In Situ Synthesis of Highly Fluorescent, Phosphorus-Doping Carbon-Dot-Functionalized, Dendritic Silica Nanoparticles Applied for Multi-Component Lateral Flow Immunoassay
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
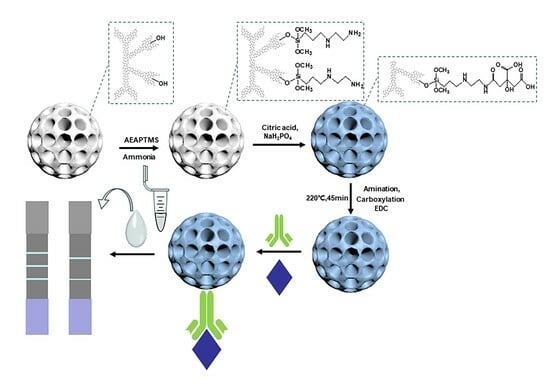
2.2. Synthesis of DMSNs-BCDs
2.3. Measurement of Fluorescence Quantum Yield
2.4. Preparation of Sample (DMSNs-BCDs Label Antibody)
2.5. Detection of DMSNs-BCDs-Based LFIA
3. Results and Discussion
3.1. Synthesis and Characterization of DMSNs-BCDs
3.2. Performance of DMSNs-BCDs-LFIA for PCT, CA199, and AFP Detection
3.3. Stability and Specificity Detection of DMSNs-BCDs-LFIA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fernandes, E.; Sores, J.; Cotton, S.; Peixoto, A.; Ferreira, D.; Freitas, R.; Reis, C.A.; Santos, L.L.; Ferreira, J.A. Esophageal, gastric and colorectal cancers: Looking beyond classical serological biomarkers towards glycoproteomics-assisted precision oncology. Theranostics 2020, 10, 4903–4928. [Google Scholar] [CrossRef]
- Liu, Z.W.; Yang, D.Y.; Gao, J.L.; Xiang, X.H.; Hu, X.R.; Li, S.Y.; Wu, W.W.; Cai, J.; Tang, C.Y.; Zhang, D.S.; et al. Discovery and validation of miR-452 as an effective biomarker for acute kidney injury in sepsis. Theranostics 2020, 10, 11963–11975. [Google Scholar] [CrossRef]
- Gan, N.; Xie, L.; Zhang, K.; Cao, Y.; Hu, F.; Li, T. An endonuclease-linked multiplex immunoassay for tumor markers detection based on microfluidic chip electrophoresis for DNA analysis. Sens. Actuators B Chem. 2018, 272, 526–533. [Google Scholar] [CrossRef]
- Zhou, C.Q.; Fang, Z.C.; Zhao, C.Y.; Mai, X.Y.; Emami, S.; Taha, A.Y.; Sun, G.; Pan, T.R. Sample-to-answer robotic ELISA. Anal. Chem. 2021, 93, 11424–11432. [Google Scholar] [CrossRef]
- Chen, P.P.; Qiao, X.Y.; Liu, J.H.; Xia, F.Q.; Tian, D.; Zhou, C.L. A dual-signals response electrochemiluminescence immunosensor based on PTC-DEPA/KCC-1 NCs for detection of procalcitonin. Sens. Actuators B Chem. 2018, 267, 525–532. [Google Scholar] [CrossRef]
- Feng, X.; Gan, N.; Zhou, J.; Li, T.; Cao, Y.; Hu, F.; Yu, H.; Jiang, Q. A novel dual-template molecularly imprinted electrochemiluminescence immunosensor array using Ru(bpy)32+-Silica@Poly-L-lysine-Au composite nanoparticles as labels for near-simultaneous detection of tumor markers. Electrochim. Acta 2014, 139, 127–136. [Google Scholar] [CrossRef]
- Wang, R.; Feng, J.J.; Liu, W.D.; Jiang, L.Y.; Wang, A.J. A novel label-free electrochemical immunosensor based on the enhanced catalytic currents of oxygen reduction by AuAg hollow nanocrystals for detecting carbohydrate antigen 199. Biosens. Bioelectron. 2017, 96, 152–158. [Google Scholar] [CrossRef]
- Sun, J.; Tian, D.; Guo, Q.; Zhang, L.; Jiang, W.; Yang, M. A label-free electrochemical immunosensor for the detection of cancer biomarker α-fetoprotein (AFP) based on hydroxyapatite induced redox current. Anal. Methods 2016, 8, 7319–7323. [Google Scholar] [CrossRef]
