Mechanosensor Channels in Mammalian Somatosensory Neurons
Abstract
:1. Introduction
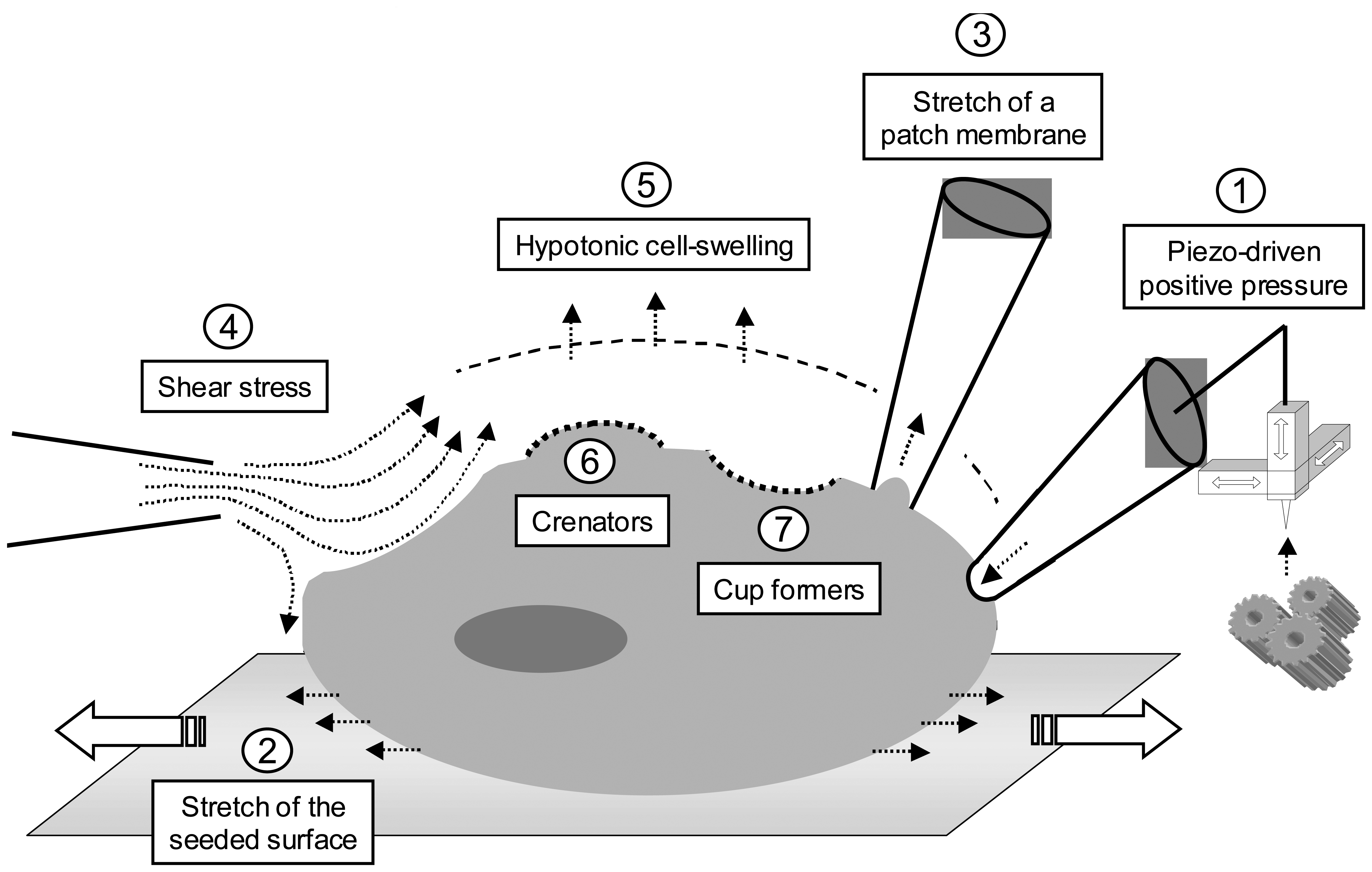
2. Probing mechanosensor channels
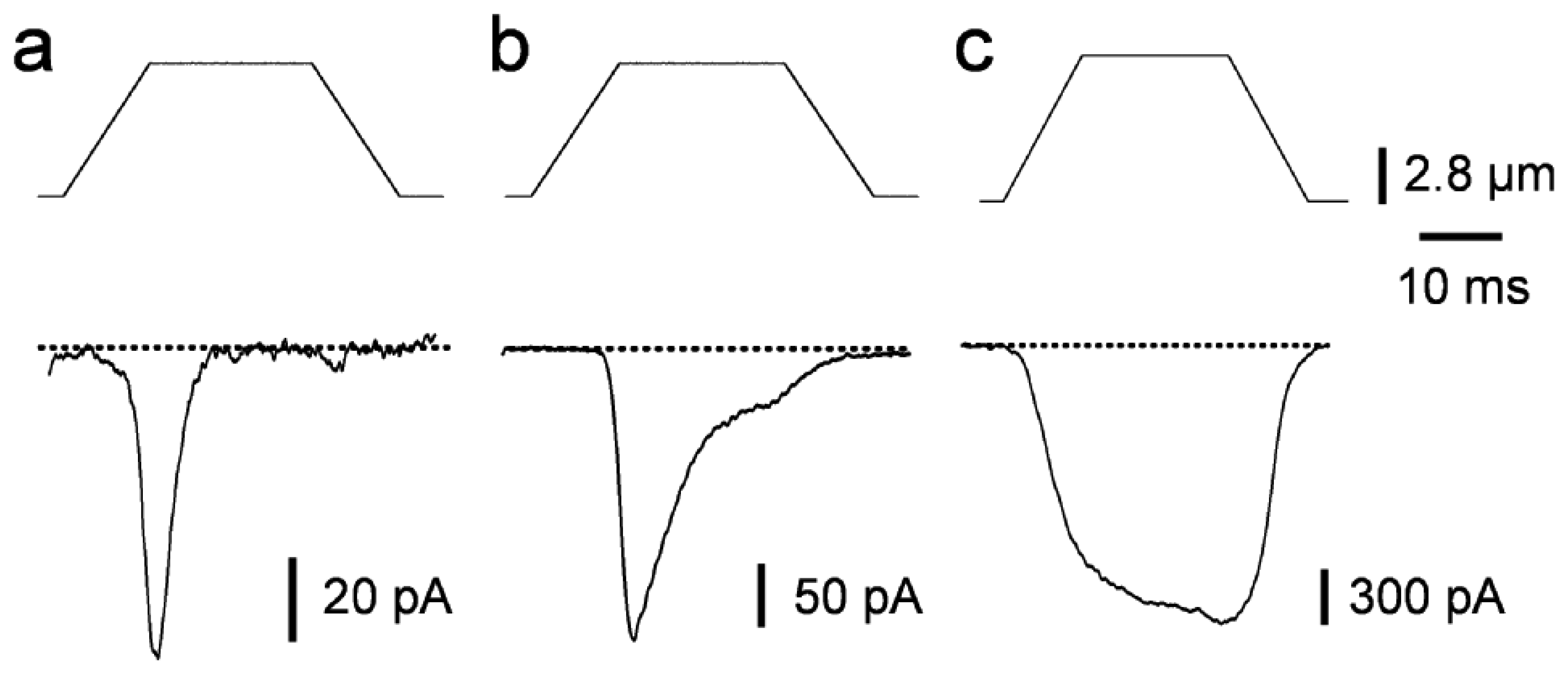
2.1. Piezo-driven pressure
2.2. Cell stretch
2.3. Fluid shear stress
2.4. Osmotic challenges
2.5. Crenators and cup formers
3. Biophysical properties of macroscopic MS currents in sensory neurons
4. Candidate channels
4.1. The DEG/ENaC superfamily
Acid Sensing Ion Channels
Stomatin
4.2. The TRP superfamily
TRPV channels
TRPC1 channels
TRPA1 channels
5. Conclusion
Acknowledgments
References and Notes
- Olausson, H.; Lamarre, Y.; Backlund, H.; Morin, C.; Wallin, B.G.; Starck, G.; Ekholm, S.; Strigo, I.; Worsley, K.; Vallbo, A.B.; Bushnell, M.C. Unmyelinated tactile afferents signal touch and project to insular cortex. Nat. Neurosci. 2002, 5, 900–904. [Google Scholar]
- McCarter, G.C.; Reichling, D.B.; Levine, J.D. Mechanical transduction by rat dorsal root ganglion neurons in vitro. Neurosci. Lett. 1999, 273, 179–182. [Google Scholar]
- Drew, L.J.; Wood, J.N.; Cesare, P. Distinct mechanosensitive properties of capsaicin-sensitive and -insensitive sensory neurons. J. Neurosci. 2002, 22, 228. [Google Scholar]
- Drew, L.J.; Rohrer, D.K.; Price, M.P.; Blaver, K.E.; Cockayne, D.A.; Cesare, P.; Wood, J.N. Acid-sensing ion channels ASIC2 and ASIC3 do not contribute to mechanically activated currents in mammalian sensory neurons. J. Physiol. 2004, 556, 691–710. [Google Scholar]
- Hu, J.; Lewin, G.R. Mechanosensitive currents in the neurites of cultured mouse sensory neurons. J. Physiol. 2006, 577, 815–828. [Google Scholar]
- McCarter, G.C.; Levine, J.D. Ionic basis of a mechanotransduction current in adult rat dorsal root ganglion neurons. Mol. Pain 2006, 2, 28. [Google Scholar]
- Coste, B.; Crest, M.; Delmas, P. Pharmacological dissection and distribution of NaN/Nav1.9, T-type Ca2+ currents, and mechanically activated cation currents in different populations of DRG neurons. J. Gen. Physiol. 2007, 129, 57–77. [Google Scholar]
- Hamill, O.P. Twenty odd years of stretch-sensitive channels. Pflügers Arch. 2006, 453, 333–351. [Google Scholar]
- Cho, H.; Shi, J.; Shin, C.Y.; Lee, S.Y.; Oh, U. Mechanosensitive ion channels in cultured sensory neurons of neonatal rats. J. Neurosci. 2002, 22, 1238–1247. [Google Scholar]
