Dopamine Measurement Using Engineered CNT–CQD–Polymer Coatings on Pt Microelectrodes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Solution and Chemicals
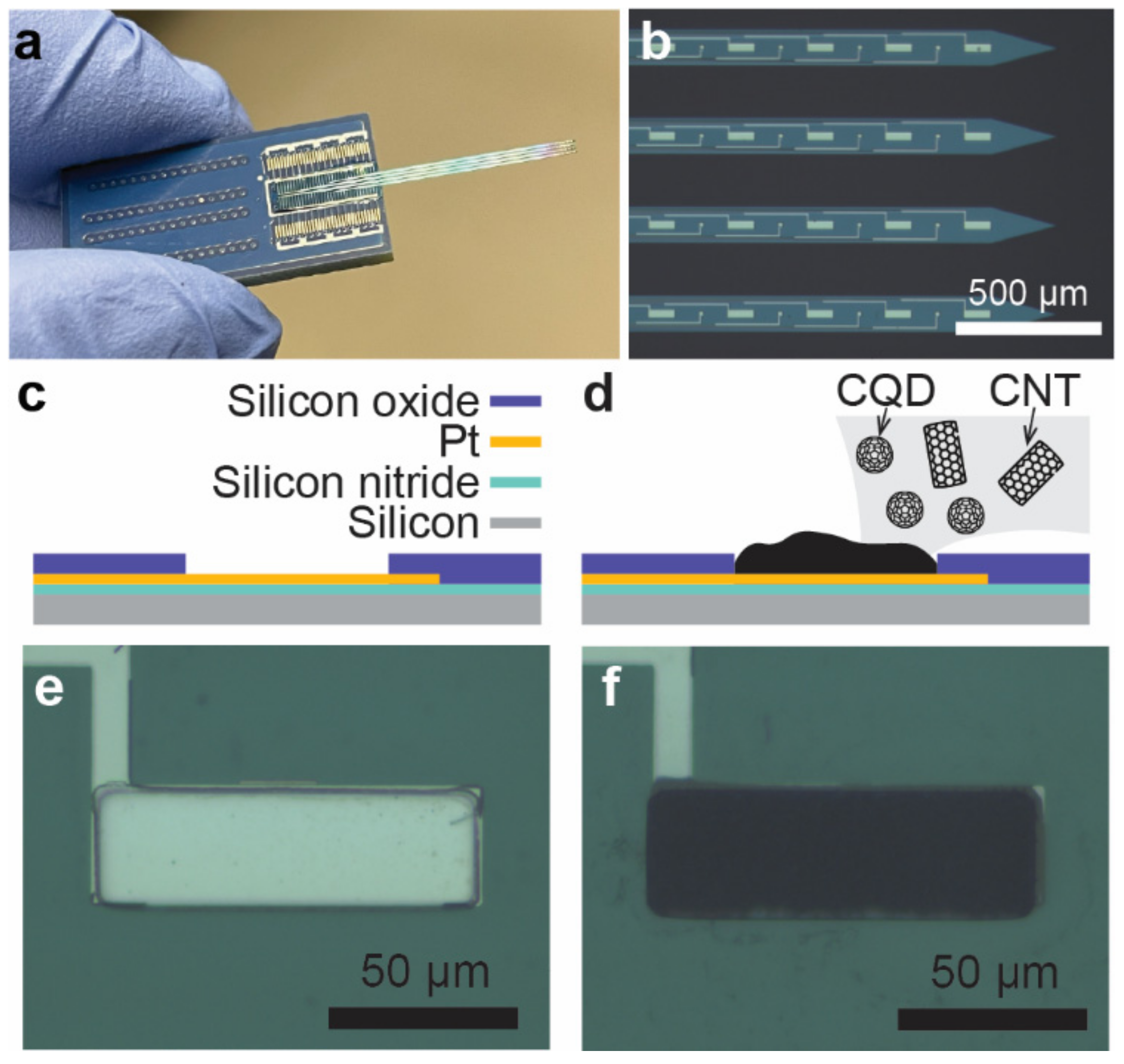
2.2. Preparation of Carbonized Nanocomposite Modified Microelectrodes
2.3. Preparation of CNT–PEDOT and CNT–CQD–PEDOT Modified Microelectrodes
2.4. Preparation of CNT–PPy and CNT–CQD–PPy Modified Microelectrodes
2.5. Electrochemical Analysis
3. Results
3.1. Surface Characterization
3.2. Electrochemical Characterization
3.3. Electrochemical Recordings of Dopamine
3.4. Dopamine Sensitivity
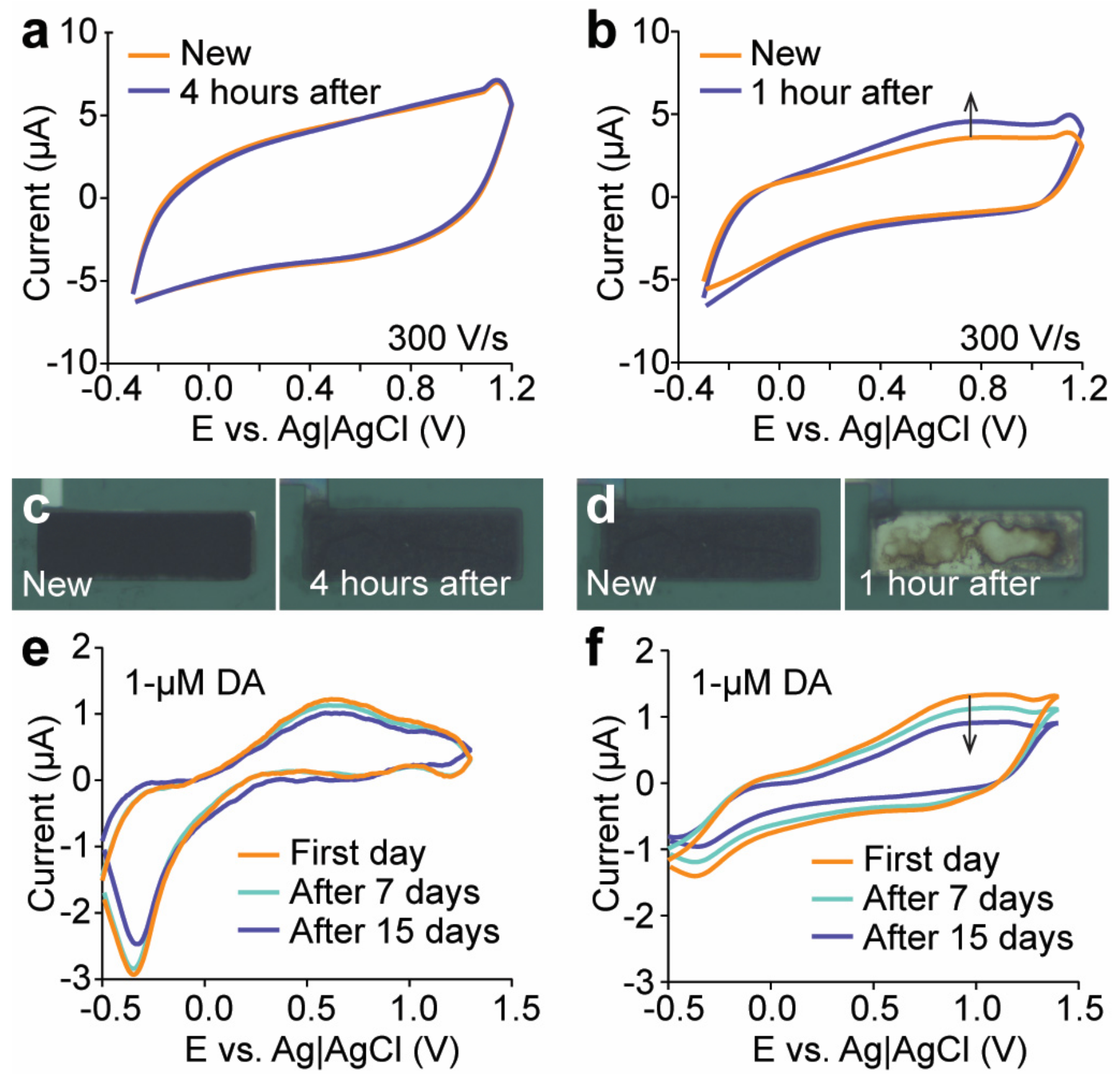
3.5. Stability of Engineered Microelectrodes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Robinson, D.L.; Hermans, A.; Seipel, A.T.; Wightman, R.M. Monitoring Rapid Chemical Communication in the Brain. Chem. Rev. 2008, 108, 2554–2584. [Google Scholar] [CrossRef]
- Ganesana, M.; Lee, S.T.; Wang, Y.; Venton, B.J. Analytical Techniques in Neuroscience: Recent Advances in Imaging, Separation, and Electrochemical Methods. Anal. Chem. 2017, 89, 314–341. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Venton, B.J. Optimization of Graphene Oxide-Modified Carbon-Fiber Microelectrode for Dopamine Detection. Anal. Methods 2020, 12, 2893–2902. [Google Scholar] [CrossRef] [PubMed]
- Puthongkham, P.; Venton, B.J. Recent Advances in Fast-Scan Cyclic Voltammetry. Analyst 2020, 145, 1087–1102. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.G.; Sombers, L.A. Fast-Scan Cyclic Voltammetry: Chemical Sensing in the Brain and Beyond. Anal. Chem. 2018, 90, 490–504. [Google Scholar] [CrossRef] [PubMed]
- Bucher, E.S.; Wightman, R.M. Electrochemical Analysis of Neurotransmitters. Annu. Rev. Anal. Chem. 2015, 8, 239–261. [Google Scholar] [CrossRef] [PubMed]
- Wilson, L.R.; Panda, S.; Schmidt, A.C.; Sombers, L.A. Selective and Mechanically Robust Sensors for Electrochemical Measurements of Real-Time Hydrogen Peroxide Dynamics In Vivo. Anal. Chem. 2018, 90, 888–895. [Google Scholar] [CrossRef] [PubMed]
- Wightman, R.M. (Invited) Exploring Brain Chemistry with Microelectrodes. ECS Meet. Abstr. 2017, MA2017-02, 2302. [Google Scholar] [CrossRef]
- Yang, C.; Denno, M.E.; Pyakurel, P.; Venton, B.J. Recent Trends in Carbon Nanomaterial-Based Electrochemical Sensors for Biomolecules: A Review. Anal. Chim. Acta 2015, 887, 17–37. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R.; Wiley, J. Electrochemical Methods Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2001. [Google Scholar]