- Wang, G.; Wan, Y.; Lin, G.F.; Li, Z.X.; Dong, Z.N.; Liu, T.C. Development of a novel chemiluminescence immunoassay for the detection of procalcitonin. J. Immunol. Methods 2020, 484, 4. [Google Scholar] [CrossRef]
- Chu, W.R.; Chen, Y.; Liu, W.; Zhao, M.; Li, H.F. Paper-based chemiluminescence immunodevice with temporal controls of reagent transport technique. Sens. Actuators B Chem. 2017, 250, 324–332. [Google Scholar] [CrossRef]
- Zong, C.; Wu, J.; Wang, C.; Ju, H.X.; Yan, F. Chemiluminescence imaging immunoassay of multiple tumor markers for cancer screening. Anal. Chem. 2012, 84, 2410–2415. [Google Scholar] [CrossRef]
- Liu, H.; Cao, J.; Ding, S.N. Simultaneous detection of two ovarian cancer biomarkers in human serums with biotin-enriched dendritic mesoporous silica nanoparticles-labeled multiplex lateral flow immunoassay. Sens. Actuators B Chem. 2022, 371, 8. [Google Scholar] [CrossRef]
- Song, C.M.; Zhi, A.M.; Liu, Q.T.; Yang, J.F.; Jia, G.C.; Shervin, J.; Tang, L.; Hu, X.F.; Deng, R.G.; Xu, C.L.; et al. Rapid and sensitive detection of β-agonists using a portable fluorescence biosensor based on fluorescent nanosilica and a lateral flow test strip. Biosens. Bioelectron. 2013, 50, 62–65. [Google Scholar] [CrossRef]
- Yan, Z.Q.; Zhou, L.; Zhao, Y.K.; Wang, J.; Huang, L.H.; Hu, K.X.; Liu, H.H.; Wang, H.; Guo, Z.B.; Song, Y.J.; et al. R Rapid quantitative detection of Yersinia pestis by lateral-flow immunoassay and up-converting phosphor technology-based biosensor. Sens. Actuators B Chem. 2006, 119, 656–663. [Google Scholar] [CrossRef]
- Wang, C.W.; Shen, W.Z.; Rong, Z.; Liu, X.X.; Gu, B.; Xiao, R.; Wang, S.Q. Layer-by-layer assembly of magnetic-core dual quantum dot-shell nanocomposites for fluorescence lateral flow detection of bacteria. Nanoscale 2020, 12, 795–807. [Google Scholar] [CrossRef]
- Parolo, C.; Sena-Torralba, A.; Bergua, J.F.; Calucho, E.; Fuentes-Chust, C.; Hu, L.M.; Rivas, L.; Alvarez-Diduk, R.; Nguyen, E.P.; Cinti, S.; et al. Tutorial: Design and fabrication of nanoparticle-based lateral-flow immunoassays. Nat. Protoc. 2020, 15, 3788–3816. [Google Scholar] [CrossRef]
- Jiao, X.; Peng, T.; Liang, Z.; Hu, Y.; Meng, B.; Zhao, Y.; Xie, J.; Gong, X.; Jiang, Y.; Fang, X.; et al. Lateral flow immunoassay based on time-resolved fluorescence microspheres for rapid and quantitative screening CA199 in human serum. Int. J. Mol. Sci. 2022, 23, 9991. [Google Scholar] [CrossRef]
- Lu, L.C.; Yu, J.L.; Liu, X.X.; Yang, X.S.; Zhou, Z.H.; Jin, Q.; Xiao, R.; Wang, C.W. Rapid, quantitative and ultra-sensitive detection of cancer biomarker by a SERRS-based lateral flow immunoassay using bovine serum albumin coated Au nanorods. RSC Adv. 2020, 10, 271–281. [Google Scholar] [CrossRef]
