, A.; Wiecheć, E. Stem Cells and Biomaterials for Regenerative Medicine; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar] [CrossRef]
- Pedrosa, P.; Fiedler, P.; Lopes, C.; Alves, E.; Barradas, N.P.; Haueisen, J.; Machado, A.V.; Fonseca, C.; Vaz, F. Ag:TiN-Coated Polyurethane for Dry Biopotential Electrodes: From Polymer Plasma Interface Activation to the First EEG Measurements. Plasma Process. Polym. 2015, 13, 341–354. [Google Scholar] [CrossRef]
- Calleja, G.; Jourdan, A.; Ameduri, B.; Habas, J.-P. Where is the glass transition temperature of poly(tetrafluoroethylene)? A new approach by dynamic rheometry and mechanical tests. Eur. Polym. J. 2013, 49, 2214–2222. [Google Scholar] [CrossRef]
- Barradas, N.; Alves, E.; Jeynes, C.; Tosaki, M. Accurate simulation of backscattering spectra in the presence of sharp resonances. Nucl. Instruments Methods Phys. Res. Sect. B: Beam Interactions Mater. Atoms 2006, 247, 381–389. [Google Scholar] [CrossRef]
- Barradas, N.; Jeynes, C. Advanced physics and algorithms in the IBA DataFurnace. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2008, 266, 1875–1879. [Google Scholar] [CrossRef]
- Matsunami, N.; Yamamura, Y.; Itikawa, Y.; Itoh, N.; Kazumata, Y.; Miyagawa, S.; Morita, K.; Shimizu, R.; Tawara, H. Energy dependence of the ion-induced sputtering yields of monatomic solids. At. Data Nucl. Data Tables 1984, 31, 1–80. [Google Scholar] [CrossRef]
