Measuring the Canopy Architecture of Young Vegetation Using the Fastrak Polhemus 3D Digitizer
Abstract
:1. Introduction
2. Materials and Methods
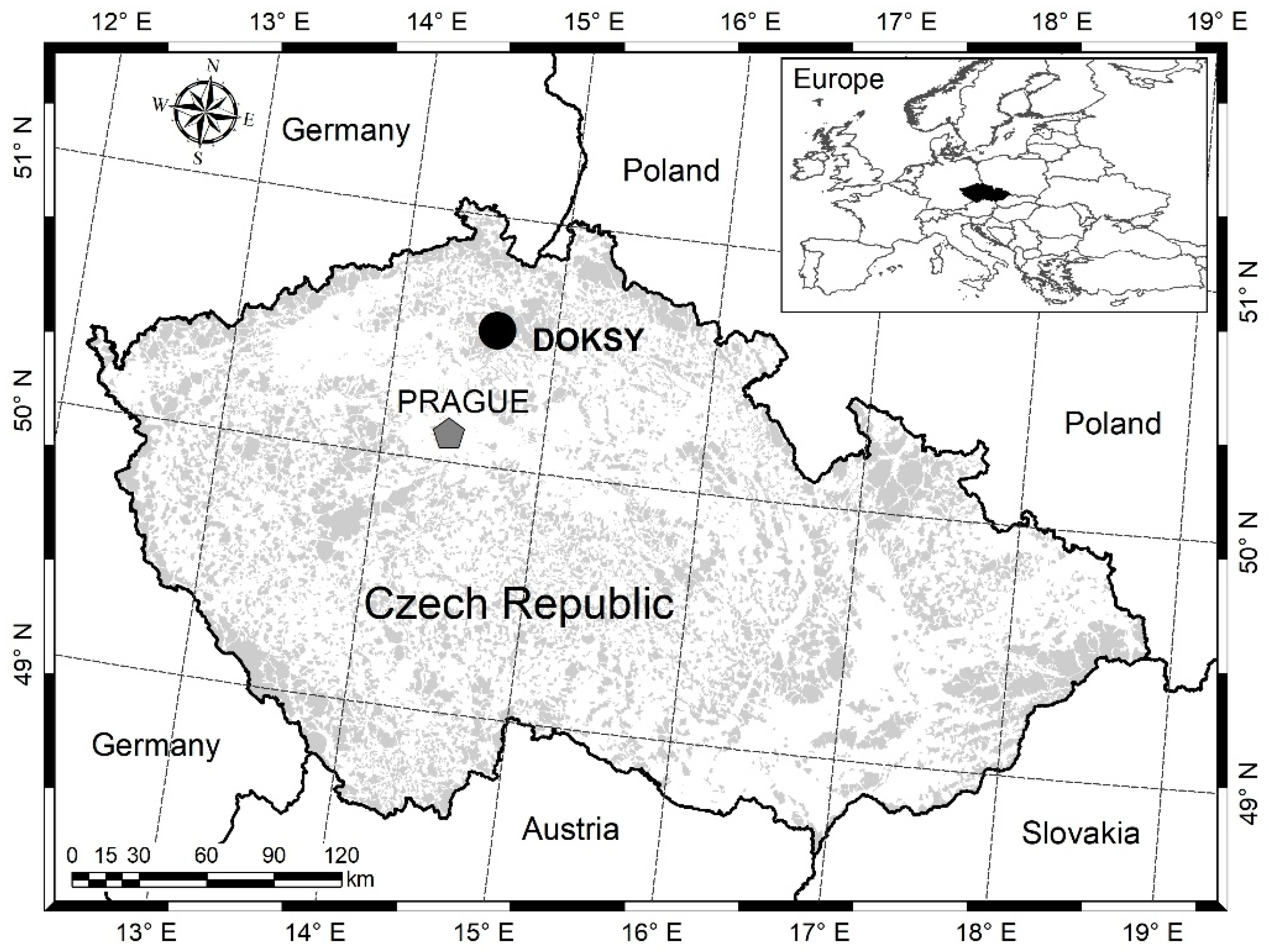
2.1. Study Area
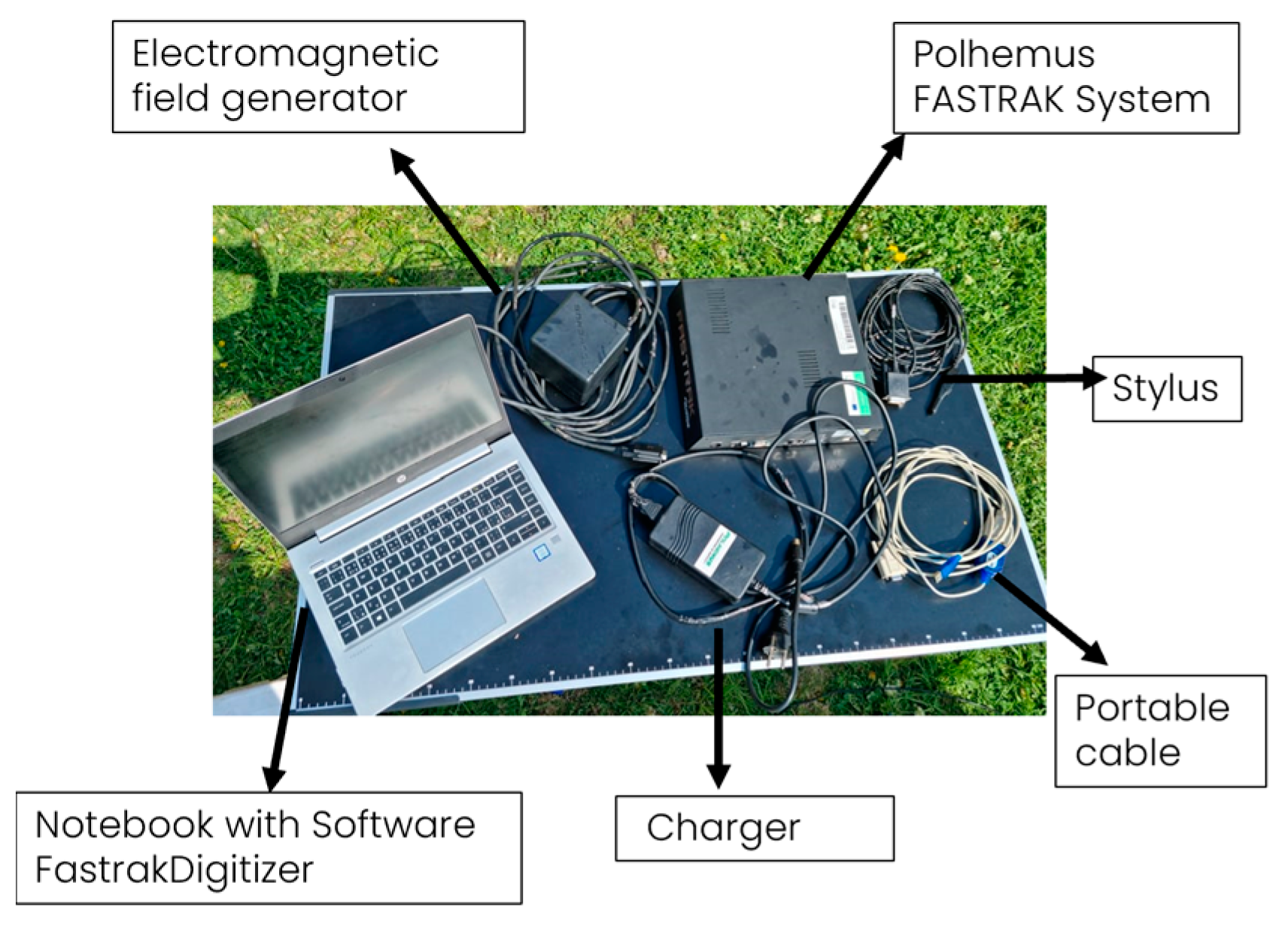
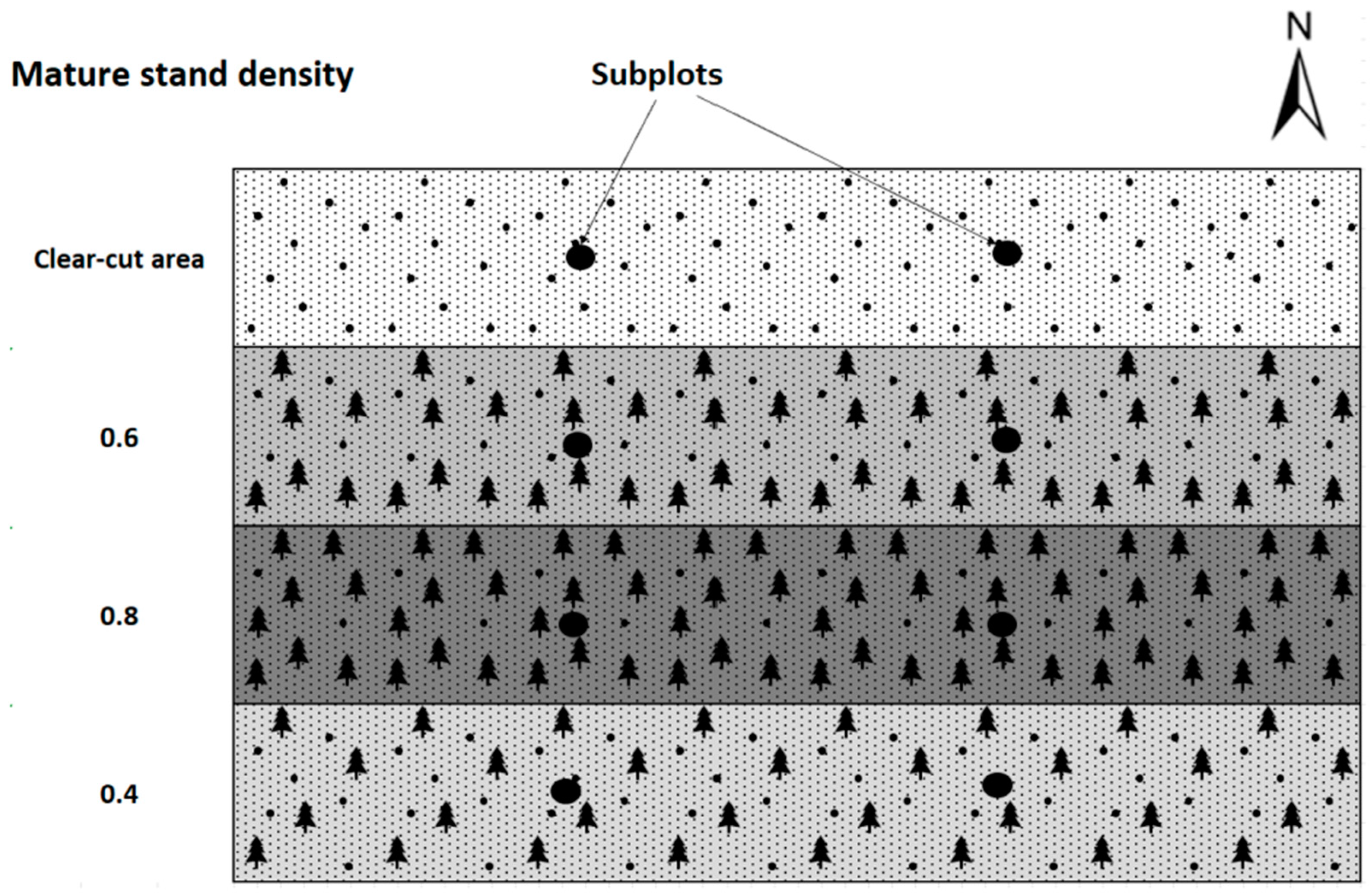
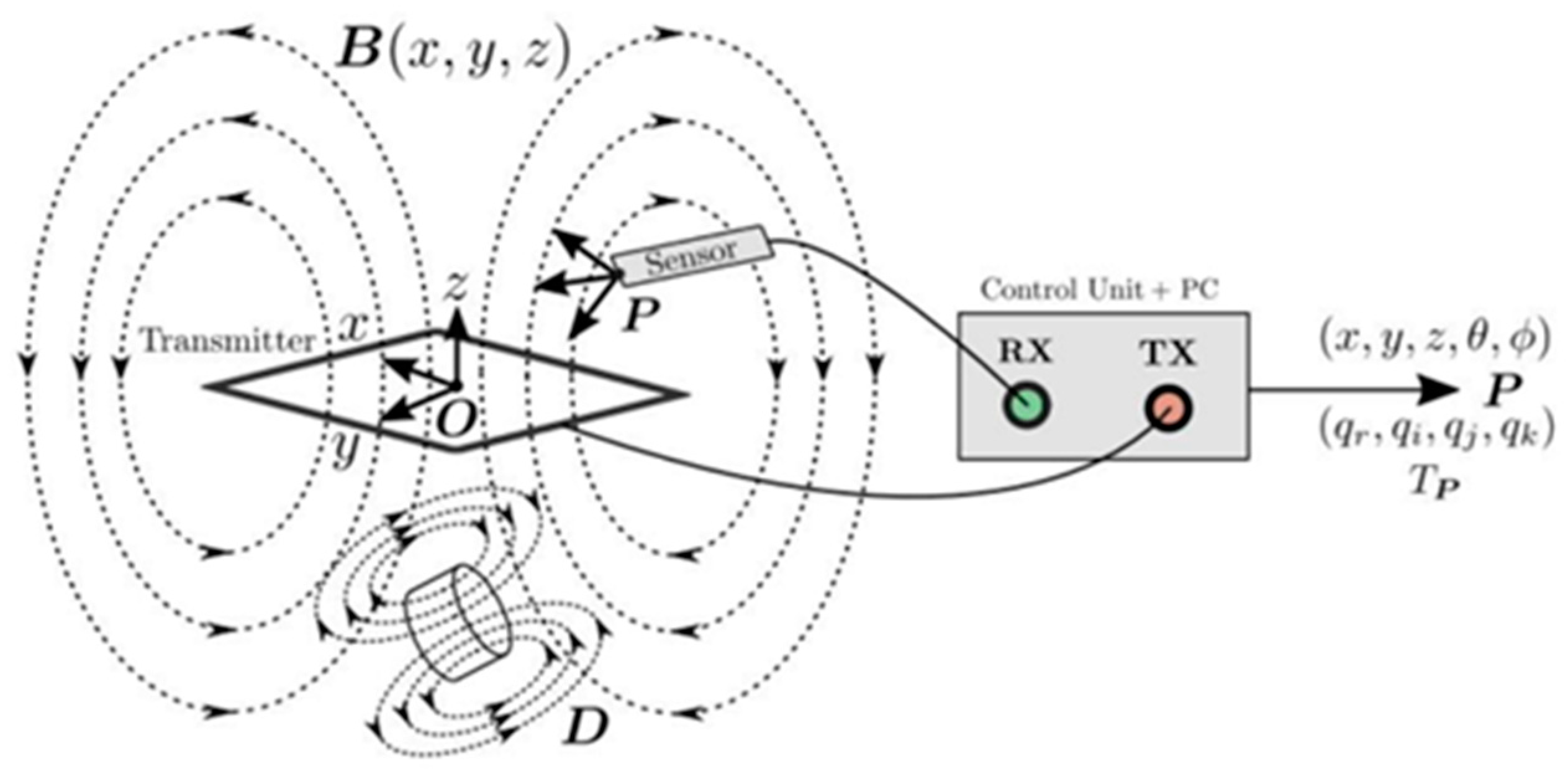
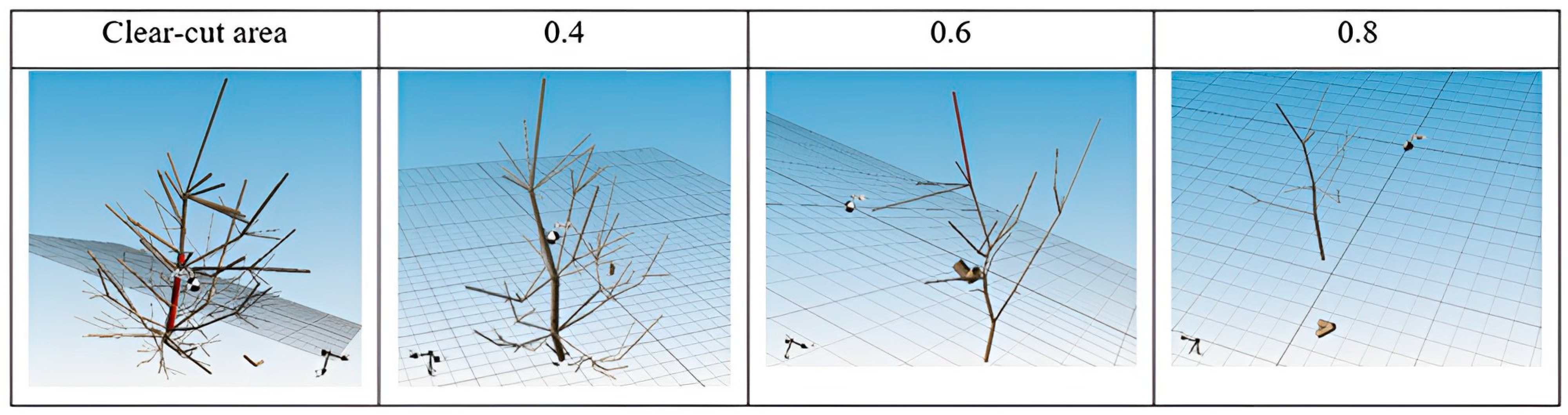
2.2. Data Collection
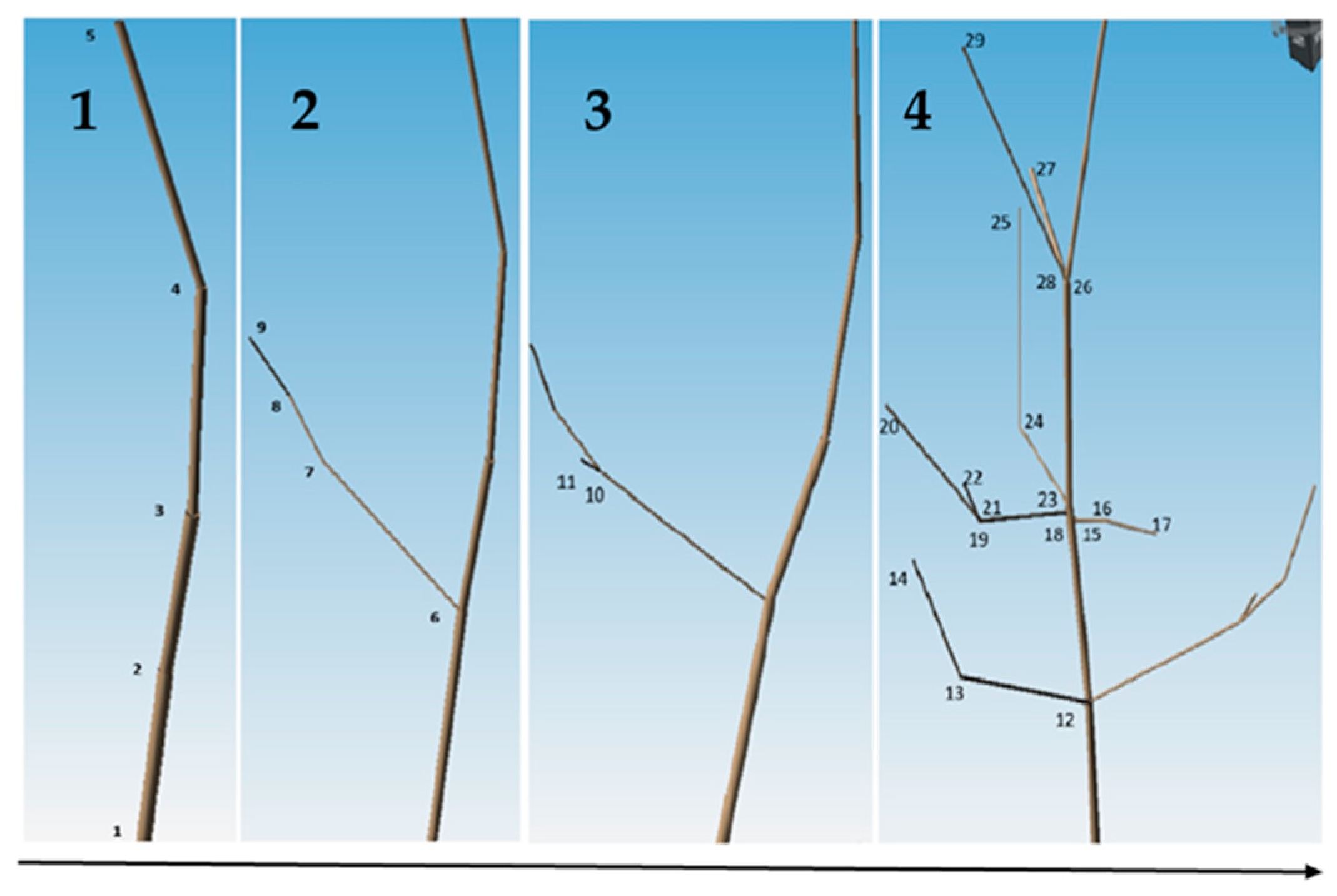
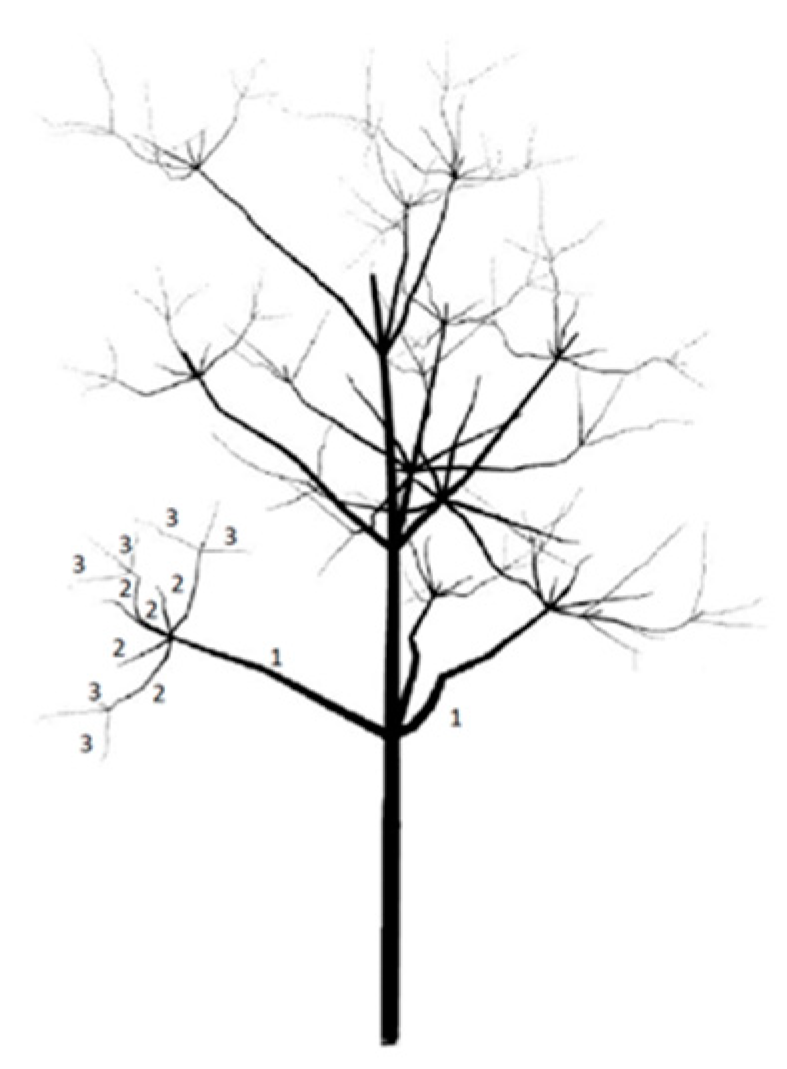
2.3. Data Analysis
3. Results
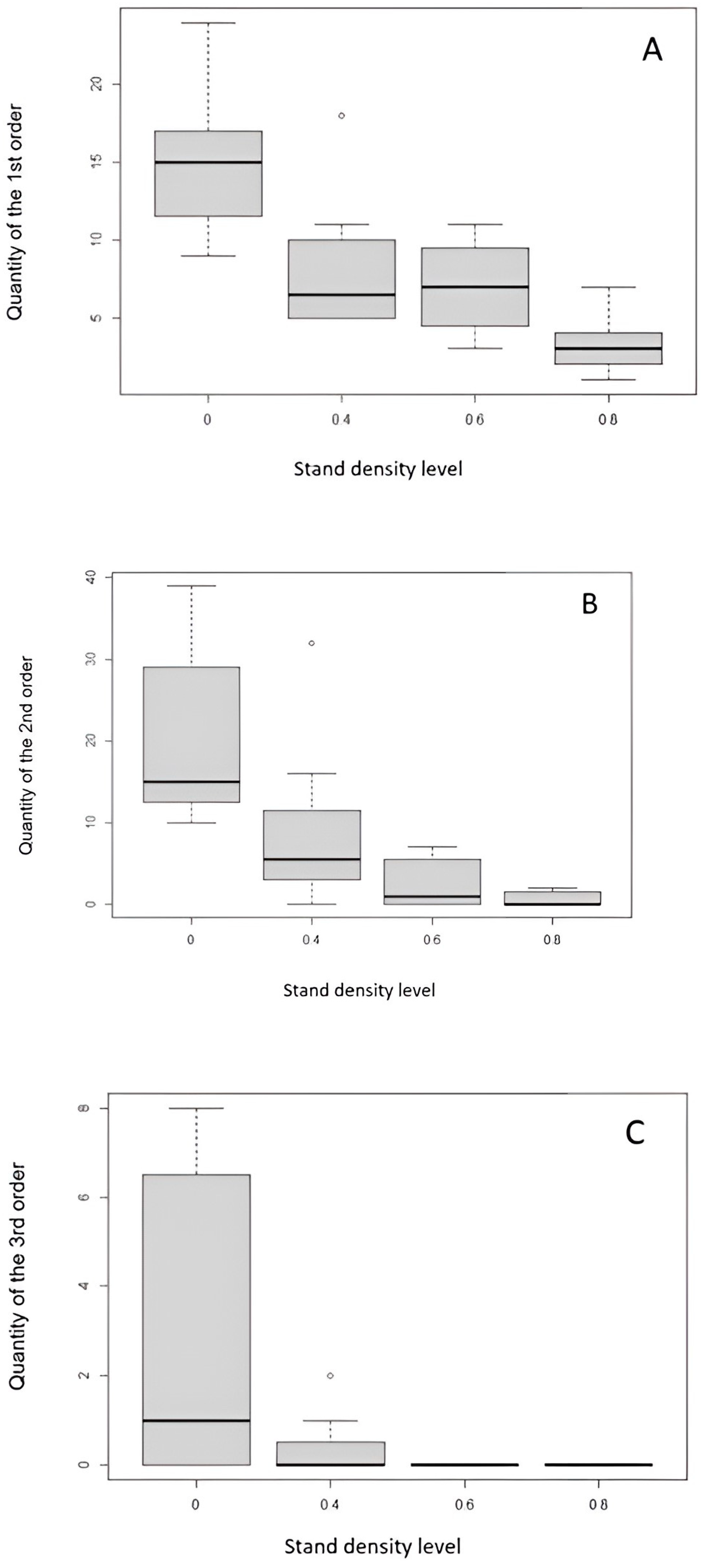
3.1. Dependence of Branching Regarding Stand Density
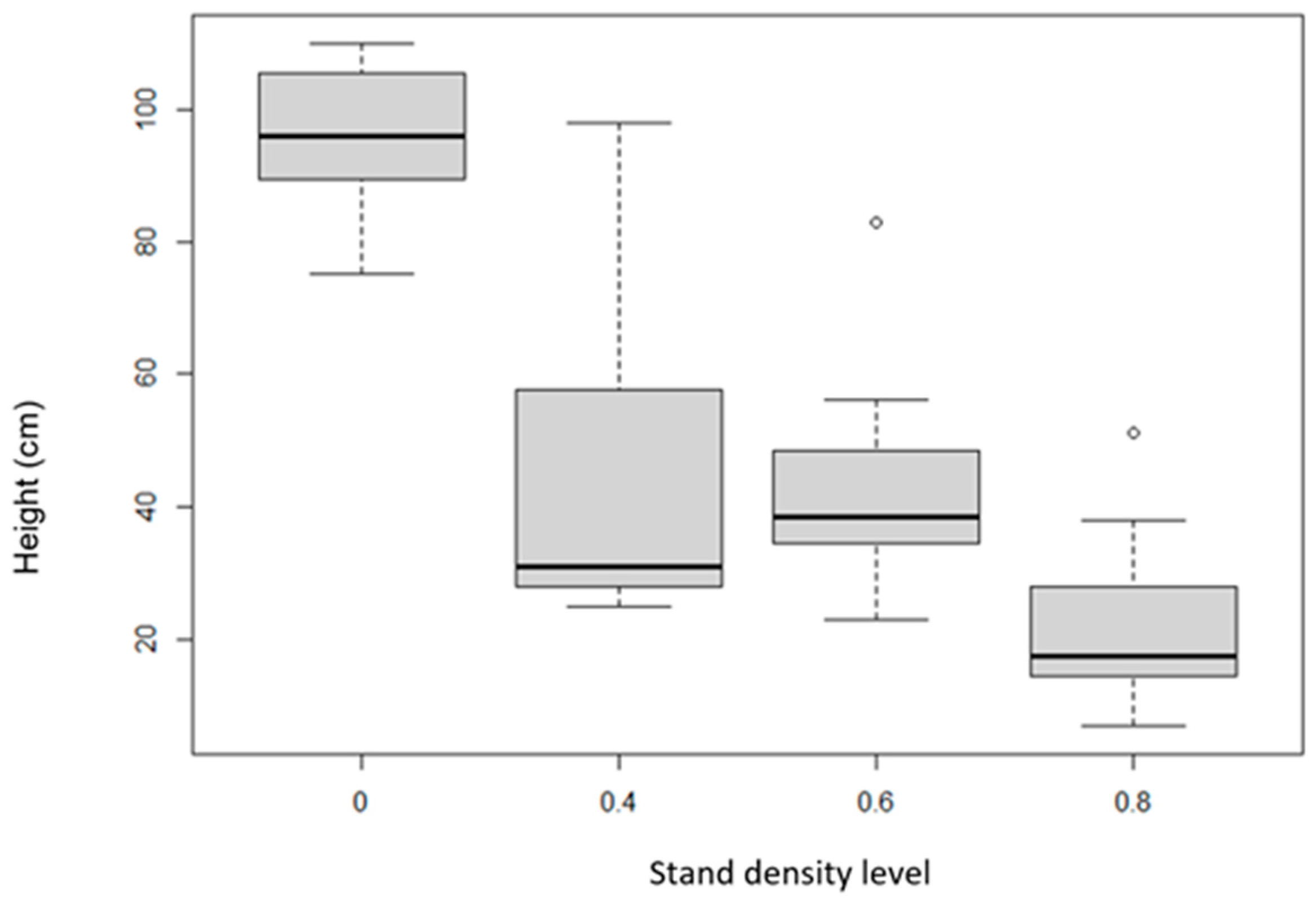
3.2. Dependence of Height of Natural Regeneration Individuals on Stand Density
3.3. Dependence of the Height of Natural Regeneration Individuals on the Biomass of Orders
3.4. Evaluation of the Time and Complexity of Measurements
4. Discussion
4.1. Dependence of Branching on Stand Density
4.2. Dependence of Height of Natural Regeneration Individuals on Stand Density
4.3. Dependence of the Height of Natural Regeneration Individuals on the Biomass of Orders
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


References
- Sharma, R.P.; Bílek, L.; Vacek, Z.; Vacek, S. Modelling crown width-diameter relationship for Scots pine in the central Europe. Trees 2017, 31, 1875–1889. [Google Scholar] [CrossRef]
- Lundqvist, L.; Aahlström, M.A.; Axelsson, E.P.; Mörling, T.; Valinger, E. Multi-layered Scots pine forests in boreal Sweden result from mass regeneration and size stratification. Forest Ecol. Manag. 2019, 441, 176–181. [Google Scholar] [CrossRef]
- Liška, J.; Knížek, M.; Véle, A. Evaluation of insect pest occurrence in areas of calamitous mortality of Scots pine. Cent. Eur. For. 2021, 67, 85–90. [Google Scholar] [CrossRef]
- Sydorenko, S.; Voron, V.; Koval, I.; Sydorenko, S.; Rumiantsev, M.; Hurzhii, R. Postfire tree mortality and fire resistance patterns in pine forests of Ukraine. Cent. Eur. For. J. 2021, 67, 21–29. [Google Scholar] [CrossRef]
- Ministerstvo zemědělství. Zpráva o Stavu Lesa a Lesního Hospodářství; eAGRI, 2021; ISBN 978-80-7434-625-5. [Google Scholar]
