Peak Tibiofemoral Contact Forces Estimated Using IMU-Based Approaches Are Not Significantly Different from Motion Capture-Based Estimations in Patients with Knee Osteoarthritis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Dataset Used to Develop and Validate the InCap Workflow
2.2. Data Processing
2.3. InCap-Based Workflow Overview
2.4. Musculoskeletal Model
2.5. PPCA Model Training Phase
2.6. PPCA Model Validation Phase
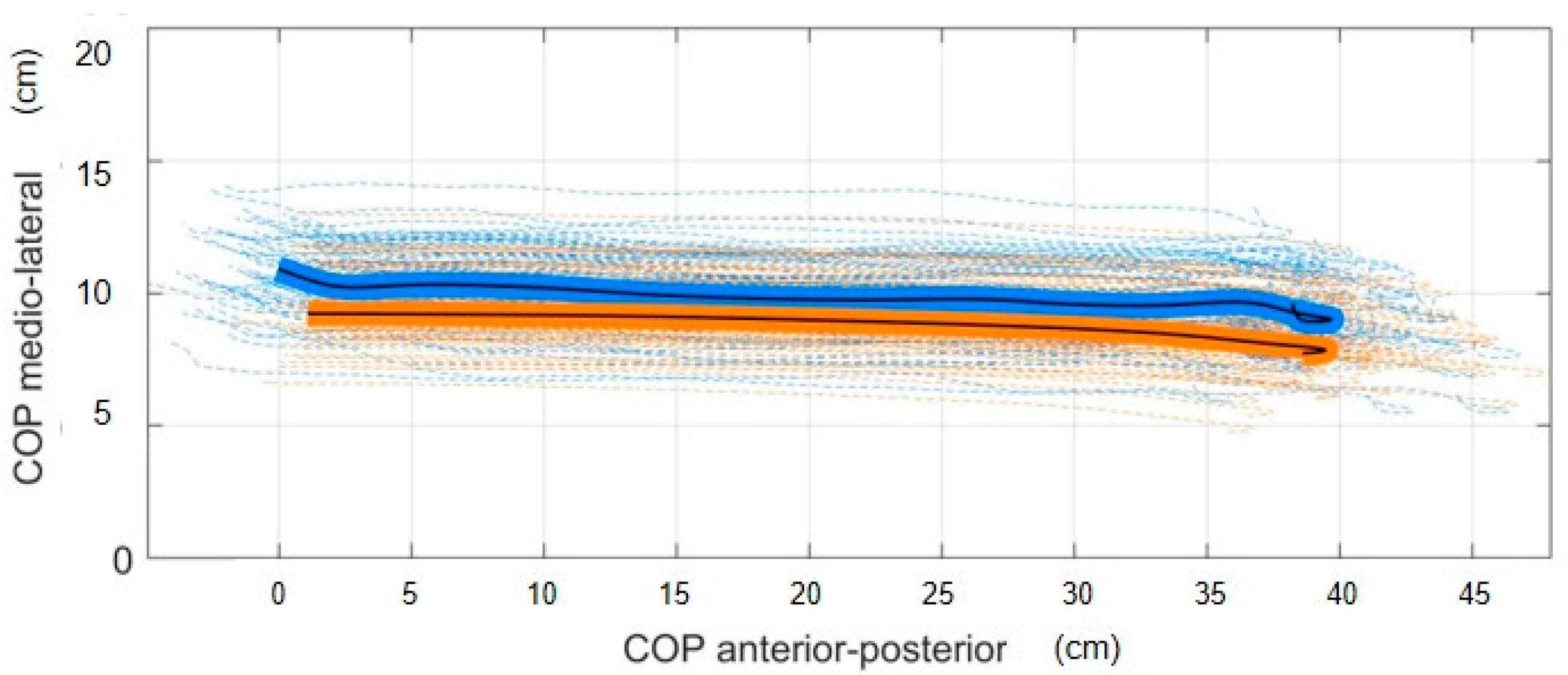
2.7. COP Estimation
2.8. Knee Contact Forces and Joint Moments Estimation
2.9. Statistics
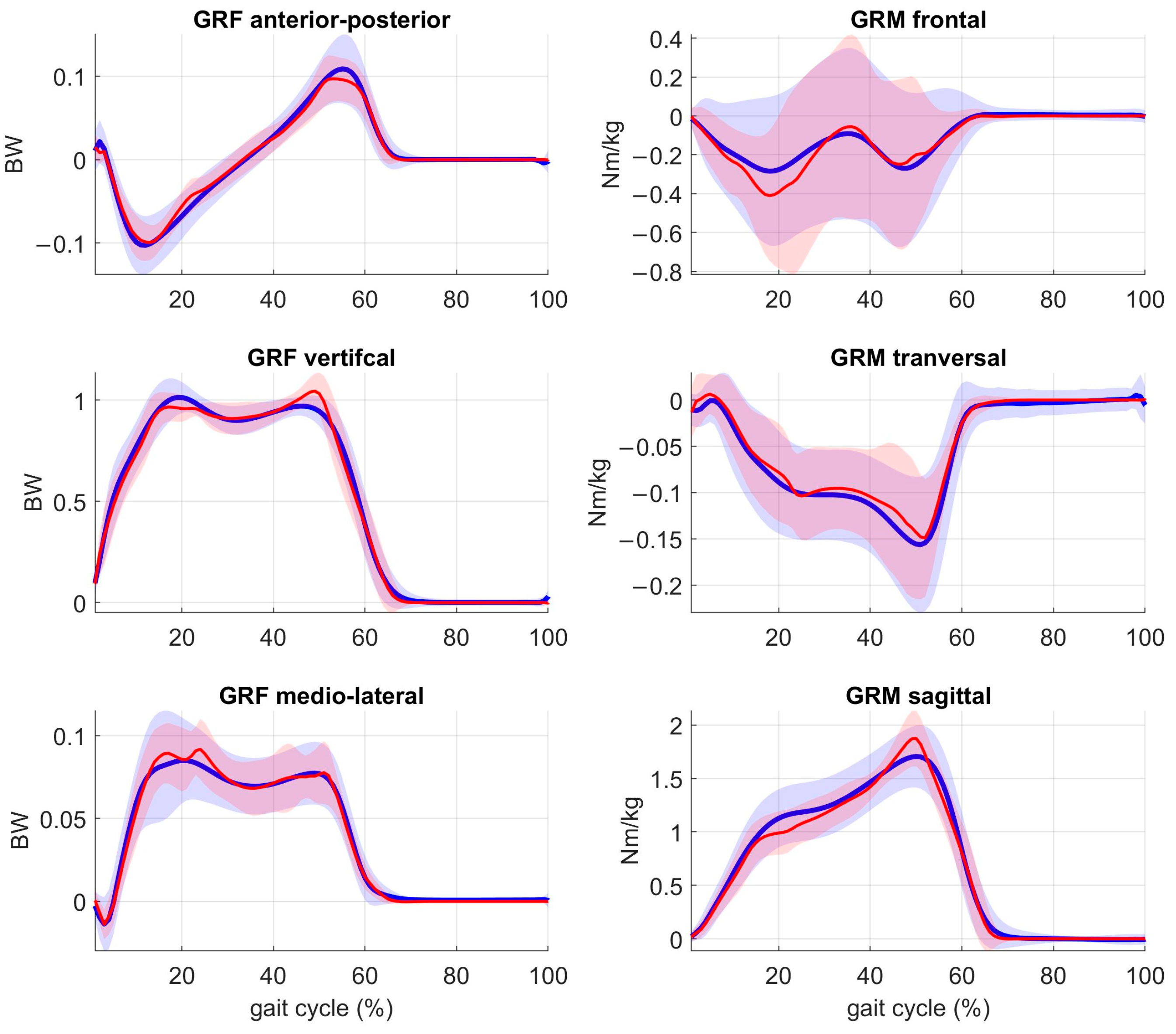
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McAlindon, T.E.; Bannuru, R.R.; Sullivan, M.C.; Arden, N.K.; Berenbaum, F.; Bierma-Zeinstra, S.M.; Hawker, G.A.; Henrotin, Y.; Hunter, D.J.; Kawaguchi, H.; et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthr. Cartil. 2014, 22, 363–388. [Google Scholar] [CrossRef] [PubMed]
- March, L.; Cross, M.; Lo, C.; Arden, N.K.; Gates, L.; Leyland, K.M.; Hawker, G.; King, L.; Leyland, K. Osteoarthritis: A Serious Disease: Submitted to the U.S. Food and Drug Administration; United States Food and Drug Administration: Silver Spring, MD, USA, 2016; pp. 1–103. Available online: https://research-information.bris.ac.uk/en/publications/osteoarthritis-a-serious-disease-submitted-to-the-us-food-and-dru?utm_medium=email&utm_source=transaction (accessed on 30 March 2023).