- Chawla, V.; Jayaganthan, R.; Chawla, A.; Chandra, R. Microstructural characterizations of magnetron sputtered Ti films on glass substrate. J. Mater. Process. Technol. 2009, 209, 3444–3451. [Google Scholar] [CrossRef]
- Fazio, M.; Vega, D.; Kleiman, A.; Colombo, D.; Arias, L.F.; Márquez, A. Study of the structure of titanium thin films deposited with a vacuum arc as a function of the thickness. Thin Solid Film. 2015, 593, 110–115. [Google Scholar] [CrossRef]
- Petrov, I.; Barna, P.B.; Hultman, L.; Greene, J.E. Microstructural evolution during film growth. J. Vac. Sci. Technol. A 2003, 21, S117–S128. [Google Scholar] [CrossRef]
- Köppers, M.; Herzig, C.; Friesel, M.; Mishin, Y. Intrinsic self-diffusion and substitutional Al diffusion in α-Ti. Acta Mater. 1997, 45, 4181–4191. [Google Scholar] [CrossRef]
- Speight, J. Lange’s Handbook of Chemistry; McGraw-Hill Education: New York, NY, USA, 2005. [Google Scholar]
- Mishin, Y.; Herzig, C. Diffusion in the Ti–Al system. Acta Mater. 2000, 48, 589–623. [Google Scholar] [CrossRef]
- de Lopes, C.J.R. Development of Ti-Based Intermetallic Thin Films for Enhanced Biomedical Sensing Performance. Ph.D. Thesis, Universidade do Minho, Braga, Portugal, 2018. [Google Scholar]