- Bílek, L.; Vacek, S.; Vacek, Z.; Remeš, J.; Král, J.; Bulušek, D.; Gallo, J. How close to nature is close-to-nature pine silviculture? J. For. Sci. 2016, 62, 24–34. [Google Scholar] [CrossRef]
- Podrázský, V.; Vacek, Z.; Vacek, S.; Vítámvás, J.; Gallo, J.; Prokůpková, A.; D’Andrea, G. Production potential and structural variability of pine stands in the Czech Republic: Scots pine (Pinus sylvestris L.) vs. introduced pines–case study and problem review. J. For. Sci. 2020, 66, 197–207. [Google Scholar] [CrossRef]
- Poleno, Z.; Vacek, S.; Podrázský, V.; Remeš, J.; Štefančík, I.; Mikeska, M.; Kobliha, J.; Kupka, I.; Malík, V.; Turčáni, M.; et al. Pěstování lesů III. Praktické postupy pěstování lesů.[Silviculture III. Practical methods in silviculture]. Kostelec nad Černými lesy. Lesn. Práce. 2009, 952. [Google Scholar]
- Kurz, W.; Dymond, C.; White, T.; Stinson, G.; Shaw, C.; Rampley, G.; Smyth, C.; Simpson, B.; Neilson, E.; Trofymow, J.; et al. CBM-CFS3: A model of carbon-dynamics in forestry and land-use change implementing IPCC standards. Ecol. Model. 2009, 220, 480–504. [Google Scholar] [CrossRef]
- Lin, Y.; Hyyppä, J.; Kukko, A.; Jaakkola, A.; Kaartinen, H. Tree height growth measurement with single-scan airborne, static terrestrial and mobile laser scanning. Sensors 2012, 12, 12798–12813. [Google Scholar] [CrossRef]
- Côté, J.F.; Fournier, R.A.; Egli, R. An architectural model of trees to estimate forest structural attributes using terrestrial LiDAR. Environ. Model. Softw. 2011, 26, 761–777. [Google Scholar] [CrossRef]
- Brovkina, O.; Novotny, J.; Cienciala, E.; Zemek, F.; Russ, R. Mapping forest aboveground biomass using airborne hyperspectral and LiDAR data in the mountainous conditions of Central Europe. Ecol. Eng. 2017, 100, 219–230. [Google Scholar] [CrossRef]
- Polhemus Incorporated: 3space Fastrak User Manual. Available online: http://www.polhemus.com/fastrak.html (accessed on 5 December 2022).
- Mutke, S.; Nikinmaa, E.; Perttunen, J.; Gil, L.; Sievänen, R. Crown architecture of grafted Stone pine (Pinus pinea L.): Shoot growth and bud differentiation. Trees 2005, 19, 15–25. [Google Scholar] [CrossRef]
- Lang, A. Leaf orientation of a cotton plant. Agric. Meteorol. 1973, 11, 37–51. [Google Scholar] [CrossRef]
