Compound Acoustic Radiation Force Impulse Imaging of Bovine Eye by Using Phase-Inverted Ultrasound Transducer
Abstract
:1. Introduction
2. Materials and Methods
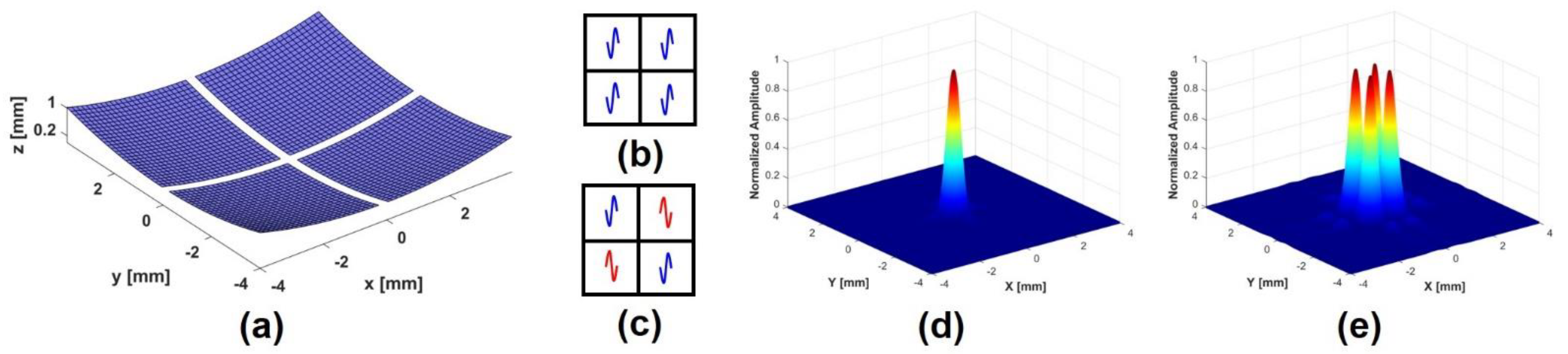
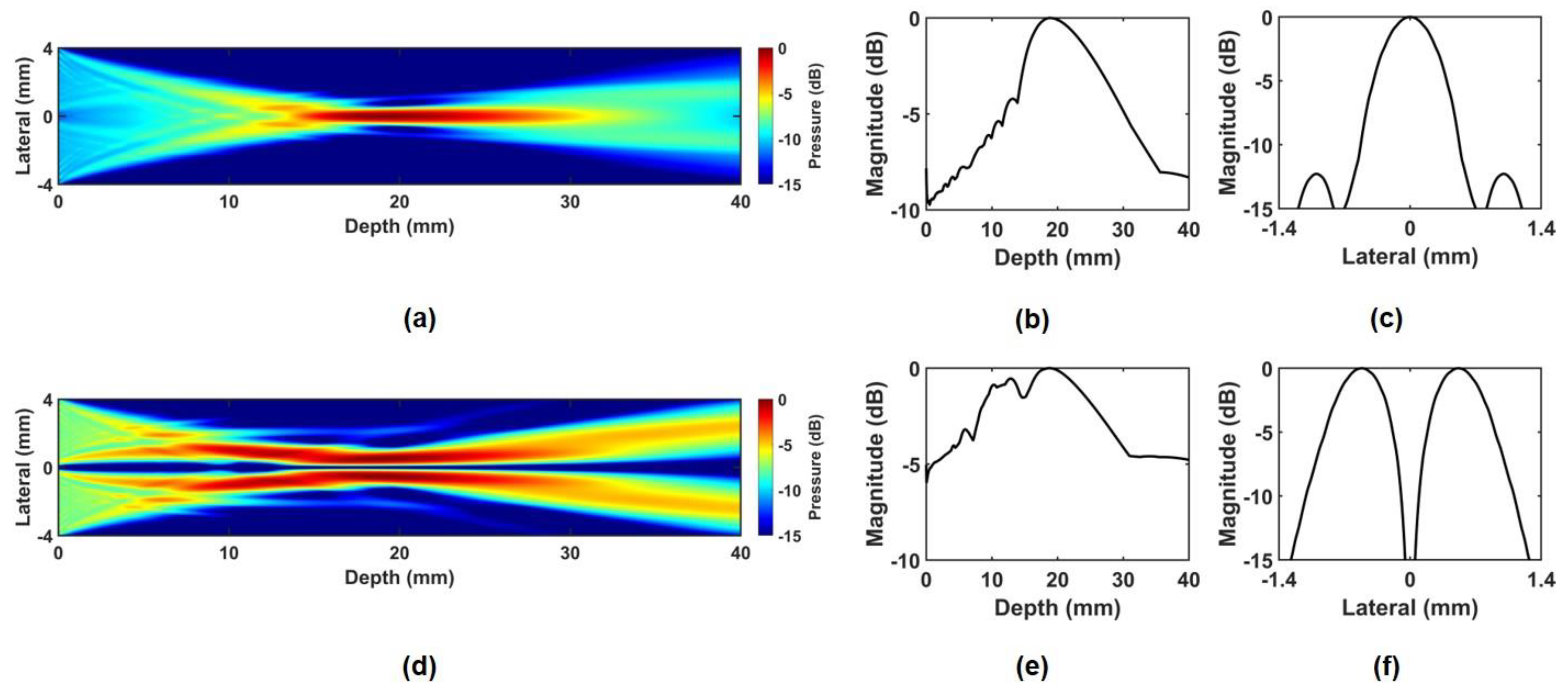
2.1. Principle and Simulation of Phase-Inverted Ultrasound Transducer
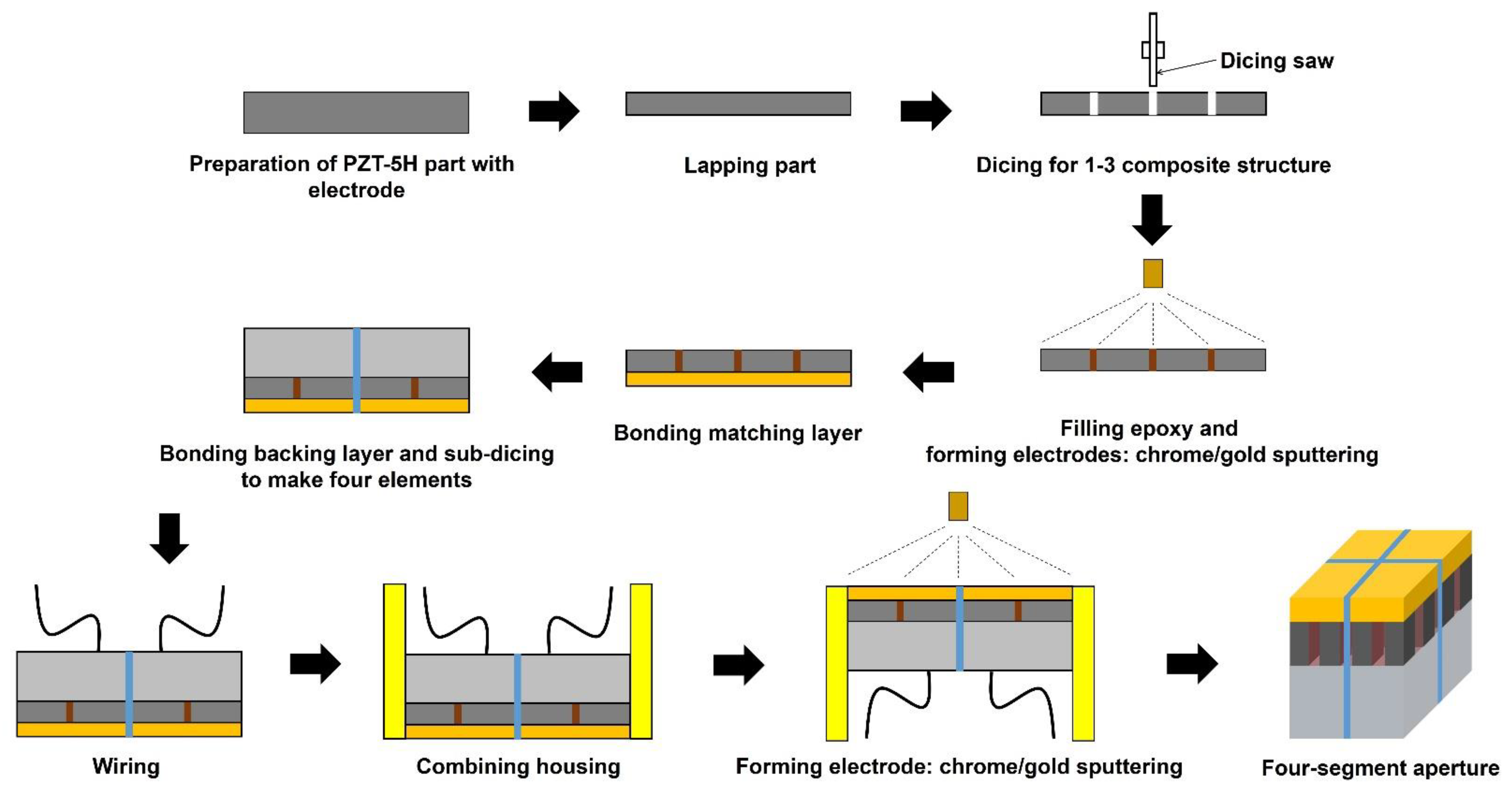
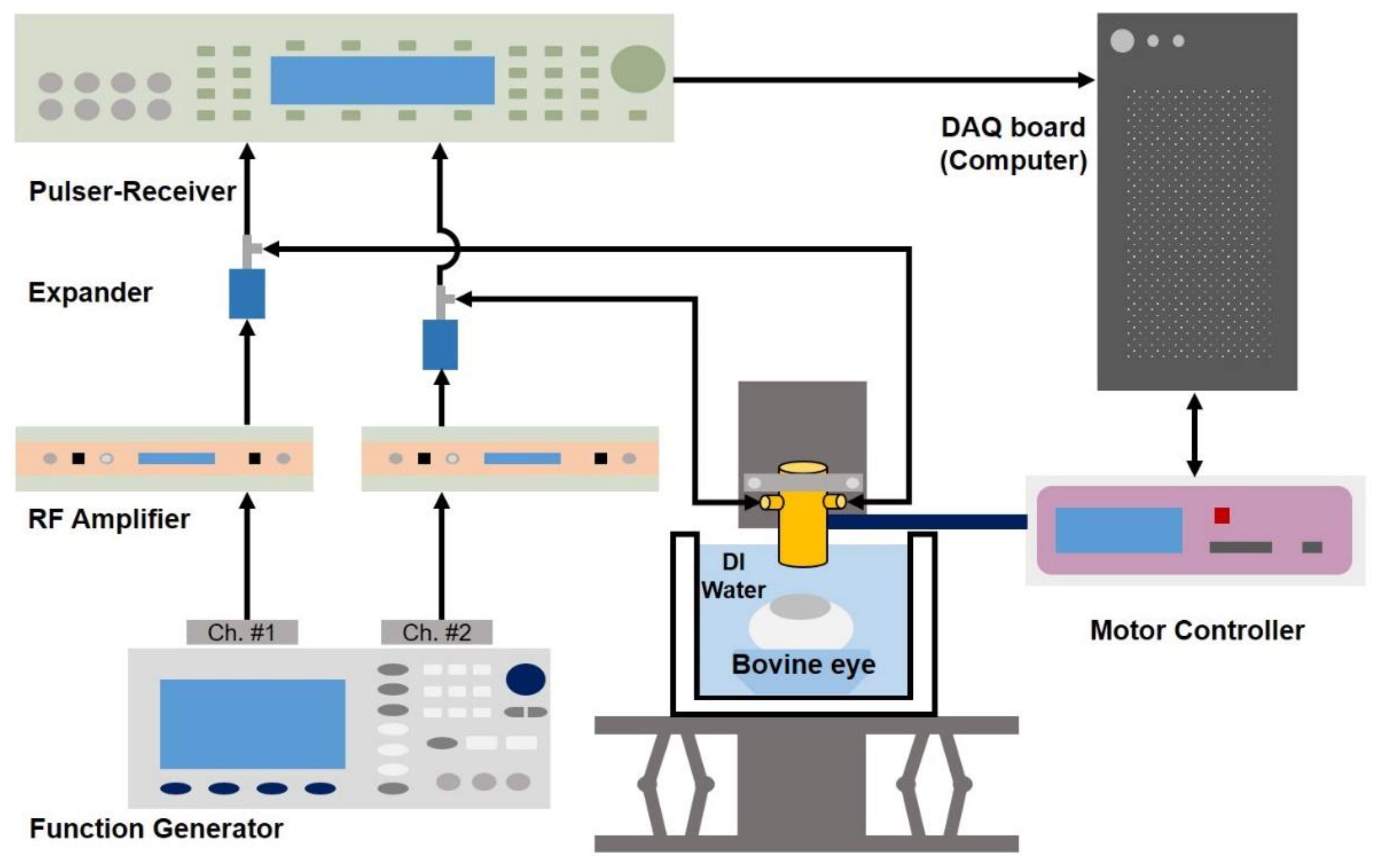
2.2. Transducer Fabrication and Experimental Setup
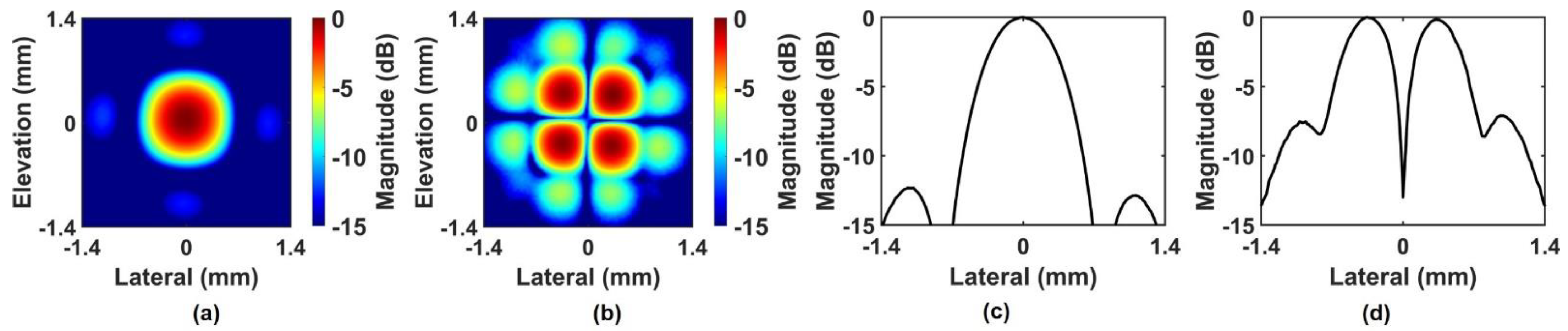
3. Experimental Results
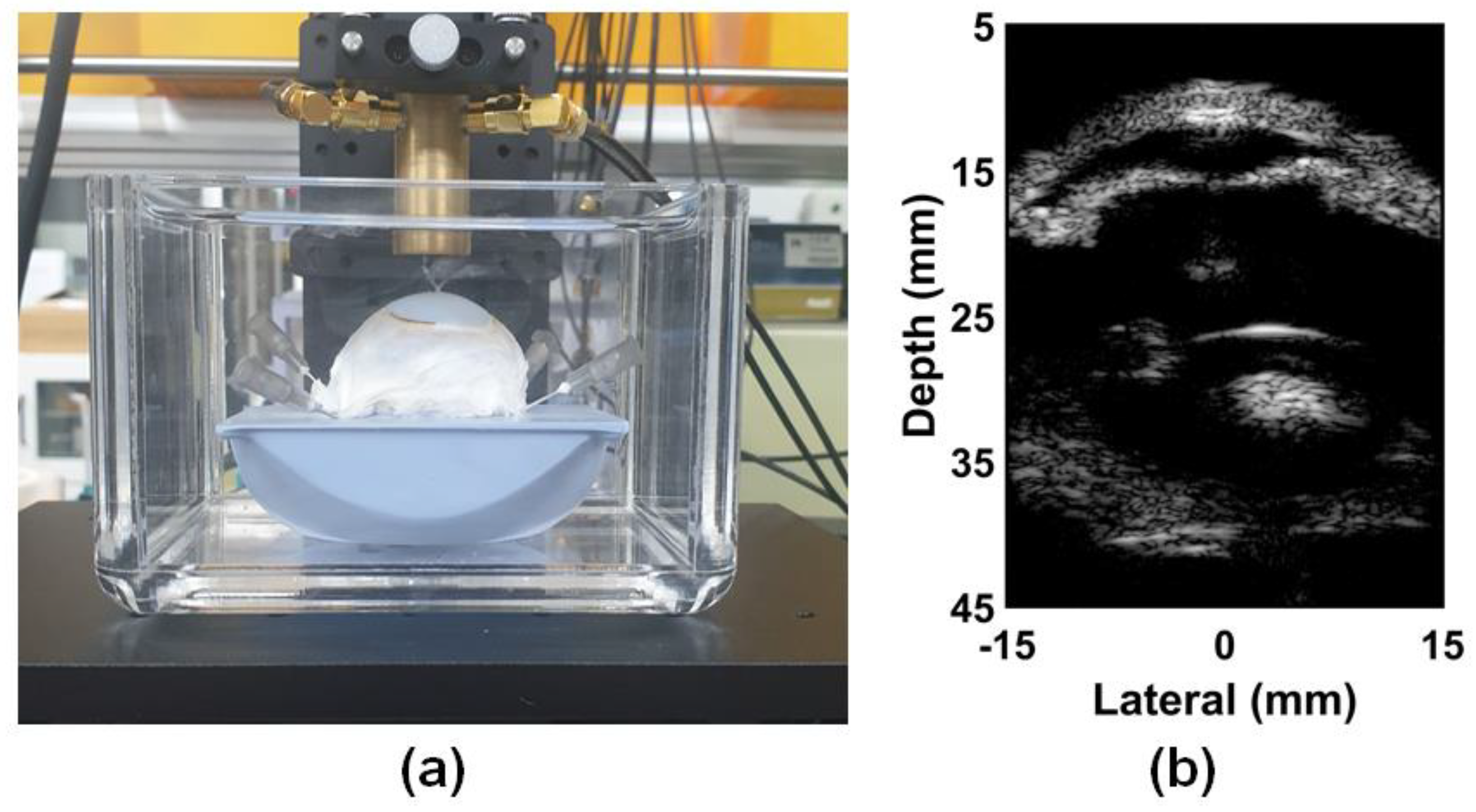
3.1. ARFI Imaging of Bovine Eye
3.2. Performance Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Zhu, J.; Chen, J.J.; Yu, J.; Jin, Z.; Miao, Y.; Browne, A.W.; Zhou, Q.; Chen, Z. Simultaneously imaging and quantifying in vivo mechanical properties of crystalline lens and cornea using optical coherence elastography with acoustic radiation force excitation. APL Photonics 2019, 4, 106104. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Qian, X.; Gong, C.; Zhang, J.; Liu, Y.; Xu, B.; Humayun, M.S.; Zhou, Q. Simultaneous assessment of the whole eye biomechanics using ultrasonic elastography. IEEE Trans. Biomed. Eng. 2023, 70, 1310–1317. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Yoon, H.; Larin, K.V.; Emelianov, S.Y.; Aglyamov, S.R. The impact of intraocular pressure on elastic wave velocity estimates in the crystalline lens. Phys. Med. Biol. 2017, 62, N45–N57. [Google Scholar] [CrossRef] [PubMed]
- Weng, C.C.; Chen, P.Y.; Chou, D.; Shih, C.C.; Huang, C.C. High frequency ultrasound elastography for estimating the viscoelastic properties of the cornea using lamb wave model. IEEE Trans. Biomed. Eng. 2021, 68, 2637–2644. [Google Scholar] [CrossRef] [PubMed]
- Kwok, S.; Pan, X.; Liu, W.; Hendershot, A.; Liu, J. High-frequency ultrasound detects biomechanical weaking in keratoconus with lower stiffness at higher grade. PLoS ONE 2022, 17, e0271749. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.C.; Huang, C.C.; Zhou, Q.; Shung, K.K. High-resolution acoustic-radiation-force-impulse imaging for assessing corneal sclerosis. IEEE Trans. Med. Imaging 2013, 32, 1316–1324. [Google Scholar] [CrossRef] [PubMed]