- Tisone, T.C.; Drobek, J. Diffusion in Thin Film Ti–Au, Ti–Pd, and Ti–Pt Couples. J. Vac. Sci. Technol. 1972, 9, 271–275. [Google Scholar] [CrossRef]
- Lee, K.-H.; Takai, O.; Lee, M.-H. Tribological and corrosive properties of silver thin films prepared by e-beam ion plating method. Surf. Coat. Technol. 2003, 169, 695–698. [Google Scholar] [CrossRef]
- Ergun, M.; Turan, A.Y. Pitting potential and protection potential of carbon steel for chloride ion and the effectiveness of different inhibiting anions. Corros. Sci. 1991, 32, 1137–1142. [Google Scholar] [CrossRef]
- Fovet, Y.; Gal, J.-Y.; Toumelin-Chemla, F. Influence of pH and fluoride concentration on titanium passivating layer: Stability of titanium dioxide. Talanta 2001, 53, 1053–1063. [Google Scholar] [CrossRef]
- Mokma, D.L. Encyclopedia of SOILS in the Environment; Academic Press: Cambridge, MA, USA, 2005. [Google Scholar]
- Laroche, G.; Fitremann, J.; Gherardi, N. FTIR-ATR spectroscopy in thin film studies: The importance of sampling depth and deposition substrate. Appl. Surf. Sci. 2013, 273, 632–637. [Google Scholar] [CrossRef]
- Kazarian, S.G.; Chan, K.L.A. ATR-FTIR spectroscopic imaging: Recent advances and applications to biological systems. Analyst 2013, 138, 1940–1951. [Google Scholar] [CrossRef]
- Piwowarczyk, J.; Jędrzejewski, R.; Moszyński, D.; Kwiatkowski, K.; Niemczyk, A.; Baranowska, J. XPS and FTIR Studies of Polytetrafluoroethylene Thin Films Obtained by Physical Methods. Polymers 2019, 11, 1629. [Google Scholar] [CrossRef]