- Serebrennikova, K.V.; Samsonova, J.V.; Osipov, A.P. A semi-quantitative rapid multi-range gradient lateral flow immunoassay for procalcitonin. Mikrochim. Acta 2019, 186, 8. [Google Scholar] [CrossRef]
- Zhou, S.; Peng, Y.L.; Hu, J.; Duan, H.; Ma, T.T.; Hou, L.; Li, X.M.; Xiong, Y.H. Quantum dot nanobead-based immunochromatographic assay for the quantitative detection of the procalcitonin antigen in serum samples. Microchem. J. 2020, 159, 7. [Google Scholar] [CrossRef]
- Ashoka, A.H.; Aparin, I.O.; Reisch, A.; Klymchenko, A.S. Brightness of fluorescent organic nanomaterials. Chem. Soc. Rev. 2023, 52, 4525–4548. [Google Scholar] [CrossRef]
- Guo, J.; Lu, Y.; Xie, A.Q.; Li, G.; Liang, Z.B.; Wang, C.F.; Yang, X.; Chen, S. Yellow-emissive carbon dots with high solid-state photoluminescence. Adv. Funct. Mater. 2022, 32, 2110393. [Google Scholar] [CrossRef]
- Li, X.C.; Zhao, S.J.; Li, B.L.; Yang, K.; Lan, M.H.; Zeng, L.T. Advances and perspectives in carbon dot-based fluorescent probes: Mechanism, and application. Coord. Chem. Rev. 2021, 431, 22. [Google Scholar] [CrossRef]
- Lu, S.; Xiao, G.; Sui, L.; Feng, T.; Yong, X.; Zhu, S.; Li, B.; Liu, Z.; Zou, B.; Jin, M.; et al. Piezochromic carbon dots with two-photon fluorescence. Angew. Chem. Int. Ed. 2017, 56, 6187–6191. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Q.; Wang, S.; Yu, X.; Wang, B.; Chen, G.; Ren, L.; Li, J.; Jin, M.; Yu, J. Confining carbon dots in amino-functionalized mesoporous silica: N-π* interaction triggered deep-red solid-state fluorescence. Nano Res. 2022, 16, 4170–4177. [Google Scholar] [CrossRef]
- Kong, Y.; Jing, Y.; Sun, H.; Zhou, S. The diagnostic value of contrast-enhanced ultrasound and enhanced CT combined with tumor markers AFP and CA199 in liver cancer. J. Healthc. Eng. 2022, 2022, 5074571. [Google Scholar] [CrossRef]
- Wang, B.; Lu, S. The light of carbon dots: From mechanism to applications. Matter 2022, 5, 110–149. [Google Scholar] [CrossRef]
- Jiang, L.; Ding, H.; Lu, S.; Geng, T.; Xiao, G.; Zou, B.; Bi, H. Photoactivated fluorescence enhancement in F, N-doped carbon dots with piezochromic behavior. Angew. Chem. Int. Ed. 2019, 59, 9986–9991. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Y.; Cheng, H.; Hu, Y.; Shi, G.; Dai, L.; Qu, L. Nitrogen-doped graphene quantum dots with oxygen-rich functional groups. J. Am. Chem. Soc. 2011, 134, 15–18. [Google Scholar] [CrossRef]
- Gong, Y.; Yu, B.; Yang, W.; Zhang, X. Phosphorus, and nitrogen co-doped carbon dots as a fluorescent probe for real-time measurement of reactive oxygen and nitrogen species inside macrophages. Biosens. Bioelectron. 2016, 79, 822–828. [Google Scholar] [CrossRef]
- Wang, C.; Wu, X.; Li, X.; Wang, W.; Wang, L.; Gu, M.; Li, Q. Upconversion fluorescent carbon nanodots enriched with nitrogen for light harvesting. J. Mater. Chem. 2012, 22, 15522–15525. [Google Scholar] [CrossRef]