- Ruberti, J.W.; Roy, A.S.; Roberts, C.J. Corneal biomechanics and biomaterials. Annu. Rev. Biomed. Eng. 2011, 15, 269–295. [Google Scholar] [CrossRef] [PubMed]
- Strenk, S.A.; Strenk, L.M.; Koretz, J.F. The mechanism of presbyopia. Prog. Retin. Eye Res. 2005, 24, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Brian, G.; Taylor, H. Cataract blindness—Challenges for the 21st century. Bull. World Health Organ. 2001, 79, 249–256. [Google Scholar]
- Allen, D.; Vasavada, A. Cataract and surgery for cataract. BMJ 2006, 333, 128–132. [Google Scholar] [CrossRef]
- Yoo, S.H.; Zein, M. Vision Restoration: Cataract Surgery and Surgical Correction of Myopia, Hyperopia, and Presbyopia. Med. Clin. North Am. 2021, 105, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Truscott, R.J.W.; Zhu, X. Presbyopia and cataract: A question of heat and time. Prog. Retin. Eye Res. 2010, 29, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Singh, M.; Nair, A.; Larin, K.V.; Aglyamov, S.R. Elasticity changes in the crystalline lens during oxidative damage and the antioxidant effect of alpha-lipoic acid measured by optical coherence elastography. Photonics 2021, 8, 207. [Google Scholar] [CrossRef]
- Krag, S.; Andreassen, T.T. Biomechanical measurements of the porcine lens capsule. Exp. Eye Res. 1996, 62, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Weeber, H.A.; Eckert, G.; Pechhold, W.; van der Heijde, R.G.L. Stiffness gradient in the crystalline lens. Graefes Arch. Clin. Exp. Ophthalmol. 2007, 245, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Sarvazyan, A.; Hall, T.J.; Urban, M.W.; Fatemi, M.; Aglyamov, S.R.; Garra, B.S. An overview of elastography-an emerging branch of medical imaging. Curr. Med. Imaging Rev. 2011, 7, 255–282. [Google Scholar] [CrossRef] [PubMed]