- Stamate, M.D. Dielectric properties of TiO2 thin films deposited by a DC magnetron sputtering system. Thin Solid Film. 2000, 372, 246–249. [Google Scholar] [CrossRef]
- Yildiz, A.; Lisesivdin, S.; Kasap, M.; Mardare, D. Electrical properties of TiO2 thin films. J. Non-Cryst. Solids 2008, 354, 4944–4947. [Google Scholar] [CrossRef]
- Rao, M.C.; Ravindranadh, K.; Shekhawat, M.S. Structural and electrical properties of TiO2 thin films. AIP Conf. Proc. 2016, 1728, 020077. [Google Scholar] [CrossRef]
- Apreutesei, M.; Lopes, C.; Borges, J.; Vaz, F.; Macedo, F. Modulated IR radiometry for determining thermal properties and basic characteristics of titanium thin films. J. Vac. Sci. Technol. A Vac. Surf. Film. 2014, 32, 041511. [Google Scholar] [CrossRef]
- Nagarjuna, S.; Balasubramanian, K.; Sarma, D. Effect of Ti additions on the electrical resistivity of copper. Mater. Sci. Eng. A 1997, 225, 118–124. [Google Scholar] [CrossRef]
- Forootan, A.; Sjöback, R.; Björkman, J.; Sjögreen, B.; Linz, L.; Kubista, M. Methods to determine limit of detection and limit of quantification in quantitative real-time PCR (qPCR). Biomol. Detect. Quantif. 2017, 12, 1–6. [Google Scholar] [CrossRef] [PubMed]

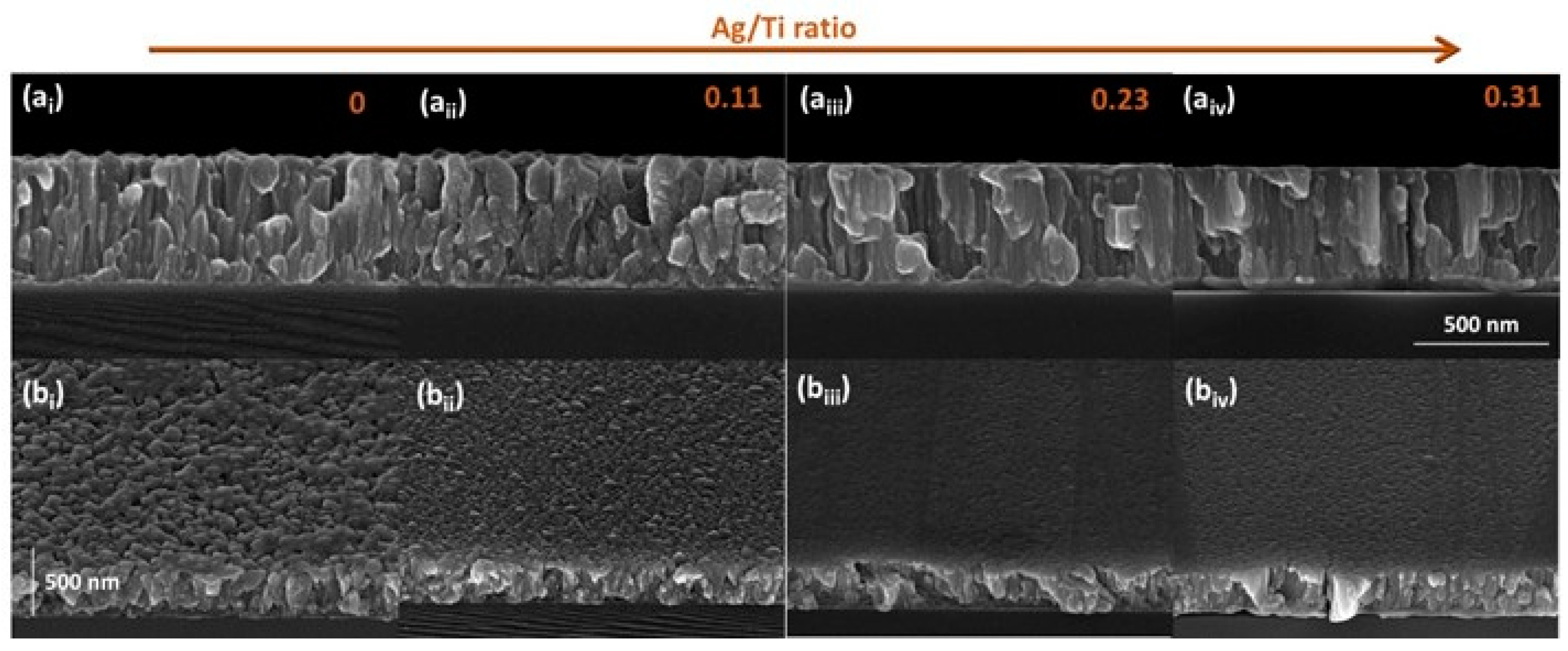



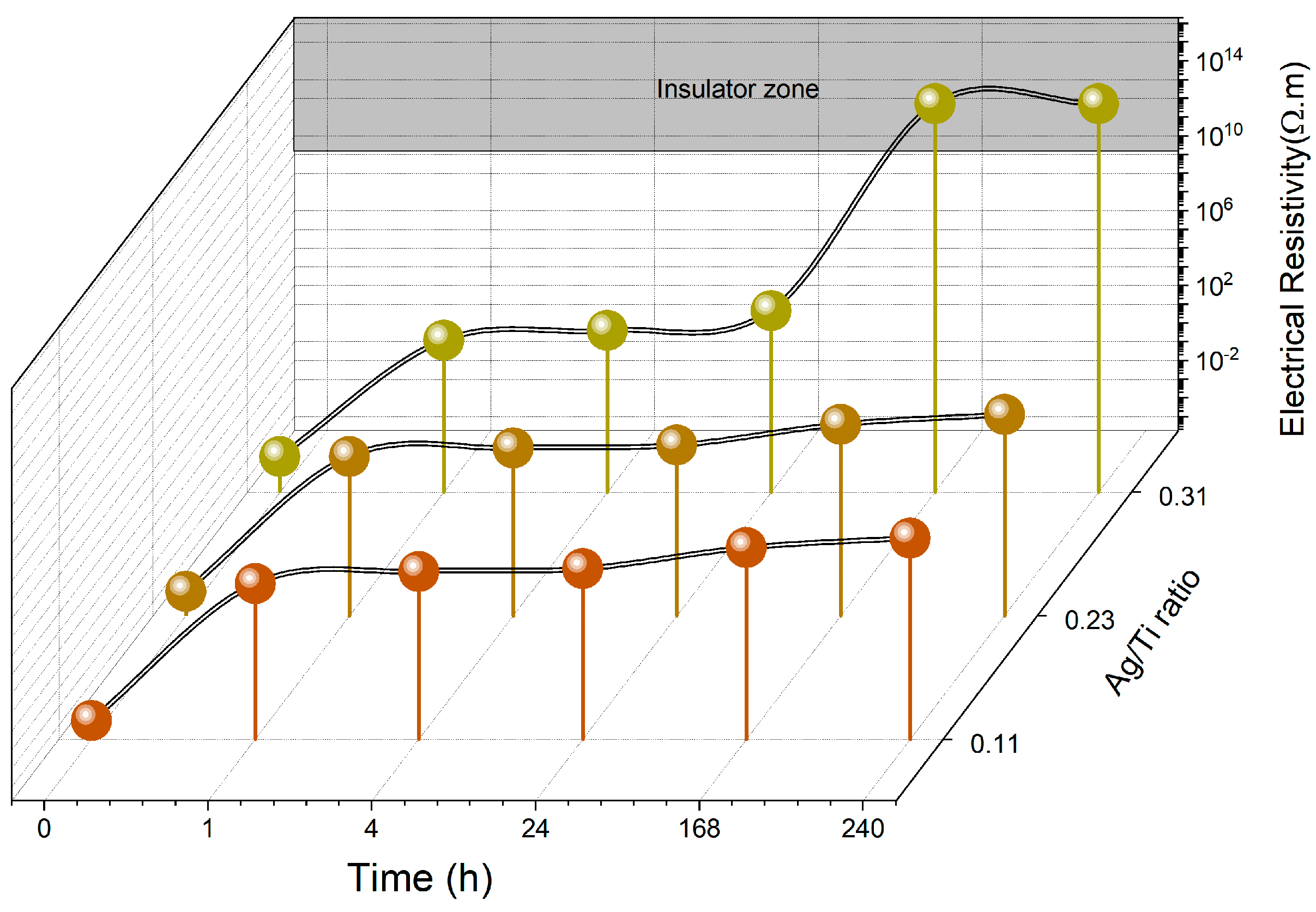