- Emery, C.A.; Whittaker, J.L.; Mahmoudian, A.; Lohmander, L.S.; Roos, E.M.; Bennell, K.L.; Toomey, C.M.; Reimer, R.A.; Thompson, D.; Ronsky, J.L.; et al. Establishing outcome measures in early knee osteoarthritis. Nat. Rev. Rheumatol. 2019, 15, 438–448. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Knee Injury and Osteoarthritis Outcome Score. Definitions 2020, 1–5. [Google Scholar] [CrossRef]
- Baumbach, L.; List, M.; Grønne, D.T.; Skou, S.T.; Roos, E.M. Individualized predictions of changes in knee pain, quality of life and walking speed following patient education and exercise therapy in patients with knee osteoarthritis—A prognostic model study. Osteoarthr. Cartil. 2020, 28, 1191–1201. [Google Scholar] [CrossRef] [PubMed]
- Hawker, G.A. Osteoarthritis is a serious disease. Clin. Exp. Rheumatol. 2019, 37, 3–6. [Google Scholar]
- De Pieri, E.; Nüesch, C.; Pagenstert, G.; Viehweger, E.; Egloff, C.; Mündermann, A. High tibial osteotomy effectively redistributes compressive knee loads during walking. J. Orthop. Res. 2022, 41, 591–600. [Google Scholar] [CrossRef]
- Wu, D.D.; Burr, D.B.; Boyd, R.D.; Radin, E.L. Bone and cartilage changes following experimental varus or valgus tibial angulation. J. Orthop. Res. 1990, 8, 572–585. [Google Scholar] [CrossRef]
- Hunt, M.A.; Takacs, J. Effects of a 10-week toe-out gait modification intervention in people with medial knee osteoarthritis: A pilot, feasibility study. Osteoarthr. Cartil. 2014, 22, 904–911. [Google Scholar] [CrossRef]
- Ro, D.H.; Lee, J.; Lee, J.; Park, J.Y.; Han, H.S.; Lee, M.C. Effects of Knee Osteoarthritis on Hip and Ankle Gait Mechanics. Adv. Orthop. 2019, 2019, 9757369. [Google Scholar] [CrossRef]
- Federolf, P.A.; Boyer, K.A.; Andriacchi, T.P. Application of principal component analysis in clinical gait research: Identification of systematic differences between healthy and medial knee-osteoarthritic gait. J. Biomech. 2013, 46, 2173–2178. [Google Scholar] [CrossRef]
- Fregly, B.J.; Besier, T.F.; Lloyd, D.G.; Delp, S.L.; Banks, S.A.; Pandy, M.G.; D’Lima, D.D. Grand challenge competition to predict in vivo knee loads. J. Orthop. Res. 2012, 30, 503–513. [Google Scholar] [CrossRef]
- Kutzner, I.; Heinlein, B.; Graichen, F.; Bender, A.; Rohlmann, A.; Halder, A.; Beier, A.; Bergmann, G. Loading of the knee joint during activities of daily living measured in vivo in five subjects. J. Biomech. 2010, 43, 2164–2173. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Phan, C.B.; Koo, S. Intra-articular knee contact force estimation during walking using force-reaction elements and subject-specific joint model. J. Biomech. Eng. 2016, 138, 021016. [Google Scholar] [CrossRef] [PubMed]
- Ancillao, A.; Aertbeliën, E.; De Schutter, J. Effect of the soft tissue artifact on marker measurements and on the calculation of the helical axis of the knee during a gait cycle: A study on the CAMS-Knee data set. Hum. Mov. Sci. 2021, 80, 102866. [Google Scholar] [CrossRef] [PubMed]
- Iosa, M.; Picerno, P.; Paolucci, S.; Morone, G. Wearable inertial sensors for human movement analysis. Expert Rev. Med. Devices 2016, 13, 641–659. [Google Scholar] [CrossRef]
- Prill, R.; Walter, M.; Królikowska, A.; Becker, R. A systematic review of diagnostic accuracy and clinical applications of wearable movement sensors for knee joint rehabilitation. Sensors 2021, 21, 8221. [Google Scholar] [CrossRef]