- Gottlieb, P.A.; Suchyna, T.M.; Ostrow, L.W.; Sachs, F. Mechanosensitive ion channels as drug targets. Curr. Drug Targets CNS Neurol. Disord. 2004, 3, 287–295. [Google Scholar]
- Suchyna, T.M.; Tape, S.E.; Koeppe, R.E.; Andersen, O.S.; Sachs, F.; Gottlieb, P.A. Bilayer-dependent inhibition of mechanosensitive channels by neuroactive peptide enantiomers. Nature 2004, 430, 235–240. [Google Scholar]
- Yuan, X.; Luo, S.; Lin, Z.; Wu, Y. Cyclic stretch translocates the alpha2-subunit of the Na pump to plasma membrane in skeletal muscle cells in vitro. Biochem. Biophys. Res. Commun. 2006, 348, 750–757. [Google Scholar]
- Guharay, F.; Sachs, F. Stretch-activated single ion channel currents in tissue-cultured embryonic chick skeletal muscle. J. Physiol. 1984, 352, 685–701. [Google Scholar]
- Takahashi, A.; Gotoh, H. Mechanosensitive whole-cell currents in cultured rat somatosensory neurons. Brain Res. 2000, 869, 225–230. [Google Scholar]
- Praetorius, H.A.; Spring, K.R. Bending the MDCK cell primary cilium increases intracellular calcium. J. Membr. Biol. 2001, 184, 71–79. [Google Scholar]
- Giamarchi, A.; Delmas, P. Activation mechanisms and functional roles of TRPP2 cation channels. In TRP ion channel function in sensory transduction and cellular signaling cascades; Boca Raton, F.L., Ed.; Frontiers in Neuroscience: New York, NY, 2006; pp. 189–202. [Google Scholar]
- Giamarchi, A.; Padilla, F.; Coste, B.; Raoux, M.; Crest, M.; Honoré, E.; Delmas, P. The versatile nature of the calcium-permeable cation channel TRPP2. Embo Rep. 2006, 7, 787–793. [Google Scholar]
- Davies, P.F.; Mundel, T.; Barbee, K.A. A mechanism for heterogeneous endothelial responses to flow in vivo and in vitro. J. Biomech. 1995, 28, 1553–1560. [Google Scholar]
- Tulis, D.A.; Unthank, J.L.; Prewitt, R.L. Flow-induced arterial remodeling in rat mesenteric vasculature. Am. J. Physiol. 1998, 274, 874–882. [Google Scholar]
- Spungin, B.; Silberberg, A. Stimulation of mucus secretion, ciliary activity, and transport in frog palate epithelium. Am. J. Physiol. 1984, 247, 299–308. [Google Scholar]
- Andrade, Y.N.; Fernandes, J.; Vazquez, E.; Fernandez-Fernandez, J.M.; Arniges, M.; Sanchez, T.M.; Villalon, M.; Valverde, M.A. TRPV4 channel is involved in the coupling of fluid viscosity changes to epithelial ciliary activity. J. Cell. Biol. 2005, 168, 869–874. [Google Scholar]