- Ophir, J.; Alam, S.K.; Garra, B.; Kallel, F.; Konofagou, E.; Krouskop, T.; Varghese, T. Elastography: Ultrasonic estimation and imaging of the elastic properties of tissues. Proc. Inst. Mech. Eng. H. 1999, 213, 203–233. [Google Scholar] [CrossRef] [PubMed]
- Muthupillai, R.; Lomas, D.J.; Rossman, P.J.; Greenleaf, J.F.; Manduca, A.; Ehman, R.L. Magnetic resonance elastography by direct visualization of propagating acoustic strain waves. Science 1995, 269, 1854–1857. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Clayton, E.H.; Chang, Y.; Okamoto, R.J.; Bayly, P.V. Viscoelastic properties of the ferret brain measured in vivo at multiple frequencies by magnetic resonance elastography. J. Biomech. 2013, 46, 863–870. [Google Scholar] [CrossRef]
- Kirby, M.A.; Pelivanov, I.; Song, S.; Ambrozinski, L.; Yoon, S.J.; Gao, L.; Li, D.; Shen, T.T.; Wang, R.K.; O’Donnell, M. Optical coherence elastography in ophthalmology. J. Biomed. Opt. 2017, 22, 121720. [Google Scholar] [CrossRef]
- Larin, K.V.; Sampson, D.D. Optical coherence elastography–OCT at work in tissue biomechanics. Biomed. Opt. Express 2017, 8, 1172–1202. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Ma, T.; He, Y.; Zhu, J.; Dai, C.; Yu, M.; Huang, S.; Lu, F.; Shung, K.K.; Zhou, Q.; et al. Acoustic Radiation Force Optical Coherence Elastography of Corneal Tissue. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Han, Z.; Wang, S.; Li, J.; Singh, M.; Liu, C.H.; Aglyamov, S.; Emelianov, S.; Manns, F.; Larfin, K.V. Assessing age-related changes in the biomechanical properties of rabbit lens using a coaligned ultrasound and optical coherence elastography system. Invest. Ophthalmol. Vis. Sci. 2015, 56, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Q.; Lyu, Z.; Gao, X.; Zhang, P.; Lin, H.; Guo, Y.; Wang, T.; Chen, S.; Chen, X. Noninvasive assessment of age-related stiffness of crystalline lenses in a rabbit model using ultrasound elastography. Biomed. Eng. Online 2018, 17, 75. [Google Scholar] [CrossRef] [PubMed]