- Armstrong-James, M.; Millar, J. Carbon Fibre Microelectrodes. J. Neurosci. Methods 1979, 1, 279–287. [Google Scholar] [CrossRef]
- Armstrong-James, M.; Millar, J.; Kruk, Z.L. Quantification of Noradrenaline Iontophoresis. Nature 1980, 288, 181–183. [Google Scholar] [CrossRef] [PubMed]
- Baur, J.E.; Kristensen, E.W.; May, L.J.; Wiedemann, D.J.; Mark Wightman, R. Fast-Scan Voltammetry of Biogenic Amines. Anal. Chem. 1988, 60, 1268–1272. [Google Scholar] [CrossRef] [PubMed]
- White, K.A.; Darroudi, M.; Park, J.; Kim, B.N. A 128-Ch Area-Efficient Neurochemical-Sensing Front-End for FSCV Recordings of Dopamine. IEEE Sens. J. 2024, 1. [Google Scholar] [CrossRef]
- White, K.A.; Kim, B.N. Quantifying Neurotransmitter Secretion at Single-Vesicle Resolution Using High-Density Complementary Metal–Oxide–Semiconductor Electrode Array. Nat. Commun. 2021, 12, 431. [Google Scholar] [CrossRef] [PubMed]
- Venton, B.J.; Cao, Q. Fundamentals of Fast-Scan Cyclic Voltammetry for Dopamine Detection. Analyst 2020, 145, 1158–1168. [Google Scholar] [CrossRef]
- Johnson, J.A.; Hobbs, C.N.; Wightman, R.M. Removal of Differential Capacitive Interferences in Fast-Scan Cyclic Voltammetry. Anal. Chem. 2017, 89, 6166–6174. [Google Scholar] [CrossRef]
- Johnson, J.A.; Wightman, R.M. Cyclic Voltammetric Measurements of Neurotransmitters. Electrochem. Soc. Interface 2017, 26, 53–57. [Google Scholar] [CrossRef]
- Cho, Y.W.; Park, J.H.; Lee, K.H.; Lee, T.; Luo, Z.; Kim, T.H. Recent Advances in Nanomaterial-Modified Electrical Platforms for the Detection of Dopamine in Living Cells. Nano Converg. 2020, 7, 40. [Google Scholar] [CrossRef]
- Walton, L.R.; Verber, M.; Lee, S.-H.; Chao, T.-H.H.; Wightman, R.M.; Shih, Y.-Y.I. Simultaneous FMRI and Fast-Scan Cyclic Voltammetry Bridges Evoked Oxygen and Neurotransmitter Dynamics across Spatiotemporal Scales. Neuroimage 2021, 244, 118634. [Google Scholar] [CrossRef]
- Zachek, M.K.; Hermans, A.; Wightman, R.M.; McCarty, G.S. Electrochemical Dopamine Detection: Comparing Gold and Carbon Fiber Microelectrodes Using Background Subtracted Fast Scan Cyclic Voltammetry. J. Electroanal. Chem. 2008, 614, 113. [Google Scholar] [CrossRef]