- Watanabe, T.; Hanan, J.S.; Room, P.M.; Hasegawa, T.; Nakagawa, H.; Takahashi, W. Rice morphogenesis and plant architecture: Measurement, specification and the reconstruction of structural development by 3D architectural modelling. Ann. Bot. 2005, 95, 1131–1143. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, A.; Surový, P.; Konoshima, M.; Kurth, W. Constructing tree stem form from digitized surface measurements by a programming approach within discrete mathematics. Trees 2014, 28, 1577–1588. [Google Scholar] [CrossRef]
- Han, X.; Hao, X.; Qiu, S.; Guan, S.; Zhan, H.; Yu, J.; Wang, S.; Lu, X. Strigolactone regulates plant architecture by inhibiting lateral branch growth in Quercus mongolica seedlings. Scand. J. For. Res. 2021, 36, 333–343. [Google Scholar] [CrossRef]
- Hu, B.G.; Jaeger, M. Analysis and Modeling of the Root System Architecture of Winter Wheat Seedling; Springer and Tsinghua University Press: Beijing, China, 2003. [Google Scholar]
- Sinoquet, H.; Rivet, P.; Hanan, J.; Měch, R. Measurement and visualization of the architecture of an adult tree based on a three-dimensional digitising device. Trees 1997, 11, 265–270. [Google Scholar] [CrossRef]
- Dawson, T.E. Determining water use by trees and forests from isotopic, energy balance and transpiration analyses: The roles of tree size and hydraulic lift. Tree Physiol. 1996, 16, 263–272. [Google Scholar] [CrossRef]
- Vertessy, R.A.; Benyon, R.G.; O’sullivan, S.K.; Gribben, P.R. Relationships between stem diameter, sapwood area, leaf area and transpiration in a young mountain ash forest. Tree Physiol. 1995, 15, 559–567. [Google Scholar] [CrossRef]
- Vertessy, R.A.; Hatton, T.J.; Reece, P.; O'Sullivan, S.K.; Benyon, R.G. Estimating stand water use of large mountain ash trees and validation of the sap flow measurement technique. Tree Physiol. 1997, 17, 747–756. [Google Scholar] [CrossRef]
- Tfwala, C.M.; Van Rensburg, L.D.; Schall, R.; Zietsman, P.C.; Dlamini, P. Whole tree water use: Effects of tree morphology and environmental factors. Ecol. Indic. 2019, 102, 366–373. [Google Scholar] [CrossRef]