- Picerno, P. 25 years of lower limb joint kinematics by using inertial and magnetic sensors: A review of methodological approaches. Gait Posture 2017, 51, 239–246. [Google Scholar] [CrossRef]
- Seel, T.; Raisch, J.; Schauer, T. IMU-based joint angle measurement for gait analysis. Sensors 2014, 14, 6891–6909. [Google Scholar] [CrossRef]
- Favre, J.; Erhart-Hledik, J.C.; Chehab, E.F.; Andriacchi, T.P. Baseline ambulatory knee kinematics are associated with changes in cartilage thickness in osteoarthritic patients over 5 years. J. Biomech. 2016, 49, 1859–1864. [Google Scholar] [CrossRef]
- Fasel, B.; Sporri, J.; Chardonnens, J.; Kroll, J.; Muller, E.; Aminian, K. Joint Inertial Sensor Orientation Drift Reduction for Highly Dynamic Movements. IEEE J. Biomed. Health Inform. 2018, 22, 77–86. [Google Scholar] [CrossRef]
- Picerno, P.; Cereatti, A.; Cappozzo, A. Joint kinematics estimate using wearable inertial and magnetic sensing modules. Gait Posture 2008, 28, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Teufl, W.; Miezal, M.; Taetz, B.; Frohlichi, M.; Bleser, G. Validity of inertial sensor based 3D joint kinematics of static and dynamic sport and physiotherapy specific movements. PLoS ONE 2019, 14, e0213064. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Lim, H.; Park, S. Spring-loaded inverted pendulum modeling improves neural network estimation of ground reaction forces. J. Biomech. 2020, 113, 110069. [Google Scholar] [CrossRef]
- Robert-Lachaine, X.; Mecheri, H.; Larue, C.; Plamondon, A. Accuracy and repeatability of single-pose calibration of inertial measurement units for whole-body motion analysis. Gait Posture 2017, 54, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.S.; Tippaya, S.; Binnie, T.; Davey, P.; Napier, K.; Caneiro, J.P.; Kent, P.; Smith, A.; O’sullivan, P.; Campbell, A. Predicting Knee Joint Kinematics from Wearable Sensor Data in People with Knee Osteoarthritis and Clinical Considerations for Future Machine Learning Models. Sensors 2022, 22, 446. [Google Scholar] [CrossRef]
- Ancillao, A.; Tedesco, S.; Barton, J.; O’Flynn, B. Indirect measurement of ground reaction forces and moments by means of wearable inertial sensors: A systematic review. Sensors 2018, 18, 2564. [Google Scholar] [CrossRef]
- De Brabandere, A.; Emmerzaal, J.; Timmermans, A.; Jonkers, I.; Vanwanseele, B.; Davis, J. A Machine Learning Approach to Estimate Hip and Knee Joint Loading Using a Mobile Phone-Embedded IMU. Front. Bioeng. Biotechnol. 2020, 8, 320. [Google Scholar] [CrossRef]
- Emmerzaal, J.; De Brabandere, A.; Vanrompay, Y.; Vranken, J.; Storms, V.; De Baets, L.; Corten, K.; Davis, J.; Jonkers, I.; Vanwanseele, B.; et al. Towards the monitoring of functional status in a free-living environment for people with hip or knee osteoarthritis: Design and evaluation of the jolo blended care app. Sensors 2020, 20, 6967. [Google Scholar] [CrossRef]
- Tanghe, K.; Afschrift, M.; Jonkers, I.; De Groote, F.; De Schutter, J.; Aertbeliën, E. A probabilistic method to estimate gait kinetics in the absence of ground reaction force measurements. J. Biomech. 2019, 96, 109327. [Google Scholar] [CrossRef]
- Xiang, Y.; Arora, J.S.; Rahmatalla, S.; Abdel-Malek, K. Optimization-based dynamic human walking prediction: One step formulation. Int. J. Numer. Methods Eng. 2009, 79, 667–695. [Google Scholar] [CrossRef]