- Huang, S.; Yang, E.; Liu, Y.; Yao, J.; Su, W.; Xiao, Q. Low-temperature rapid synthesis of nitrogen and phosphorus dual-doped carbon dots for multicolor cellular imaging and hemoglobin probing in human blood. Sens. Actuators B Chem. 2018, 265, 326–334. [Google Scholar] [CrossRef]
- Gao, F.; Lei, C.; Liu, Y.; Song, H.; Kong, Y.Q.; Wan, J.J.; Yu, C.Z. Rational design of dendritic mesoporous silica nanoparticles’ surface chemistry for quantum dot enrichment and an ultrasensitive lateral flow immunoassay. ACS Appl. Mater. Interfaces 2021, 13, 21507–21515. [Google Scholar] [CrossRef]
- Gao, F.; Liu, Y.; Lei, C.; Liu, C.; Song, H.; Gu, Z.; Jiang, P.; Jing, S.; Wan, J.; Yu, C. The role of dendritic mesoporous silica nanoparticles’ size for quantum dots enrichment and lateral flow immunoassay performance. Small Methods 2021, 5, e2000924. [Google Scholar] [CrossRef]
- Gao, F.; Liu, C.; Yao, Y.; Lei, C.; Li, S.; Yuan, L.; Song, H.; Yang, Y.; Wan, J.; Yu, C. Quantum dots’ size matters for balancing their quantity and quality in label materials to improve lateral flow immunoassay performance for C-reactive protein determination. Biosens. Bioelectron. 2022, 199, 113892. [Google Scholar] [CrossRef]
- Huang, L.; Liao, T.; Wang, J.; Ao, L.J.; Su, W.; Hu, J. Brilliant pitaya-type silica colloids with central-radial and high-density quantum dots incorporation for ultrasensitive fluorescence immunoassays. Adv. Funct. Mater. 2018, 28, 11. [Google Scholar] [CrossRef]
- Sun, X.; Bruckner, C.; Lei, Y. One-pot and ultrafast synthesis of nitrogen and phosphorus co-doped carbon dots possessing bright dual wavelength fluorescence emission. Nanoscale 2015, 7, 17278–17282. [Google Scholar] [CrossRef]
- Zhao, L.; Cheng, M.; Liu, G.; Lu, H.; Gao, Y.; Yan, X.; Liu, F.; Sun, P.; Lu, G. A fluorescent biosensor based on molybdenum disulfide nanosheets and protein aptamer for sensitive detection of carcinoembryonic antigen. Sens. Actuators B Chem. 2018, 273, 185–190. [Google Scholar] [CrossRef]
- Jiao, Y.; Du, C.; Zong, L.; Guo, X.; Han, Y.; Zhang, X.; Li, L.; Zhang, C.; Ju, Q.; Liu, J.; et al. 3D vertical-flow paper-based device for simultaneous detection of multiple cancer biomarkers by fluorescent immunoassay. Sens. Actuators B Chem. 2020, 306. [Google Scholar] [CrossRef]
- Yang, L.Y.; Du, L.; Hou, B.L.; Niu, X.Q.; Wang, W.; Shen, W.F. Clinical value of combined multi-indicator tests in diagnosis of benign ovarian. Int. J. Gen. Med. 2023, 16, 2047–2053. [Google Scholar] [CrossRef]
- Xu, L.D.; Wang, S.W.; Zhu, J.; Zhou, T.T.; Ding, S.N. Dendritic silica nanospheres loaded with red-emissive enhanced carbon dots for Zika virus immunoassay. ChemistrySelect 2021, 6, 9787–9793. [Google Scholar] [CrossRef]
- Xu, L.D.; Zhu, J.; Ding, S.N. Highly-fluorescent carbon dots grown onto dendritic silica nanospheres for anthrax protective antigen detection. Anal. Methods 2022, 14, 1836–1840. [Google Scholar] [CrossRef]