- Kurth, W.; Anzola Jürgenson, G. Triebwachstum und Verzweigung junger Fichten in Abhängigkeit von den beiden Einflussgrößen “Beschattung” und “Wuchsdichte”: Datenaufbereitung undanalyse mit GROGRA. Dtsch. Verb. Forstl. Forschungsanstalten Sekt. Forstl. Biom. Und Inform. 1997, 10, 89–108. [Google Scholar]
- White, J. The plant as a metapopulation. Annu. Rev. Ecol. Syst. 1979, 10, 109–145. [Google Scholar] [CrossRef]
- Kellomäki, S.; Strandman, H. A model for the structural growth of young Scotch pine crowns based on light interception byshoots. Ecol. Model. 1995, 80, 237–2501. [Google Scholar] [CrossRef]
- Sievänen, R.; Nikinmaa, E.; Nygren, P.; Ozier-Lafontaine, H.; Perttunen, J.; Hakula, H. Components of functional-structural tree models. Ann. For. Sci. 2000, 57, 399–412. [Google Scholar] [CrossRef]
- Gavrikov, V.L.; Sekretenko, O.P. Shoot-based three-dimensional model of young Scots pine growth. Ecol. Model. 1996, 88, 183–193. [Google Scholar] [CrossRef]
- Kellomäki, S.; Ikonen, V.P.; Peltola, H.; Kolström, T. Modelling the structural growth of Scots pine with implications for wood quality. Ecol. Model. 1999, 122, 117–134. [Google Scholar] [CrossRef]
- Paletto, A.; Tosi, V. Forest canopy cover and canopy closure: Comparison of assessment techniques. Eur. J. For. Res. 2009, 128, 265–272. [Google Scholar] [CrossRef]
- James, K.R.; Dahle, G.A.; Grabosky, J.; Kane, B.; Detter, A. Tree biomechanics literature review: Dynamics. Arboric. Urban. For. 2014, 40, 1–15. [Google Scholar] [CrossRef]
- Schönfelder, O.; Zeidler, A.; Borůvka, V.; Bílek, L.; Vítámvás, J. Effect of Shelterwood and Clear-Cutting Regeneration Method on Wood Density of Scots Pine. Forests 2020, 11, 868. [Google Scholar] [CrossRef]
- Lance, S. EvansZella Kahn-Jetter Jessica Torres Mabel Martinez Paul. Tarsia 2008 Mechanical stresses of primary branches: A survey of 40 woody tree and shrub species. Trees 2008, 22, 283–289. [Google Scholar] [CrossRef]
- Danjon, F.; Sinoquet, H.; Godin, C.; Colin, F.; Drexhage, M. Characterisation of structural tree root architecture using 3D digitising and AMAPmod software. Plant Soil 1999, 211, 241–258. [Google Scholar] [CrossRef]
- Långström, B.; Hellqvist, C. Effects of different pruning regimes on growth and sapwood area of Scots pine. For. Ecol. Manag. 1991, 44, 239–254. [Google Scholar] [CrossRef]
