Design of Bio-Optical Transceiver for In Vivo Biomedical Sensor Applications †
Abstract
:1. Introduction
- We provide the decision feedback version of our proposed ISD receiver.
- We evaluate the decision feedback version of our proposed ISD receiver analytically and compare with the non-feedback version.
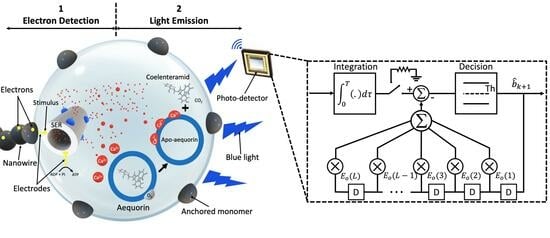
2. System Design
2.1. Bio-Optical Transceiver
2.2. ISD Receiver
3. System Model
3.1. Bit Error Probability of the ISD Receiver
3.2. Decision Feedback Receiver
3.3. Markov Chain
3.4. Bit Error Probability of the DF Version
4. Numerical Results
Bit Error Probability
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BER | Bit Error Rate |
| DbMC | Diffusion-based Molecular Communication |
| DF | Decision Feedback |
| IoBNT | Internet of BioNanoThings |
| IoT | Internet of Things |
| ISD | Integrate, Sample, and Dump |
| ISI | InterSymbol Interference |
| MC | Markov Chain |
| OSK | Optical Shift Keying |
| PDMS | Polydimethylsiloxane |
| SER | Smooth Endoplasmic Reticulum |
| SNR | Signal-to-Noise Ratio |
| WANNET | Wired Ad hoc NanoNETwork |
References
- Akyildiz, I.F.; Jornet, J.M. The Internet of nano-things. IEEE Wirel. Commun. 2010, 17, 58–63. [Google Scholar] [CrossRef]
- Akyildiz, I.F.; Pierobon, M.; Balasubramaniam, S.; Koucheryavy, Y. The internet of bio-nano things. IEEE Commun. Mag. 2015, 53, 32–40. [Google Scholar] [CrossRef]
- Pierobon, M.; Akyildiz, I.F. A physical end-to-end model for molecular communication in nanonetworks. IEEE J. Sel. Areas Commun. 2010, 28, 602–611. [Google Scholar] [CrossRef]
- Tepekule, B.; Pusane, A.E.; Yilmaz, H.B.; Chae, C.B.; Tugcu, T. ISI Mitigation Techniques in Molecular Communication. IEEE Trans. Mol. Biol. Multi-Scale Commun. 2015, 1, 202–216. [Google Scholar] [CrossRef]
- Kadloor, S.; Adve, R.S.; Eckford, A.W. Molecular Communication Using Brownian Motion with Drift. IEEE Trans. NanoBiosci. 2012, 11, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Arjmandi, H.; Ahmadzadeh, A.; Schober, R.; Kenari, M.N. Ion Channel Based Bio-Synthetic Modulator for Diffusive Molecular Communication. IEEE Trans. Nanobiosci. 2016, 15, 418–432. [Google Scholar] [CrossRef] [PubMed]
- Mahfuz, M.U.; Makrakis, D.; Mouftah, H.T. A comprehensive analysis of strength-based optimum signal detection in concentration-encoded molecular communication with spike transmission. IEEE Trans. Nanobiosci. 2015, 14, 67–83. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.; Lin, L.; Yan, H. Adaptive Detection and ISI Mitigation for Mobile Molecular Communication. IEEE Trans. Nanobiosci. 2018, 17, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Noel, A.; Cheung, K.C.; Schober, R. Improving Receiver Performance of Diffusive Molecular Communication with Enzymes. IEEE Trans. NanoBiosci. 2014, 13, 31–43. [Google Scholar] [CrossRef]
- Assaf, S.S.; Salehi, S.; Cid-Fuentes, R.G.; Solé-Pareta, J.; Alarcón, E. Influence of neighboring absorbing receivers upon the inter-symbol interference in a diffusion-based molecular communication system. Nano Commun. Netw. 2017, 14, 40–47. [Google Scholar] [CrossRef]
- Koo, B.H.; Lee, C.; Yilmaz, H.B.; Farsad, N.; Eckford, A.; Chae, C.B. Molecular MIMO: From Theory to Prototype. IEEE J. Sel. Areas Commun. 2016, 34, 600–614. [Google Scholar] [CrossRef]
- Dambri, O.A.; Cherkaoui, S. Modeling self-assembly of polymer-based wired nano-communication channel. IEEE Trans. Mol. Biol. Multi-Scale Commun. 2022, 8, 107–118. [Google Scholar] [CrossRef]
- Dambri, O.A.; Cherkaoui, S. Toward a wired ad hoc nanonetwork. In Proceedings of the ICC 2020—IEEE International Conference on Communications ICC, Dublin, Ireland, 7–11 June 2020; pp. 1–6. [Google Scholar]
- Asghari, M. Intrabody hybrid perpetual nanonetworks based on simultaneous wired and wireless nanocommunications. Nano Commun. Netw. 2022, 32–33, 100406. [Google Scholar] [CrossRef]
- Michelusi, N.; Pirbadian, S.; El-Naggar, M.Y.; Mitra, U. A stochastic model for electron transfer in bacterial cables. IEEE J. Sel. Areas Commun. 2014, 32, 2402–2416. [Google Scholar] [CrossRef]