- Gupta, D.; Donnelly, C.; Reinbolt, J. Physics-Based Guidelines for Accepting Reasonable Dynamic Simulations of Movement. IEEE Trans. Biomed. Eng. 2022, 69, 1194–1201. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, E.J.; Gutierrez-Farewik, E.M. Computation of ground reaction force using Zero Moment Point. J. Biomech. 2015, 48, 3776–3781. [Google Scholar] [CrossRef] [PubMed]
- Nüesch, C.; Ismailidis, P.; Koch, D.; Pagenstert, G.; Ilchmann, T.; Eckardt, A.; Stoffel, K.; Egloff, C.; Mündermann, A. Assessing site specificity of osteoarthritic gait kinematics with wearable sensors and their association with patient reported outcome measures (Proms): Knee versus hip osteoarthritis. Sensors 2021, 21, 5363. [Google Scholar] [CrossRef]
- Van Rossom, S.; Wesseling, M.; Smith, C.R.; Thelen, D.G.; Vanwanseele, B.; Dieter, V.A.; Jonkers, I. The influence of knee joint geometry and alignment on the tibiofemoral load distribution: A computational study. Knee 2019, 26, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Meireles, S.; Wesseling, M.; Smith, C.R.; Thelen, D.G.; Verschueren, S.; Jonkers, I. Medial knee loading is altered in subjects with early osteoarthritis during gait but not during step-up-and-over task. PLoS ONE 2017, 12, e0187583. [Google Scholar] [CrossRef]
- Meireles, S.; De Groote, F.; Van Rossom, S.; Verschueren, S.; Jonkers, I. Differences in knee adduction moment between healthy subjects and patients with osteoarthritis depend on the knee axis definition. Gait Posture 2017, 53, 104–109. [Google Scholar] [CrossRef]
- Davis, R.B.; Õunpuu, S.; Tyburski, D.; Gage, J.R. A gait analysis data collection and reduction technique. Hum. Mov. Sci. 1991, 10, 575–587. [Google Scholar] [CrossRef]
- Schepers, M.; Giuberti, M.; Bellusci, G. Xsens MVN: Consistent tracking of human motion using inertial sensing. Xsens Technol. 2018, 1, 1–8. [Google Scholar] [CrossRef]
- Paulich, M.; Schepers, M.; Rudigkeit, N.; Bellusci, G. Xsens MTw Awinda: Miniature Wireless Inertial-Magnetic Motion Tracker for Highly Accurate 3D Kinematic Applications. Xsens Technol. 2018, 1–9. [Google Scholar] [CrossRef]
- Di Raimondo, G.; Vanwanseele, B.; van der Have, A.; Emmerzaal, J.; Willems, M.; Killen, B.A.; Jonkers, I. Inertial Sensor-to-Segment Calibration for Accurate 3D Joint Angle Calculation for Use in OpenSim. Sensors 2022, 22, 3259. [Google Scholar] [CrossRef]
- Smith, C.R.; Won Choi, K.; Negrut, D.; Thelen, D.G. Efficient computation of cartilage contact pressures within dynamic simulations of movement. Comput. Methods Biomech. Biomed. Eng. Imaging Vis. 2018, 6, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Lenhart, R.L.; Kaiser, J.; Smith, C.R.; Thelen, D.G. Prediction and Validation of Load-Dependent Behavior of the Tibiofemoral and Patellofemoral Joints During Movement. Ann. Biomed. Eng. 2015, 43, 2675–2685. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.R.; Vignos, M.F.; Lenhart, R.L.; Kaiser, J.; Thelen, D.G. The influence of component alignment and ligament properties on tibiofemoral contact forces in total knee replacement. J. Biomech. Eng. 2016, 138, 021017. [Google Scholar] [CrossRef] [PubMed]