- Soil Map. Available online: http://www.geology.cz/extranet-eng/services/web-applications/ (accessed on 11 March 2020).
- Brichta, J.; Linda, R.; Bílek, L.; Vitamvas, J. Does shelterwood regeneration on natural Scots pine sites under changing environmental conditions represent a viable alternative to traditional clear-cut management? Cent. Eur. For. J. 2020, 66, 9. [Google Scholar] [CrossRef]
- Bílek, L.; Vacek, Z.; Vacek, S.; Bulušek, D.; Linda, R.; Král, J. Are clearcut borders an effective tool for Scots pine (Pinus sylvestris L.) natural regeneration? For. Syst. 2018, 27, e010. [Google Scholar] [CrossRef]
- Köppen, W. Das Geographische System der Klimate, Handbuch der Klimatologie; Gebrüder Borntraeger: Berlin, Germany, 1936. [Google Scholar]
- Raab, F.H.; Blood, E.B.; Steiner, T.O.; Jones, H.R. Magnetic position and orientation tracking system. IEEE Trans. Aerosp. Electron. Syst. 1979, AES-15, 709–718. [Google Scholar] [CrossRef]
- Jaeger, H.A.; Cantillon-Murphy, P. Distorter characterisation using mutual inductance in electromagnetic tracking. Sensors 2018, 18, 3059. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Vaughan, I.; Lello, J. Data analysis with R statistical software. A guidebook for scientists. Eco-Explore 2017, 80. [Google Scholar]
- Kurth, W. Specification of morphological models with L-systems and relational growth grammars. IMAGE. Z. Für Interdiszip. Bild. 2007, 3, 50–79. [Google Scholar]
- Lefsky, M.A.; Harding, D.J.; Keller, M.; Cohen, W.B.; Carabajal, C.C.; Del Bom, F.; Hunter, M.O.; de Oliveira, R., Jr. Estimates of forest canopy height and aboveground biomass using ICESat. Geophys. Res. Lett. 2005, 32, 1–4. [Google Scholar] [CrossRef]
- Lefsky, M.A.; Harding, D.; Cohen, W.; Parker, G.; Shugart, H. Surface lidar remote sensing of basal area and biomass in deciduous forests of eastern maryland, USA. Remote Sens. Environ. 1999, 67, 83–98. [Google Scholar] [CrossRef]
- Lefsky, M.A.; Cohen, W.B.; Spies, T.A. An evaluation of alternate remote sensing products for forest inventory, monitoring, and mapping of Douglas-fir forests in western Oregon. Can. J. For. Res. 2001, 31, 78–87. [Google Scholar] [CrossRef]