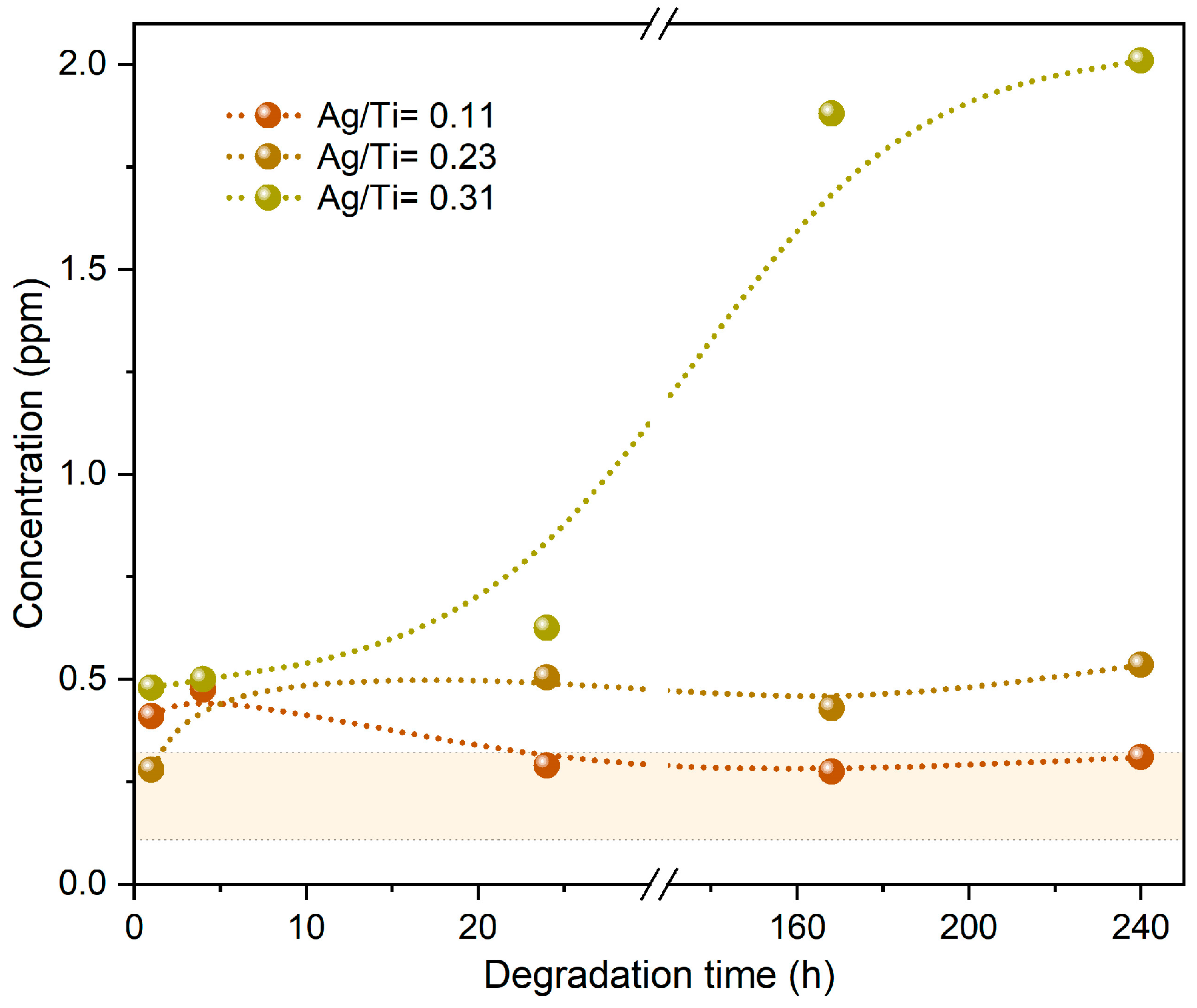
| Sample | Nº of Ag Pellets | Pellet Area (cm2) | Ti (at.%) | Ag (at.%) | Ag/Ti |
|---|---|---|---|---|---|
| Ti | 0 | 0 | 100 | 0 | - |
| Ti-Ag1 | 14 | 2.2 | 89.9 | 10.1 | 0.11 |
| Ti-Ag2 | 28 | 4.5 | 81.6 | 18.4 | 0.23 |
| Ti-Ag3 | 41 | 6.6 | 76.3 | 23.7 | 0.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, D.; Marques, S.; Siqueira, G.; Ferreira, A.; Santos, J.; Geraldo, D.; Castro, C.R.; Machado, A.V.; Vaz, F.; Lopes, C. Enhancing the Longevity and Functionality of Ti-Ag Dry Electrodes for Remote Biomedical Applications: A Comprehensive Study. Sensors 2023, 23, 8321. https://doi.org/10.3390/s23198321
Carvalho D, Marques S, Siqueira G, Ferreira A, Santos J, Geraldo D, Castro CR, Machado AV, Vaz F, Lopes C. Enhancing the Longevity and Functionality of Ti-Ag Dry Electrodes for Remote Biomedical Applications: A Comprehensive Study. Sensors. 2023; 23(19):8321. https://doi.org/10.3390/s23198321
Chicago/Turabian StyleCarvalho, Daniel, Sandra Marques, Giorgia Siqueira, Armando Ferreira, João Santos, Dulce Geraldo, Cidália R. Castro, Ana V. Machado, Filipe Vaz, and Cláudia Lopes. 2023. "Enhancing the Longevity and Functionality of Ti-Ag Dry Electrodes for Remote Biomedical Applications: A Comprehensive Study" Sensors 23, no. 19: 8321. https://doi.org/10.3390/s23198321