- Sigrist, R.M.S.; Liau, J.; Kaffas, A.E.; Chammas, M.C.; Willmann, J.K. Ultrasound elastography: Review of techniques and clinical applications. Theranostics 2017, 7, 1303–1329. [Google Scholar] [CrossRef] [PubMed]
- Nightingale, K.; Soo, M.S.; Nightingale, R.; Trahey, G. Acoustic radiation force impulse imaging: In vivo demonstration of clinical feasibility. Ultrasound Med. Biol. 2002, 28, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Nightingale, K. Acoustic radiation force impulse (ARFI) imaging: A review. Curr. Med. Imaging Rev. 2011, 7, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Dahl, J.J.; Pinhton, G.F.; Mark, L.; Agrawal, V.; Nightingale, K.R.; Trahey, G.E. A parallel tracking method for acoustic radiation force impulse imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2007, 54, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Parker, K.J.; Doyley, M.M.; Rubens, D.J. Imaging the elastic properties of tissue: The 20 year perspective. Phys. Med. Biol. 2011, 56, R1–R29. [Google Scholar] [CrossRef]
- Shiina, T.; Nightingale, K.R.; Palmeri, M.L.; Hall, T.J.; Bamber, J.C.; Barr, R.G.; Castera, L.; Choi, B.I.; Chou, Y.H.; Cosgrove, D.; et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 1: Basic principles and terminology. Ultrasound Med. Biol. 2015, 41, 1126–1147. [Google Scholar] [CrossRef]
- Barr, R.G. Future of breast elastography. Ultrasonography 2019, 38, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Yeh, M.L.; Huang, C.I.; Yang, J.F.; Liang, P.C.; Huang, C.F.; Dai, C.Y.; Lin, Z.Y.; Chen, S.C.; Huang, J.F.; et al. The performance of acoustic radiation force impulse imaging in predicting liver fibrosis in chronic liver diseases. Kaohsiung J. Med. Sci. 2016, 32, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.; Madden, J.; Foo, W.C.; Palmeri, M.L.; Mouraviev, V.; Polascik, T.J.; Nightingale, K.R. Acoustic radiation force impulse imaging of human prostates ex vivo. Ultrasound Med. Biol. 2010, 36, 576–588. [Google Scholar] [CrossRef] [PubMed]