- Berberian, K.; Kisler, K.; Qinghua, F.; Lindau, M. Improved Surface Patterned Platinum Microelectrodes for the Study of Exocytotic Events. Anal. Chem. 2009, 81, 8734. [Google Scholar] [CrossRef]
- Castagnola, E.; Thongpang, S.; Hirabayashi, M.; Nava, G.; Nimbalkar, S.; Nguyen, T.; Lara, S.; Oyawale, A.; Bunnell, J.; Moritz, C.; et al. Glassy Carbon Microelectrode Arrays Enable Voltage-Peak Separated Simultaneous Detection of Dopamine and Serotonin Using Fast Scan Cyclic Voltammetry. Analyst 2021, 146, 3955–3970. [Google Scholar] [CrossRef]
- Castagnola, E.; Robbins, E.M.; Wu, B.; Pwint, M.Y.; Garg, R.; Cohen-Karni, T.; Cui, X.T. Flexible Glassy Carbon Multielectrode Array for In Vivo Multisite Detection of Tonic and Phasic Dopamine Concentrations. Biosensors 2022, 12, 540. [Google Scholar] [CrossRef]
- Pokrajac, L.; Abbas, A.; Chrzanowski, W.; Dias, G.M.; Eggleton, B.J.; Maguire, S.; Maine, E.; Malloy, T.; Nathwani, J.; Nazar, L.; et al. Nanotechnology for a Sustainable Future: Addressing Global Challenges with the International Network4Sustainable Nanotechnology. ACS Nano 2021, 15, 18608–18623. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Shin, M.; Lavrik, N.V.; Venton, B.J. 3D-Printed Carbon Nanoelectrodes for In Vivo Neurotransmitter Sensing. Nano Lett. 2020, 20, 6831–6836. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.E. State-of-the-Art Developments in Carbon-Based Metal Nanocomposites as a Catalyst: Photocatalysis. Nanoscale Adv. 2021, 3, 1887–1900. [Google Scholar] [CrossRef] [PubMed]
- Maehashi, K.; Matsumoto, K. Label-Free Electrical Detection Using Carbon Nanotube-Based Biosensors. Sensors 2009, 9, 5368–5378. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.; Wang, Y.; Borgus, J.R.; Venton, B.J. Electrochemistry at the Synapse. Annu. Rev. Anal. Chem. 2019, 12, 297–321. [Google Scholar] [CrossRef]
- Shin, M.; Copeland, J.M.; Venton, B.J. Real-Time Measurement of Stimulated Dopamine Release in Compartments of the Adult Drosophila Melanogaster Mushroom Body. Anal. Chem. 2020, 92, 14398–14407. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Geng, Y.; Li, D.; Yao, H.; Huo, Z.; Li, Y.; Zhang, K.; Zhu, S.; Wei, H.; Xu, W.; et al. Deep Red Emissive Carbonized Polymer Dots with Unprecedented Narrow Full Width at Half Maximum. Adv. Mater. 2020, 32, 1906641. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, R.; Yang, B. Carbon Dots: A New Type of Carbon-Based Nanomaterial with Wide Applications. ACS Cent. Sci. 2020, 6, 2179–2195. [Google Scholar] [CrossRef]