- Millard, M.; Uchida, T.; Seth, A.; Delp, S.L. Flexing computational muscle: Modeling and simulation of musculotendon dynamics. J. Biomech. Eng. 2013, 135, 021005. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, B.; Santos, A.N.; Jolles, B.M.; Benninger, D.H.; Favre, J. Gait events during turning can be detected using kinematic features originally proposed for the analysis of straight-line walking. J. Biomech. 2019, 91, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Lee, D. Development of an IMU-based foot-ground contact detection (FGCD) algorithm. Ergonomics 2017, 60, 384–403. [Google Scholar] [CrossRef]
- Di Raimondo, G.; van der Have, T.; Van Rossom, S.; Willems, M.; Emmerzaal, J.; Ancillao, A.; Vanwanseele, B.; Jonkers, I. Towards optimised IMU-based monitoring of joint kinematics and loading in osteoarthritis subjects. Gait Posture 2021, 90, 44–45. [Google Scholar] [CrossRef]
- Karatsidis, A.; Bellusci, G.; Schepers, H.M.; de Zee, M.; Andersen, M.S.; Veltink, P.H. Estimation of ground reaction forces and moments during gait using only inertial motion capture. Sensors 2017, 17, 75. [Google Scholar] [CrossRef]
- Catelli, D.; Di Raimondo, G.; Jonkers, I.; Vanwanseele, B. Scaling simplification approaches to estimate musculotendon lengths during running using inertial measurement units. Gait Posture 2022, 97, S305–S306. [Google Scholar] [CrossRef]
- Delp, S.L.; Anderson, F.C.; Arnold, A.S.; Loan, P.; Habib, A.; John, C.T.; Guendelman, E.; Thelen, D.G. OpenSim: Open-source software to create and analyze dynamic simulations of movement. IEEE Trans. Biomed. Eng. 2007, 54, 1940–1950. [Google Scholar] [CrossRef]
- Miller, R.H.; Esterson, A.Y.; Shim, J.K. Joint contact forces when minimizing the external knee adduction moment by gait modification: A computer simulation study. Knee 2015, 22, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Van Rossom, S.; Smith, C.R.; Thelen, D.G.; Vanwanseele, B.; Van Assche, D.; Jonkers, I. Knee joint loading in healthy adults during functional exercises: Implications for rehabilitation guidelines. J. Orthop. Sports Phys. Ther. 2018, 48, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Meireles, S.; De Groote, F.; Reeves, N.D.; Verschueren, S.; Maganaris, C.; Luyten, F.; Jonkers, I. Knee contact forces are not altered in early knee osteoarthritis. Gait Posture 2016, 45, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Jones, R.K.; Howard, D. Whole body inverse dynamics over a complete gait cycle based only on measured kinematics. J. Biomech. 2008, 41, 2750–2759. [Google Scholar] [CrossRef]
- Fluit, R.; Andersen, M.S.; Kolk, S.; Verdonschot, N.; Koopman, H.F.J.M. Prediction of ground reaction forces and moments during various activities of daily living. J. Biomech. 2014, 47, 2321–2329. [Google Scholar] [CrossRef]
- Oh, S.E.; Choi, A.; Mun, J.H. Prediction of ground reaction forces during gait based on kinematics and a neural network model. J. Biomech. 2013, 46, 2372–2380. [Google Scholar] [CrossRef]
- Oubre, B.; Lane, S.; Holmes, S.; Boyer, K.; Lee, S.I. Estimating Ground Reaction Force and Center of Pressure Using Low-Cost Wearable Devices. IEEE Trans. Biomed. Eng. 2022, 69, 1461–1468. [Google Scholar] [CrossRef]