- Czernuszewicz, T.J.; Streeter, J.E.; Dayton, P.A.; Gallippi, C.M. Experimental validation of displacement underestimation in ARFI ultrasound. Ultrason. Imaging 2013, 35, 196–213. [Google Scholar] [CrossRef] [PubMed]
- Byram, B.; Trahey, G.E.; Palmeri, M. Bayesian speckle tracking. Part II: Biased ultrasound displacement estimation. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2013, 60, 144–157. [Google Scholar] [CrossRef] [PubMed]
- Palmeri, M.L.; McAleavey, S.A.; Trahey, G.E.; Nightingale, K.R. Ultrasonic tracking of acoustic radiation force-induced displacements in homogeneous media. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2006, 53, 1300–1313. [Google Scholar] [CrossRef] [PubMed]
- Walker, W.F.; Trahey, G.E. A fundamental limit on delay estimation using partially correlated speckle signals. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1995, 42, 301–308. [Google Scholar] [CrossRef]
- Palmeri, M.L.; Sharma, A.C.; Bouchard, R.R.; Nightingale, R.W.; Nightingale, K.R. A finite-element method model of soft tissue response to impulsive acoustic radiation force. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2005, 52, 1699–1712. [Google Scholar] [CrossRef] [PubMed]
- McAleavey, S.A.; Nightingale, K.R.; Trahey, G.E. Estimates of echo correlation and measurement bias in acoustic radiation force impulse imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2003, 50, 631–641. [Google Scholar] [CrossRef]
- Rao, M.; Varghese, T. Correlation analysis of three-dimensional strain imaging using ultrasound two-dimensional array transducers. J. Acoust. Soc. Am. 2008, 124, 1858–1865. [Google Scholar] [CrossRef]
- Shih, C.C.; Lai, T.Y.; Huang, C.C. Evaluating the intensity of the acoustic radiation force impulse (ARFI) in intravascular ultrasound (IVUS) imaging: Preliminary in vitro results. Ultrasonics 2016, 70, 64–74. [Google Scholar] [CrossRef]