- Xu, J.; Tao, J.; Su, L.; Wang, J.; Jiao, T. A Critical Review of Carbon Quantum Dots: From Synthesis toward Applications in Electrochemical Biosensors for the Determination of a Depression-Related Neurotransmitter. Materials 2021, 14, 3987. [Google Scholar] [CrossRef]
- Wonnenberg, P.M.; Zestos, A.G. Polymer-Modified Carbon Fiber Microelectrodes for Neurochemical Detection of Dopamine and Metabolites. ECS Trans. 2020, 97, 901–927. [Google Scholar] [CrossRef]
- Ping, Z.; Junjie, L.; Yunchun, L. Optimization of the Electrodeposition Process of a Polypyrrole/Multi-Walled Carbon Nanotube Fiber Electrode for a Flexible Supercapacitor. RSC Adv. 2022, 12, 18134–18143. [Google Scholar] [CrossRef]
- Peng, S.; Yu, Y.; Wu, S.; Wang, C.-H. Conductive Polymer Nanocomposites for Stretchable Electronics: Material Selection, Design, and Applications. ACS Appl. Mater. Interfaces 2021, 13, 43831–43854. [Google Scholar] [CrossRef]
- Adampourezare, M.; Saadati, A.; Hasanzadeh, M.; Dehghan, G.; Feizi, M.A.H. Reliable Recognition of DNA Methylation Using Bioanalysis of Hybridization on the Surface of Ag/GQD Nanocomposite Stabilized on Poly (β-Cyclodextrin): A New Platform for DNA Damage Studies Using Genosensor Technology. J. Mol. Recognit. 2022, 35, e2945. [Google Scholar] [CrossRef]
- Xu, G.; Li, B.; Luo, X. Carbon Nanotube Doped Poly(3,4-Ethylenedioxythiophene) for the Electrocatalytic Oxidation and Detection of Hydroquinone. Sens. Actuators B Chem. 2013, 176, 69–74. [Google Scholar] [CrossRef]
- Xu, G.; Li, B.; Cui, X.T.; Ling, L.; Luo, X. Electrodeposited Conducting Polymer PEDOT Doped with Pure Carbon Nanotubes for the Detection of Dopamine in the Presence of Ascorbic Acid. Sens. Actuators B Chem. 2013, 188, 405–410. [Google Scholar] [CrossRef]
- Speranza, G. Carbon Nanomaterials: Synthesis, Functionalization and Sensing Applications. Nanomaterials 2021, 11, 967. [Google Scholar] [CrossRef] [PubMed]
- Millar, J.; Stamford, J.A.; Kruk, Z.L.; Wightman, R.M. Electrochemical, Pharmacological and Electrophysiological Evidence of Rapid Dopamine Release and Removal in the Rat Caudate Nucleus Following Electrical Stimulation of the Median Forebrain Bundle. Eur. J. Pharmacol. 1985, 109, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Eom, G.; Oh, C.; Moon, J.; Kim, H.; Kim, M.K.; Kim, K.; Seo, J.W.; Kang, T.; Lee, H.J. Highly Sensitive and Selective Detection of Dopamine Using Overoxidized Polypyrrole/Sodium Dodecyl Sulfate-Modified Carbon Nanotube Electrodes. J. Electroanal. Chem. 2019, 848, 113295. [Google Scholar] [CrossRef]