- Wagner, C.V. Conditions for the start and spread of crown fire. Can. J. For. Res. 1977, 7, 23–34. [Google Scholar] [CrossRef]
- Cohen, J.D.; Sutherland, E.K. Crown fires and fire behavior in conifer forests. For. Ecol. Mat. 1996, 89, 44–55. [Google Scholar]
- Hoffman, C.M.; Morgan, P.; Charnley, S.; Collins, B.M.; Mell, W. Fire regime conditions and crown fire potential in western US forests. For. Ecol. Manag. 2016, 378, 57–67. [Google Scholar] [CrossRef]
- Hood, S.M.; Smith, S.L.; Ottmar, R.D. Crown Fire Behavior in Western Conifer Forests: A Synthesis of Research and Management Implications; General Technical Report PNW-GTR-696; United States Department of Agriculture Forest Service: Washington, DC, USA, 2014; pp. 73–74. [Google Scholar]
- Finney, M.A.; McAllister, S.S.; Cohen, J.D. Modeling crown fire initiation and spread in conifer forest stands. For Ecol. Manag. 2013, 310, 499–513. [Google Scholar] [CrossRef]
- Rauh, W. Über Gesetzmäßigkeit der Verzweigung und deren Bedeutung für die Wuchsformen der Pflanzen. Mitt. Dtsch. Dendrol. Ges. 1939, 52, 86–111. [Google Scholar]
- Hallé, F.; Oldeman, R.A.; Tomlinson, P.B. Tropical Trees and Forests: An Architectural Analysis; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Tomlinson, P.B. Chance and Design in the Construction of Plants. In Axioms and Principles of Plant Construction; Sattler, R., Ed.; Springer: Dordrecht, The Netherlands, 1982. [Google Scholar] [CrossRef]
- Konopka, B.; Šebeň, V.; Merganicova, K.; Pajtík, J. Silver birch aboveground biomass allocation pattern, stem and foliage traits with regard to intraspecific crown competition. J. For. 2020, 66, 12. [Google Scholar] [CrossRef]
- Dušek, D.; Novak, J.; Slodičak, M. Experimenty s výchovou borovice lesní na jižní Moravě–Strážnice I a Strážnice III. Zprávy Lesn. Výzkumu 2011, 56, 283–290. (In Czech) [Google Scholar]
- Surový, P.; Ribeiro, N.; Pereira, J.S. Observations on 3-dimensional crown growth of Stone pine. Agrofor. Syst. 2011, 82, 105–110. [Google Scholar] [CrossRef]
- Riikonen, J.; Kettunen, N.; Gritsevich, M.; Hakala, T.; Särkkä, L.; Tahvonen, R. Growth and development of Norway spruce and Scots pine seedlings under different light spectra. Environ. Exp. Bot. 2016, 121, 112–120. [Google Scholar] [CrossRef]