- Qian, X.; Ma, T.; Yu, M.; Chen, X.; Shung, K.K.; Zhou, Q. Multi-functional ultrasonic micro-elastography imaging system. Sci. Rep. 2017, 7, 1230. [Google Scholar] [CrossRef] [PubMed]
- Jeong, E.Y.; Sung, J.H.; Jeong, J.S. Improved acoustic radiation force impulse imaging using split-focused ultrasound transducer with phase inversion technique. IEEE Sens. J. 2021, 21, 1395–1403. [Google Scholar] [CrossRef]
- Cain, C.A.; Umemura, S.I. Concentric-Ring and Sector-Vortex Phased-Array Applicators for Ultrasound Hyperthermia. IEEE Trans. Microw. Theory Tech. 1986, 34, 542–551. [Google Scholar] [CrossRef]
- Umemura, S.I.; Cain, C.A. The sector-vortex phased array: Acoustic field synthesis for hyperthermia. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1989, 36, 249–257. [Google Scholar] [CrossRef]
- Umemura, S.I.; Cain, C.A. Analysis of temperature responses to diffused ultrasound focal fields produced by a sector-vortex phased array. Int. J. Hyperth. 1990, 6, 641–654. [Google Scholar] [CrossRef]
- Umemura, S.I.; Cain, C.A. Acoustical evaluation of a prototype sector-vortex phased-array applicator. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1992, 39, 32–38. [Google Scholar] [CrossRef]
- Sasaki, K.; Azuma, T.; Kawabata, K.I.; Shimoda, M.; Kokue, E.I.; Umemura, S.I. Effect of split-focus approach on producing larger coagulation in swine liver. Ultrasound Med. Biol. 2003, 29, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.S. Dual concentric-sectored HIFU transducer with phase-shifted ultrasound excitation for expanded necrotic region: A simulation study. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2013, 60, 924–931. [Google Scholar] [CrossRef]
- Fan, X.; Hynynen, K. Ultrasound surgery using multiple sonications—Treatment time considerations. Ultrasound Med. Biol. 1996, 22, 471–482. [Google Scholar] [CrossRef]
- Tupholme, G.E. Generation of acoustic pulses by baffled plane pistons. Mathematika 1969, 16, 209–224. [Google Scholar] [CrossRef]