- Kim, M.; Kim, M.S.; Kweon, S.H.; Jeong, S.; Kang, M.H.; Kim, M.I.; Lee, J.; Doh, J. Simple and sensitive point-of-care bioassay system based on hierarchically structured enzyme-mimetic nanoparticles. Adv. Healthc. Mater. 2015, 4, 1311–1316. [Google Scholar] [CrossRef]
- Kumar, J.V.; Kavitha, G.; Albasher, G.; Sajjad, M.; Arulmozhi, R.; Komal, M.; Nivetha, M.S.; Abirami, N. Multiplex heteroatoms doped carbon nano dots with enhanced catalytic reduction of ionic dyes and QR code security label for anti-spurious applications. Chemosphere 2022, 307, 12. [Google Scholar] [CrossRef]
- Zhi, B.; Gallagher, M.J.; Frank, B.P.; Lyons, T.Y.; Qiu, T.A.; Da, J.; Mensch, A.C.; Hamers, R.J.; Rosenzweig, Z.; Fairbrother, D.H.; et al. Investigation of phosphorous doping effects on polymeric carbon dots: Fluorescence, photostability, and environmental impact. Carbon 2018, 129, 438–449. [Google Scholar] [CrossRef]
- Shamsipur, M.; Molaei, K.; Molaabasi, F.; Hosseinkhani, S.; Alizadeh, N.; Alipour, M.; Moassess, S. One-step synthesis and characterization of highly luminescent nitrogen and phosphorus co-doped carbon dots and their application as highly selective and sensitive nanoprobes for low level detection of uranyl ion in hair and water samples and application to cellular imaging. Sens. Actuators B Chem. 2018, 257, 772–782. [Google Scholar] [CrossRef]
- Barman, M.K.; Jana, B.; Bhattacharyya, S.; Patra, A. Photophysical properties of doped carbon dots (N, P, and B) and their influence on electron/hole transfer in carbon dots–nickel (II) phthalocyanine conjugates. J. Phys. Chem. C 2014, 118, 20034–20041. [Google Scholar] [CrossRef]
- Li, H.; Shao, F.-Q.; Zou, S.-Y.; Yang, Q.-J.; Huang, H.; Feng, J.-J.; Wang, A.-J. Microwave-assisted synthesis of N, P-doped carbon dots for fluorescent cell imaging. Microchim. Acta 2015, 183, 821–826. [Google Scholar] [CrossRef]
- Wang, Z.X.; Ding, S.N. Duplex-immunoassay of ovarian cancer biomarker CA125 and HE4 based carbon dot decorated dendritic mesoporous silica nanoparticles. Analyst 2023, 148, 683–689. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.-X.; Ding, S.-N. In Situ Synthesis of Highly Fluorescent, Phosphorus-Doping Carbon-Dot-Functionalized, Dendritic Silica Nanoparticles Applied for Multi-Component Lateral Flow Immunoassay. Sensors 2024, 24, 19. https://doi.org/10.3390/s24010019
Hu J-X, Ding S-N. In Situ Synthesis of Highly Fluorescent, Phosphorus-Doping Carbon-Dot-Functionalized, Dendritic Silica Nanoparticles Applied for Multi-Component Lateral Flow Immunoassay. Sensors. 2024; 24(1):19. https://doi.org/10.3390/s24010019
Chicago/Turabian StyleHu, Jia-Xuan, and Shou-Nian Ding. 2024. "In Situ Synthesis of Highly Fluorescent, Phosphorus-Doping Carbon-Dot-Functionalized, Dendritic Silica Nanoparticles Applied for Multi-Component Lateral Flow Immunoassay" Sensors 24, no. 1: 19. https://doi.org/10.3390/s24010019