- Winters, S.L.; Davis, C.W.; Boucher, R.C. Mechanosensitivity of mouse tracheal ciliary beat frequency: roles for Ca2+, purinergic signaling, tonicity, and viscosity. Am. J. Physiol. Lung. Cell. Mol. Physiol. 2007, 292, 614–624. [Google Scholar]
- Cunningham, J.T.; Wachtel, R.E.; Abboud, F.M. Mechanosensitive currents in putative aortic baroreceptor neurons in vitro. J. Neurophysiol. 1995, 73, 2094–2098. [Google Scholar]
- Martinac, B. Mechanosensitive ion channels: molecules of mechanotransduction. J. Cell. Sci. 2004, 117, 2449–2460. [Google Scholar]
- O'Neil, R.G.; Heller, S. The mechanosensitive nature of TRPV channels. Pflügers Arch. 2005, 451, 193–203. [Google Scholar]
- Lin, S.Y.; Corey, D.P. TRP channels in mechanosensation. Curr. Opin. Neurobiol. 2005, 15, 350–357. [Google Scholar]
- Nilius, B.; Eggermont, J.; Voets, T.; Droogmans, G. Volume-activated Cl- channels. Gen. Pharmacol. 1996, 27, 1131–1140. [Google Scholar]
- Strange, K.; Emma, F.; Jackson, P.S. Cellular and molecular physiology of volume-sensitive anion channels. Am. J. Physiol. 1996, 270, 711–730. [Google Scholar]
- Okada, Y. Volume expansion-sensing outward-rectifier Cl- channel: fresh start to the molecular identity and volume sensor. Am. J. Physiol. 1997, 273, 755–789. [Google Scholar]
- Nilius, B.; Voets, T.; Prenen, J.; Barth, H.; Aktories, K.; Kaibuchi, K.; Droogmans, G.; Eggermont, J. Role of Rho and Rho kinase in the activation of volume-regulated anion channels in bovine endothelial cells. J. Physiol. 1999, 516, 67–74. [Google Scholar]
- Wiese, K.G. Electrolyte concentration, real and osmotic pressure in abscesses. Zentralbl. Chir. 1994, 119, 54–59. [Google Scholar]
- Wiese, K.G.; Merten, H.A.; Wiltfang, J.; Luhr, H.G. Clinical studies on the pathophysiology of odontogenic abscesses. Mund Kiefer Gesichtschir. 1999, 3, 242–246. [Google Scholar]
- Tsai, T.F.; Maibach, H.I. How irritant is water? An overview. Contact Dermatitis 1999, 41, 311–314. [Google Scholar]
- Misery, L.; Meyronet, D.; Pichon, M.; Brutin, J.L.; Pestre, P.; Cambazard, F. Aquadynia: a role for VIP? Ann. Dermatol. Venereol. 2003, 130, 195–198. [Google Scholar]
- Alessandri-Haber, N.; Joseph, E.; Dina, O.A.; Liedtke, W.; Levine, J.D. TRPV4 mediates pain-related behavior induced by mild hypertonic stimuli in the presence of inflammatory mediator. Pain 2005, 118, 70–79. [Google Scholar]