- Chidi, E.B.; Ebenezer, M.I.; Kenneth, E.U. Tree Crown Architecture: Approach to Tree Form, Structure and Performance: A Review. Int. J. Sci. Res. 2014, 1997, 5. [Google Scholar]
- Popescu, S.C.; Wynne, R.H.; Nelson, R.F. Estimating plot-level tree heights with lidar: Local filtering with a canopy-height based variable window size. Comput. Electron. Agric. 2002, 37, 71–95. [Google Scholar] [CrossRef]
- Wiedemann, E. Die Praktischen Erfolge des Kieferndauerwaldes. Verlag von Friedr; Vieweg & Sohn: Braunschweig, Germany, 1925; 184p. [Google Scholar]
- Barbeito, I.; LeMay, V.; Calama, R.; Canellas, I. Regeneration of Mediterranean Pinus sylvestris under two alternative shelterwood systems within a multiscale framework. Can. J. For. Res. 2011, 41, 341–351. [Google Scholar] [CrossRef]
- Aleksandrowicz-Trzcińska, M.; Drozdowski, S.; Wolczyk, Z.; Bielak, K.; Żybura, H. Effects of Reforestation and Site Preparation Methods on Early Growth and Survival of Scots Pine (Pinus sylvestris L.) in South-Eastern Poland. Forests 2017, 8, 421. [Google Scholar] [CrossRef]
- Horn, H.S. Adaptive Geometry of Trees; Princeton University Press: Princeton, NJ, USA, 1971. [Google Scholar]
- Yamamura, Y.; Ishida, A.; Hori, Y. Differences in sapling architecture between Fagus crenata and Fagus japonica. Ecol. Res. 1993, 8, 235–239. [Google Scholar] [CrossRef]
- Fournier, A. Is Architectural Radiation Adaptive. D.F.A. Dissertation, Univ. Montpellier II, 1979; 51p. [Google Scholar]
- Fisher, J.B.; Hibbs, D.E. Plasticity of tree architecture: Specific and ecological variations found in Aubreville’s model. Am. J. Bot. 1982, 69, 690–702. [Google Scholar] [CrossRef]
- Stevens, G.C.; Perkins, A.L. The branching habits and life history of woody plants. Am. Nat. 1992, 139, 267–275. [Google Scholar] [CrossRef]
- Jelonek, T.; Kopaczyk, J.; Neumann, M.; Tomczak, A.; Pazdrowski, W.; Grzywiński, W.; Klimek, K.; Naskrent, B.; Kuźmiński, R.; Szwed, T. How Wood Quality Can Be Shaped: Results of 70 Years of Experience. Forests 2022, 13, 2103. [Google Scholar] [CrossRef]