- Ko, S.H.; Kim, S.W.; Lee, Y.J. Flexible Sensor with Electrophoretic Polymerized Graphene Oxide/PEDOT:PSS Composite for Voltammetric Determination of Dopamine Concentration. Sci. Rep. 2021, 11, 21101. [Google Scholar] [CrossRef]
- Peairs, M.J.; Ross, A.E.; Venton, B.J. Comparison of Nafion- and Overoxidized Polypyrrole—Carbon Nanotube Electrodes for Neurotransmitter Detection. Anal. Methods 2011, 3, 2379–2386. [Google Scholar] [CrossRef]
- Jacobs, C.B.; Ivanov, I.N.; Nguyen, M.D.; Zestos, A.G.; Venton, B.J. High Temporal Resolution Measurements of Dopamine with Carbon Nanotube Yarn Microelectrodes. Anal. Chem. 2014, 86, 5721–5727. [Google Scholar] [CrossRef]
- Taylor, I.M.; Patel, N.A.; Freedman, N.C.; Castagnola, E.; Cui, X.T. Direct in Vivo Electrochemical Detection of Resting Dopamine Using Poly(3,4-Ethylenedioxythiophene)/Carbon Nanotube Functionalized Microelectrodes. Anal. Chem. 2019, 91, 12917–12927. [Google Scholar] [CrossRef]
- Ko, S.H.; Kim, S.W.; Lee, S.H.; Lee, Y.J. Electrodeposited Reduced Graphene Oxide-PEDOT:PSS/Nafion Hybrid Interface for the Simultaneous Determination of Dopamine and Serotonin. Sci. Rep. 2023, 13, 20274. [Google Scholar] [CrossRef] [PubMed]
- Xiao, N.; Venton, B.J. Rapid, Sensitive Detection of Neurotransmitters at Microelectrodes Modified with Self-Assembled SWCNT Forests. Anal. Chem. 2012, 84, 7816–7822. [Google Scholar] [CrossRef] [PubMed]
- Gerwig, R.; Fuchsberger, K.; Schroeppel, B.; Link, G.S.; Heusel, G.; Kraushaar, U.; Schuhmann, W.; Stett, A.; Stelzle, M.; Giugliano, M.; et al. Neuroengineering PEDOT-CNT Composite Microelectrodes for Recording and Electrostimulation Applications: Fabrication, Morphology, and Electrical Properties. Front. Neuroeng. 2012, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- McCreery, R.L. Advanced Carbon Electrode Materials for Molecular Electrochemistry. Chem. Rev. 2008, 108, 2646–2687. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Trikantzopoulos, E.; Jacobs, C.B.; Venton, B.J. Evaluation of Carbon Nanotube Fiber Microelectrodes for Neurotransmitter Detection: Correlation of Electrochemical Performance and Surface Properties. Anal. Chim. Acta 2017, 965, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Puthongkham, P.; Yang, C.; Venton, B.J. Carbon Nanohorn-Modified Carbon Fiber Microelectrodes for Dopamine Detection. Electroanalysis 2018, 30, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Castagnola, E.; Garg, R.; Rastogi, S.K.; Cohen-Karni, T.; Cui, X.T. 3D Fuzzy Graphene Microelectrode Array for Dopamine Sensing at Sub-Cellular Spatial Resolution. Biosens. Bioelectron. 2021, 191, 113440. [Google Scholar] [CrossRef] [PubMed]





| Ref. | Composite | Electrode | Method | Linear Range (µM) | LOD (nM) | Surface Area (µm2) | Sensitivity (nA/µM) |
|---|---|---|---|---|---|---|---|
| [42] | CNT–PPy | Au electrode (MEA) | DPV | 0.005–10 | 0.14 | - | 0.5 |
| [43] | GO–PEDOT | Au electrode | 0.01–100 | 8 | 1256 | 0.87 | |
| [39] | CNT–PEDOT | Carbon paste electrode | 0.1–20 | 20 | 1.25 × 107 | 44 | |
| [46] | CNT–PEDOT | Silicon-based Microelectrode Arrays (MEA) | SWV | 0.1–1 | 82 | 1200 | 108.3 |
| [24] | GO–PEDOT | Glassy carbon (MEA) | 0.01–1 | 56.2 | 1256 | 40.0 | |
| [38] | CNT–PEDOT | Carbon paste electrode | Amperometry | 1.1–125 | 300 | 1.25 × 107 | 0.25 |
| [47] | rGO–PEDOT/Nafion | Au electrode | 0.05–75 | 170 | 1256 | 9.9 | |
| [44] | CNT–PPy | CFME | FSCV | 0.05–5 | 3.3 | 1278 | 11 |
| [3] | GO | 0.025–1 | 11 | 2238 | 41 | ||
| [48] | CNT–Nafion | 0.05–100 | 0.8 | 1278 | 1840 | ||
| [45] | CNT–yarn | 0.05–25 | 10.8 | 4240 | 2.5 | ||
| [24] | GO–PEDOT | Glassy carbon | 0.1–1 | - | 1256 | 400 | |
| This work | CNT–CQD–PEDOT | Pt electrode | 0.1–1 | 40 | 3000 | 154 | |
| CNT–CQD–PPy | 0.1–1 | 35 | 3000 | 112 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darroudi, M.; White, K.A.; Crocker, M.A.; Kim, B.N. Dopamine Measurement Using Engineered CNT–CQD–Polymer Coatings on Pt Microelectrodes. Sensors 2024, 24, 1893. https://doi.org/10.3390/s24061893
Darroudi M, White KA, Crocker MA, Kim BN. Dopamine Measurement Using Engineered CNT–CQD–Polymer Coatings on Pt Microelectrodes. Sensors. 2024; 24(6):1893. https://doi.org/10.3390/s24061893
Chicago/Turabian StyleDarroudi, Mahdieh, Kevin A. White, Matthew A. Crocker, and Brian N. Kim. 2024. "Dopamine Measurement Using Engineered CNT–CQD–Polymer Coatings on Pt Microelectrodes" Sensors 24, no. 6: 1893. https://doi.org/10.3390/s24061893