- Boudreault, F.; Grygorczyk, R. Cell swelling-induced ATP release and gadolinium-sensitive channels. Am. J. Physiol. Cell. Physiol. 2002, 282, 219–226. [Google Scholar]
- Kimura, C.; Oike, M.; Ohnaka, K.; Nose, Y.; Ito, Y. Constitutive nitric oxide production in bovine aortic and brain microvascular endothelial cells: a comparative study. J. Physiol. 2004, 554, 721–730. [Google Scholar]
- Von Weikersthal, S.F.; Barrand, M.A.; Hladky, S.B. Functional and molecular characterization of a volume-sensitive chloride current in rat brain endothelial cells. J. Physiol. 1999, 516, 75–84. [Google Scholar]
- Strotmann, R.; Harteneck, C.; Nunnenmacher, K.; Schultz, G.; Plant, T.D. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat. Cell. Biol. 2000, 2, 695–702. [Google Scholar]
- Srinivas, S.P.; Maertens, C.; Goon, L.H.; Goon, L.; Satpathy, M.; Yue, B.Y.; Droogman, G.; Nilius, B. Cell volume response to hyposmotic shock and elevated cAMP in bovine trabecular meshwork cells. Exp. Eye Res. 2003, 78, 15–26. [Google Scholar]
- Liu, X.; Bandyopadhyay, B.; Nakamoto, T.; Singh, B.; Liedtke, W.; Melvin, J.E.; Ambudkar, I. A role for AQP5 in activation of TRPV4 by hypotonicity: concerted involvement of AQP5 and TRPV4 in regulation of cell volume recovery. J. Biol. Chem. 2006, 281, 15485–1595. [Google Scholar]
- Raoux, M.; Colomban, C.; Delmas, P.; Crest, M. The amine-containing cutaneous irritant heptylamine inhibits the volume-regulated anion channel and mobilizes intracellular calcium in normal human epidermal keratinocytes. Mol. Pharmacol. 2007, 71, 1685–1694. [Google Scholar]
- Sheetz, M.P.; Singer, S.J. Biological membranes as bilayer couples. A molecular mechanism of drug-erythrocyte interactions. Proc. Natl Acad. Sci. USA 1974, 71, 4457–4461. [Google Scholar]
- Sheetz, M.P.; Singer, S.J. Equilibrium and kinetic effects of drugs on the shapes of human erythrocytes. J. Cell. Biol. 1976, 70, 247–251. [Google Scholar]
- Martinac, B.; Adler, J.; Kung, C. Mechanosensitive ion channels of E. coli activated by amphipaths. Nature 1990, 348, 261–263. [Google Scholar]
- Sokabe, M.; Hasegawa, N.; Yamamori, K. Blockers and activators for stretch-activated ion channels of chick skeletal muscle. Ann. NY Acad. Sci. 1993, 707, 417–420. [Google Scholar]
- Patel, A.J.; Honore, E.; Maingret, F.; Lesage, F.; Fink, M.; Duprat, F.; Lazdunski, M. A mammalian two pore domain mechano-gated S-like K+ channel. Embo J. 1998, 17, 4283–4290. [Google Scholar]