- Šebeň, V. Indices of Tree Competition in Dense Spruces Stand Originated from Natural Regeneration; Slovak Academic Press: Lesnícký časopis Bratislava, Slovakia, 2013; Volume 59, pp. 172–179. (In Czech) [Google Scholar]
- Oliver, C.W.; Larson, B.C. Forest Stand Dynamics: Update edition; Wiley: New York, NY, USA, 1996; 520p. [Google Scholar]
- Oppelt, A.L.; Kurth, W.; Dzierzon, H.; Jentschke, G.; Godbold, D.L. Structure and fractal dimensions of root systems of four co-occurring fruit tree species from Botswana. Ann. For. Sci. 2000, 57, 463–475. [Google Scholar] [CrossRef]
- Oppelt, A.L.; Kurth, W.; Godbold, D.L. Topology, scaling relations and Leonardo’s rule in root systems from African tree species. Tree Physiol. 2001, 21, 117–128. [Google Scholar] [CrossRef] [PubMed]





| Coefficients | Intercept | Stock Density (Estimate) | STD | Error from Value | Pr (>|z|) |
|---|---|---|---|---|---|
| Order 1 | 2.73680 | −1.62223 | 0.20893 | −7.765 | 8.19 × 10−15 *** |
| Order 2 | 3.11377 | −3.28529 | 0.24351 | −13.49 | <2 × 10−16 *** |
| Order 3 | 1.1635 | −6.4741 | I.14 | −4.721 | 2.35 × 10−6 *** |
| Coefficients | Estimate | STD | Error from Value | Pr (>|z|) |
|---|---|---|---|---|
| Intercept | 2.73680 | 0.09168 | 29.852 | <2 × 10−16 *** |
| Stand density level | −1.6223 | 0.20893 | −7.765 | 8.19 × 10−15 *** |
| Coefficients | Estimate | STD | Error from Value | Pr (>|z|) |
|---|---|---|---|---|
| Order 1 | 0.087987 | 0.016221 | 5.424 | 1.10 × 10−5 *** |
| Order 2 | 0.013591 | 0.006651 | 2.043 | 0.05127 |
| Order 3 | −0.233641 | 0.080563 | −2.900 | 0.00749 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šleglová, K.; Brichta, J.; Bílek, L.; Surový, P. Measuring the Canopy Architecture of Young Vegetation Using the Fastrak Polhemus 3D Digitizer. Sensors 2024, 24, 109. https://doi.org/10.3390/s24010109
Šleglová K, Brichta J, Bílek L, Surový P. Measuring the Canopy Architecture of Young Vegetation Using the Fastrak Polhemus 3D Digitizer. Sensors. 2024; 24(1):109. https://doi.org/10.3390/s24010109
Chicago/Turabian StyleŠleglová, Kristýna, Jakub Brichta, Lukáš Bílek, and Peter Surový. 2024. "Measuring the Canopy Architecture of Young Vegetation Using the Fastrak Polhemus 3D Digitizer" Sensors 24, no. 1: 109. https://doi.org/10.3390/s24010109