- Stepanishen, P.R. The time-dependent force and radiation impedance on a piston in a rigid infinite planar baffle. J. Acoust. Soc. Am. 1971, 49, 841–849. [Google Scholar] [CrossRef]
- Lee, H.S.; Jeong, E.Y.; Sung, J.H.; Choi, B.E.; Jeong, J.S. An Intraocular Pressure Measurement Technique Based on Acoustic Radiation Force Using an Ultrasound Transducer: A Feasibility Study. Sensors 2021, 21, 1857. [Google Scholar] [CrossRef] [PubMed]
- Dhanaliwala, A.H.; Hossack, J.A.; Mauldin, F.W. Assessing and improving acoustic radiation force image quality using a 1.5-D transducer design. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2012, 59, 1602–1608. [Google Scholar] [CrossRef]
- Lee, S.A.; Kamimura, H.A.S.; Konofagou, E.E. Displacement imaging during focused ultrasound median nerve modulation: A preliminary study in human pain sensation mitigation. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2021, 68, 526–537. [Google Scholar] [CrossRef] [PubMed]
- Varghese, T.; Ophir, J. A theoretical framework for performance characterization of elastography: The strain filter. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1997, 44, 164–172. [Google Scholar] [CrossRef]
- Liu, Y.; Saharkhiz, N.; Hossain, M.M.; Konofagou, E.E. Optimization of the tracking beam sequence in harmonic motion imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2024, 71, 102–116. [Google Scholar] [CrossRef] [PubMed]
- Shamdasani, V.; Kim, Y. Two-dimensional autocorrelation method for ultrasound-based strain estimation. In Proceedings of the 26th Annual International Conference of the IEEE EMBS, San Francisco, CA, USA, 1–5 September 2004; pp. 1380–1383. [Google Scholar]
- Cespedes, I.; Ophir, J. Reduction of image noise in elastography. Ultrason. Imaging 1993, 15, 89–102. [Google Scholar] [CrossRef]
- Dikici, A.S.; Mishmanli, I.; Kilic, F.; Ozkok, A.; Kuyumcu, G.; Sultan, P.; Samanci, C.; Halit, Y.M.; Rafiee, B.; Tamcelik, N.; et al. In vivo evaluation of the biomechanical properties of the optic nerve and peripapillary structures by ultrasonic shear wave elastography in glaucoma. Iran. J. Radiol. 2016, 13, e36849. [Google Scholar] [CrossRef]