- Maingret, F.; Fosset, M.; Lesage, F.; Lazdunski, M.; Honoré, E. TRAAK is a mammalian neuronal mechano-gated K+ channel. J. Biol. Chem. 1999, 274, 1381–1387. [Google Scholar]
- Lewin, G.R.; Moshourab, R. Mechanosensation and pain. J. Neurobiol. 2004, 61, 30–44. [Google Scholar]
- Kleyman, T.R.; Cragoe, E.J. Amiloride and its analogs as tools in the study of ion transport. J. Memb. Biol. 1988, 105, 1–21. [Google Scholar]
- Delmas, P.; Nauli, S.M.; Li, X.; Coste, B.; Osorio, N.; Crest, M.; Brown, D.A.; Zhou, J. Gating of the polycystin ion channel signaling complex in neurons and kidney cells. Faseb J. 2004, 18, 740–742. [Google Scholar]
- Hamill, O.P.; McBride, D.W. The pharmacology of mechanogated membrane ion channels. Pharmacol. Rev. 1996, 48, 231–252. [Google Scholar]
- Höger, U.; Torkkeli, P.H.; Seyfarth, E.A.; French, A.S. Ionic selectivity of mechanically activated channels in spider mechanoreceptor neurons. J. Neurophysiol. 1997, 78, 2079–2085. [Google Scholar]
- Drew, L.J.; Rugiero, F.; Cesare, P.; Gale, J.E.; Abrahamsen, B.; Bowden, S.; Heinzmann, S.; Robinson, M.; Brust, A.; Colless, B.; Lewis, R.J.; Wood, J.N. High-threshold mechanosensitive ion channels blocked by a novel conopeptide mediate pressure-evoked pain. PLoS ONE 2007, 2, 515. [Google Scholar]
- Tavernarakis, N.; Driscoll, M. Molecular modeling of mechanotransduction in the nematode Caenorhabditis elegans. Annu. Rev. Physiol. 1997, 59, 659–689. [Google Scholar]
- Gillespie, P.G.; Walker, R.G. Molecular basis of mechanosensory transduction. Nature 2001, 413, 194–202. [Google Scholar]
- Bounoutas, A.; Chalfie, M. Touch sensitivity in Caenorhabditis elegans. Pflügers Arch. 2007, 454, 691–702. [Google Scholar]
- Waldmann, R.; Lazdunski, M. H+-gated cation channels: neuronal acid sensors in the NaC/DEG family of ion channels. Curr. Opin. Neurobiol. 1998, 8, 418–424. [Google Scholar]
- Lingueglia, E. Acid-sensing ion channels in sensory perception. J. Biol. Chem. 2007, 282, 17325–17329. [Google Scholar]
- García-Añoveros, J.; Samad, T.A.; Zuvela-Jelaska, L.; Woolf, C.J.; Corey, D.P. Transport and localization of the DEG/ENaC ion channel BNaC1alpha to peripheral mechanosensory terminals of dorsal root ganglia neurons. J. Neurosci. 2001, 21, 2678–2686. [Google Scholar]