- Dambri, O.A.; Cherkaoui, S.; Makrakis, D. Design and evaluation of a receiver for wired nano-communication networks. IEEE Trans. NanoBiosci. 2023, 22, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Schwarzländer, M.; Finkemeier, I. Mitochondrial energy and redox signaling in plants. Antioxid. Redox Signal. 2013, 18, 2122–2144. [Google Scholar] [CrossRef] [PubMed]
- Lems, S.; Van Der Kooi, H.J.; De Swaan Arons, J. Exergy analyses of the biochemical processes of photosynthesis. Int. J. Exergy 2010, 7, 333–351. [Google Scholar] [CrossRef]
- Hastings, J. The chemistry of bioluminescence. In Series Current Topics in Bioenergetics; Sanadi, D., Ed.; Elsevier: Amsterdam, The Netherlands, 1966; Volume 1, pp. 113–152. [Google Scholar]
- Williamson, C.D.; Wong, D.S.; Bozidis, P.; Zhang, A.; Colberg-Poley, A.M. Isolation of endoplasmic reticulum, mitochondria, and mitochondria-associated membrane and detergent resistant membrane fractions from transfected cells and from human cytomegalovirus- infected primary fibroblasts. Curr. Protoc. Cell Biol. 2015, 68, 3–27. [Google Scholar] [CrossRef]
- de Araújo, M.E.; Lamberti, G.; Huber, L.A. Homogenization of mammalian cells. Cold Spring Harb. Protoc. 2015, 2015, 1009–1012. [Google Scholar] [CrossRef]
- Swida, U.; Kreutzfeldt, C.; Ramezani-Rad, M.; Käufer, N. Isolation and characterisation of rough and smooth endoplasmic reticulum from Saccharomyces cerevisiae. FEMS Microbiol. Lett. 1982, 15, 313–318. [Google Scholar] [CrossRef]
- Padh, H. Organelle isolation and marker enzyme assay. In Tested Studies for Laboratory Teaching, Proceedings of the 13th Workshop of the Association for Biology Laboratory Education (ABLE), Laramie, Wyoming, 11–15 June 1991; Goldman, C.A., Ed.; ERIC: Washington, DC, USA, 1992; Volume 13. [Google Scholar]
- Teixeira, R.F.; van den Berg, O.; Nguyen, L.T.T.; Feher, K.; Du Prez, F.E. Microencapsulation of active ingredients using PDMS as shell material. Macromolecules 2014, 47, 8231–8237. [Google Scholar] [CrossRef]
- Wang, H.; Chen, P.; Zheng, X. Hollow permeable polysiloxane capsules: A novel approach for fabrication, guest encapsulation and morphology studies. J. Mater. Chem. 2004, 14, 1648–1651. [Google Scholar] [CrossRef]
- Katz, A.M. Physiology of the Heart, 5th ed.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2010. [Google Scholar]
- Du, P.; Li, S.; O’Grady, G.; Cheng, L.K.; Pullan, A.J.; Chen, J.D. Effects of electrical stimulation on isolated rodent gastric smooth muscle cells evaluated via a joint computational simulation and experimental approach. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 297, G672–G680. [Google Scholar] [CrossRef] [PubMed]
- Weeks, I.; Kricka, L.J.; Wild, D. Chapter 3.2—Signal generation and detection systems (excluding homogeneous assays). In The Immunoassay Handbook, 4th ed.; Wild, D., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 267–285. [Google Scholar]
- Shimomura, O.; Johnson, F.H. Properties of the bioluminescent protein Aequorin. Biochemistry 1969, 8, 3991–3997. [Google Scholar] [CrossRef]
- Kendall, J.M.; Badminton, M.N.; Dormer, R.L.; Campbell, A.K. Changes in free calcium in the endoplasmic reticulum of living cells detected using targeted aequorin. Anal. Biochem. 1994, 221, 173–181. [Google Scholar] [CrossRef]
- Sadr, R.; Hurd, W.J. Detection of signals by the digital integrate-and-dump filter with offset sampling. In The Telecommunications and Data Acquisition Report; 1987; pp. 158–173, Provided by the SAO/NASA Astrophysics Data System; Available online: https://ui.adsabs.harvard.edu/abs/1987tdar.nasa..158S (accessed on 4 February 2024).





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makrakis, D.; Dambri, O.A.; Hafid, A.S. Design of Bio-Optical Transceiver for In Vivo Biomedical Sensor Applications. Sensors 2024, 24, 2584. https://doi.org/10.3390/s24082584
Makrakis D, Dambri OA, Hafid AS. Design of Bio-Optical Transceiver for In Vivo Biomedical Sensor Applications. Sensors. 2024; 24(8):2584. https://doi.org/10.3390/s24082584
Chicago/Turabian StyleMakrakis, Dimitrios, Oussama Abderrahmane Dambri, and Abdelhakim Senhaji Hafid. 2024. "Design of Bio-Optical Transceiver for In Vivo Biomedical Sensor Applications" Sensors 24, no. 8: 2584. https://doi.org/10.3390/s24082584