- Qian, X.; Li, R.; Li, Y.; Lu, G.; He, Y.; Humayun, M.S.; Chen, Z.; Zhou, Q. In vivo evaluation of posterior eye elasticity using shaker-based optical coherence elastography. Exp. Biol. Med. 2020, 245, 282–288. [Google Scholar] [CrossRef]
- Ferrara, M.; Lugano, G.; Sandinha, M.T.; Kearns, V.R.; Geraghty, B.; Steel, D.H.W. Biomechanical properties of retina and choroid: A comprehensive review off techniques and translational relevance. Eye 2021, 35, 1818–1832. [Google Scholar] [CrossRef] [PubMed]
- Campbell, I.C.; Coudrillierm, B.; Ross, E.C. Biomechanics of the posterior eye: A critical role in health and disease. J. Biomech. Eng. 2014, 136, 21005. [Google Scholar] [CrossRef] [PubMed]




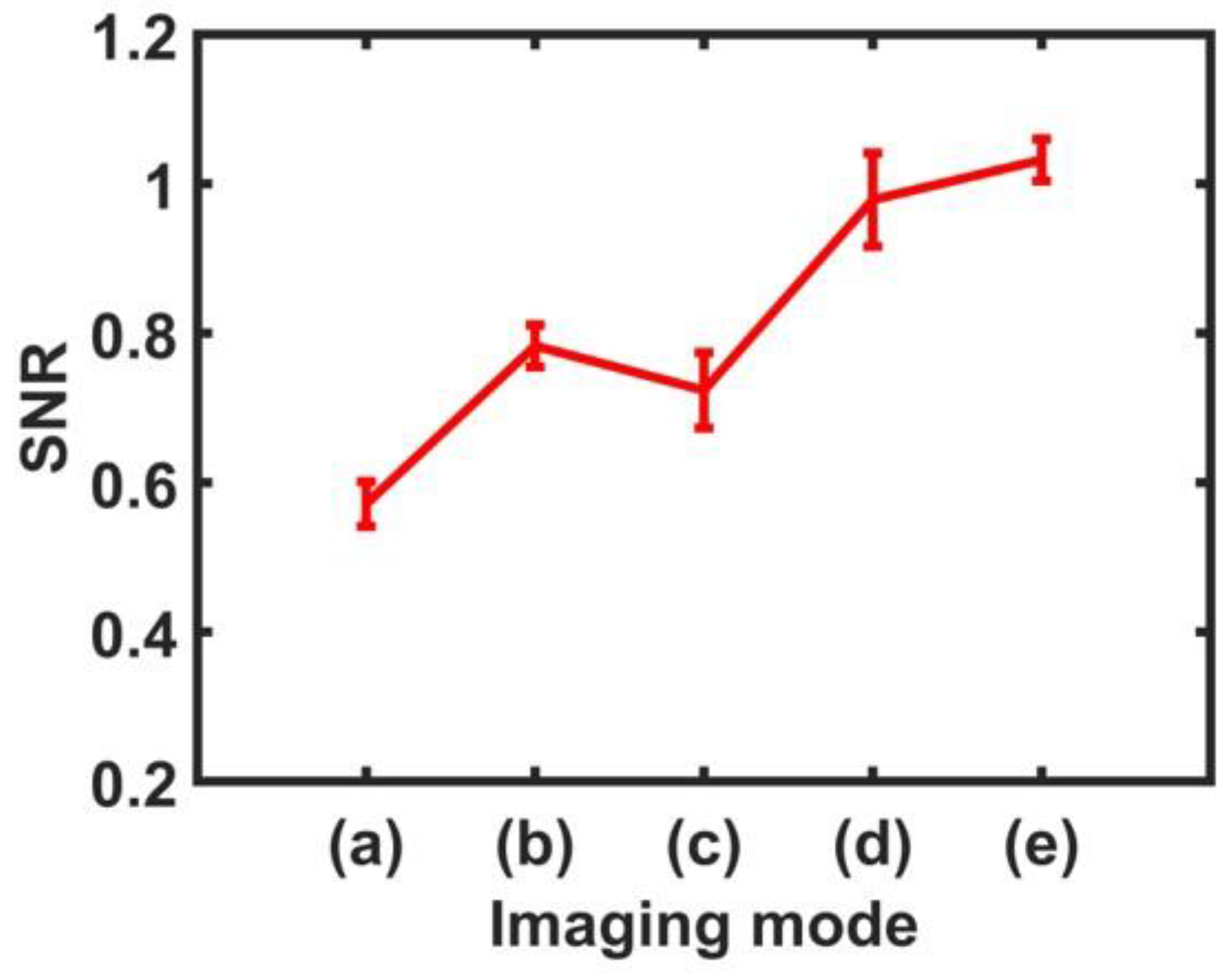
| Label * | Imaging Mode | Mean SNR | Min–Max (SNR) | Improvement (%) |
|---|---|---|---|---|
| (a) | ARFI Image (In-phase) | 0.572 | 0.544–0.604 | - |
| (b) | ARFI Image (Out-of-phase) | 0.783 | 0.762–0.818 | 36.88 |
| (c) | CARFI Image (In-phase) | 0.724 | 0.678–0.779 | 26.57 |
| (d) | CARFI Image (Out-of-phase) | 0.979 | 0.922–1.047 | 71.15 |
| (e) | CARFI Image (In-phase + Out-of-phase) | 1.032 | 1.003–1.059 | 80.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, G.S.; Moon, H.H.; Lee, H.S.; Jeong, J.S. Compound Acoustic Radiation Force Impulse Imaging of Bovine Eye by Using Phase-Inverted Ultrasound Transducer. Sensors 2024, 24, 2700. https://doi.org/10.3390/s24092700
Kim GS, Moon HH, Lee HS, Jeong JS. Compound Acoustic Radiation Force Impulse Imaging of Bovine Eye by Using Phase-Inverted Ultrasound Transducer. Sensors. 2024; 24(9):2700. https://doi.org/10.3390/s24092700
Chicago/Turabian StyleKim, Gil Su, Hak Hyun Moon, Hee Su Lee, and Jong Seob Jeong. 2024. "Compound Acoustic Radiation Force Impulse Imaging of Bovine Eye by Using Phase-Inverted Ultrasound Transducer" Sensors 24, no. 9: 2700. https://doi.org/10.3390/s24092700