- Price, M.P.; McIlwrath, S.L.; Xie, J.; Cheng, C.; Qiao, J.; Tarr, D.E.; Sluka, K.A.; Brennan, T.J.; Lewin, G.R.; Welsh, M.J. The DRASIC cation channel contributes to the detection of cutaneous touch and acid stimuli in mice. Neuron 2001, 32, 1071–1083. [Google Scholar]
- Roza, C.; Puel, J.L.; Kress, M.; Baron, A.; Diochot, S.; Lazdunski, M.; Waldmann, R. Knockout of the ASIC2 channel in mice does not impair cutaneous mechanosensation, visceral mechanonociception and hearing. J. Physiol. 2004, 558, 659–669. [Google Scholar]
- Price, M.P.; Lewin, G.R.; McIlwrath, S.L.; Cheng, C.; Xie, J.; Heppenstall, P.A.; Stucky, C.L.; Mannsfeldt, A.G.; Brennan, T.J.; Drummond, H.A.; Qiao, J.; Benson, C.J.; Tarr, D.E.; Hrstka, R.F.; Yang, B.; Williamson, R.A.; Welsh, M.J. The mammalian sodium channel BNC1 is required for normal touch sensation. Nature 2000, 407, 1007–1011. [Google Scholar]
- Huang, M.; Gu, G.; Ferguson, E.L.; Chalfie, M.A. Stomatin-like protein necessary for mechanosensation in C. elegans. Nature 1995, 378, 292–295. [Google Scholar]
- Wetzel, C.; Hu, J.; Riethmacher, D.; Benckendorff, A.; Harder, L.; Eilers, A.; Moshourab, R.; Kozlenkov, A.; Labuz, D.; Caspani, O.; Erdmann, B.; Machelska, H.; Heppenstall, P.A.; Lewin, G.R. A stomatin-domain protein essential for touch sensation in the mouse. Nature 2007, 445, 206–209. [Google Scholar]
- Stewart, G.W. Stomatin. Int. J. Biochem. Cell. Biol. 1997, 29, 271–274. [Google Scholar]
- Nilius, B.; Voets, T. TRP channels: a TR(I)P through a world of multifunctional cation channels. Pflügers Arch. 2005, 451, 1–10. [Google Scholar]
- Nilius, B.; Owsianik, G.; Voets, T.; Peters, J.A. Transient receptor potential cation channels in disease. Physiol. Rev. 2007, 87, 165–217. [Google Scholar]
- Birder, L.A.; Nakamura, Y.; Kiss, S.; Nealen, M.L.; Barrick, S.; Kanai, A.J.; Wang, E.; Ruiz, G.; De Groat, W.C.; Apodaca, G.; Watkins, S.; Caterina, M.J. Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nature Neurosci. 2002, 5, 856–860. [Google Scholar]
- Sharif Naeini, R.; Witty, M.F.; Seguela, P.; Bourque, C.W. An N-terminal variant of Trpv1 channel is required for osmosensory transduction. Nature Neurosci. 2006, 9, 93–98. [Google Scholar]
- Gunthorpe, M.J.; Benham, C.D.; Randall, A.; Davis, J.B. The diversity in the vanilloid (TRPV) receptor family of ion channels. Trends Pharmacol. Sci. 2002, 23, 183–191. [Google Scholar]