- Podobnik, J.; Kraljić, D.; Zadravec, M.; Munih, M. Centre of pressure estimation during walking using only inertial-measurement units and end-to-end statistical modelling. Sensors 2020, 20, 6136. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, J.; Zhang, Y.; Zhu, R. A wearable motion capture device able to detect dynamic motion of human limbs. Nat. Commun. 2020, 11, 5615. [Google Scholar] [CrossRef]
- Duong, T.T.H.; Zhang, H.; Lynch, T.S.; Zanotto, D. Improving the accuracy of wearable sensors for human locomotion tracking using phase-locked regression models. In Proceedings of the 2019 IEEE 16th International Conference on Rehabilitation Robotics (ICORR), Toronto, ON, Canada, 24–28 June 2019; pp. 145–150. [Google Scholar]
- Karatsidis, A.; Jung, M.; Schepers, H.M.; Bellusci, G.; de Zee, M.; Veltink, P.H.; Andersen, M.S. Musculoskeletal model-based inverse dynamic analysis under ambulatory conditions using inertial motion capture. Med. Eng. Phys. 2019, 65, 68–77. [Google Scholar] [CrossRef]
- Park, J.; Na, Y.; Gu, G.; Kim, J. Flexible insole ground reaction force measurement shoes for jumping and running. In Proceedings of the 2016 6th IEEE International Conference on Biomedical Robotics and Biomechatronics (BioRob), Singapore, 26–29 June 2016; pp. 1062–1067. [Google Scholar] [CrossRef]
- Stetter, B.J.; Ringhof, S.; Krafft, F.C.; Sell, S.; Stein, T. Estimation of knee joint forces in sport movements using wearable sensors and machine learning. Sensors 2019, 19, 3690. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, G.; Yan, Y.; Han, B.; Li, H.; Ma, J.; Wang, X. A subject-specific musculoskeletal model to predict the tibiofemoral contact forces during daily living activities. Comput. Methods Biomech. Biomed. Eng. 2023, 26, 972–985. [Google Scholar] [CrossRef] [PubMed]


| Knee Contact Forces | RMSE (BW) | R2 | ||
|---|---|---|---|---|
| Mean | Std | Mean | Std | |
| Total | 0.40 | 0.17 | 0.68 | 0.16 |
| Medial | 0.35 | 0.11 | 0.76 | 0.12 |
| Lateral | 0.15 | 0.05 | 0.58 | 0.24 |
| MAD | |||
|---|---|---|---|
| Mean | Std | ||
| Peak 1 (BW) | Total | 0.24 | 0.15 |
| Medial | 0.21 | 0.14 | |
| Lateral | 0.08 | 0.06 | |
| Peak 2 (BW) | Total | 0.19 | 0.15 |
| Medial | 0.27 | 0.14 | |
| Lateral | 0.12 | 0.13 | |
| Impulse (BWs) | Total | 0.31 | 0.18 |
| Medial | 0.30 | 0.19 | |
| Lateral | 0.31 | 0.15 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Raimondo, G.; Willems, M.; Killen, B.A.; Havashinezhadian, S.; Turcot, K.; Vanwanseele, B.; Jonkers, I. Peak Tibiofemoral Contact Forces Estimated Using IMU-Based Approaches Are Not Significantly Different from Motion Capture-Based Estimations in Patients with Knee Osteoarthritis. Sensors 2023, 23, 4484. https://doi.org/10.3390/s23094484
Di Raimondo G, Willems M, Killen BA, Havashinezhadian S, Turcot K, Vanwanseele B, Jonkers I. Peak Tibiofemoral Contact Forces Estimated Using IMU-Based Approaches Are Not Significantly Different from Motion Capture-Based Estimations in Patients with Knee Osteoarthritis. Sensors. 2023; 23(9):4484. https://doi.org/10.3390/s23094484
Chicago/Turabian StyleDi Raimondo, Giacomo, Miel Willems, Bryce Adrian Killen, Sara Havashinezhadian, Katia Turcot, Benedicte Vanwanseele, and Ilse Jonkers. 2023. "Peak Tibiofemoral Contact Forces Estimated Using IMU-Based Approaches Are Not Significantly Different from Motion Capture-Based Estimations in Patients with Knee Osteoarthritis" Sensors 23, no. 9: 4484. https://doi.org/10.3390/s23094484