- Caterina, M.J.; Leffler, A.; Malmberg, A.B.; Martin, W.J.; Trafton, J.; Petersen-Zeitz, K.R.; Koltzenburg, M.; Basbaum, A.I.; Julius, D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 2000, 288, 306–313. [Google Scholar]
- Davis, J.B.; Gray, J.; Gunthorpe, M.J.; Hatcher, J.P.; Davey, P.T.; Overend, P.; Harries, M.H.; Latcham, J.; Clapham, C.; Atkinson, K.; Hughes, S.A.; Rance, K.; Grau, E.; Harper, A.J.; Pugh, P.L.; Rogers, D.C.; Bingham, S.; Randall, A.; Sheardown, S.A. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature 2000, 405, 183–187. [Google Scholar]
- Muraki, K.; Iwata, Y.; Katanosaka, Y.; Ito, T.; Ohya, S.; Shigekawa, M.; Imaizumi, Y. TRPV2 is a component of osmotically sensitive cation channels in murine aortic myocytes. Circ. Res. 2003, 93, 829–838. [Google Scholar]
- Davis, M.J.; Hill, M.A. Signaling mechanisms underlying the vascular myogenic response. Physiol. Rev. 1999, 79, 387–423. [Google Scholar]
- Caterina, M.J.; Julius, D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu. Rev. Neurosci. 2001, 24, 487–517. [Google Scholar]
- Vriens, J.; Watanabe, H.; Janssens, A.; Droogmans, G.; Voets, T.; Nilius, B. Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proc. Natl Acad. Sci. USA 2004, 101, 396–401. [Google Scholar]
- Liedtke, W. TRPV4 as osmosensor: a transgenic approach. Pflügers Arch. 2005, 451, 176–180. [Google Scholar]
- Liedtke, W.; Choe, Y.; Martí-Renom, M.A.; Bell, A.M.; Denis, C.S.; Sali, A.; Hudspeth, A.J.; Friedman, J.M.; Heller, S. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 2000, 103, 525–535. [Google Scholar]
- Liedtke, W.; Friedman, J.M. Abnormal osmotic regulation in trpv4-/- mice. Proc. Natl Acad. Sci. USA 2003, 100, 13698–13703. [Google Scholar]
- Suzuki, M.; Mizuno, A.; Kodaira, K.; Imai, M. Impaired pressure sensation in mice lacking TRPV4. J. Biol. Chem. 2003, 278, 22664–22668. [Google Scholar]
- Alessandri-Haber, N.; Dina, O.A.; Yeh, J.J.; Parada, C.A.; Reichling, D.B.; Levine, J.D. Transient receptor potential vanilloid 4 is essential in chemotherapy-induced neuropathic pain in the rat. J. Neurosci. 2004, 24, 4444–4452. [Google Scholar]
- Parekh, A.B.; Putney, J.W. Store-operated calcium channels. Physiol. Rev. 2005, 85, 757–810. [Google Scholar]
- Maroto, R.; Raso, A.; Wood, T.G.; Kurosky, A.; Martinac, B.; Hamill, O.P. TRPC1 forms the stretch-activated cation channel in vertebrate cells. Nat. Cell. Biol. 2005, 7, 179–185. [Google Scholar]
- Hamill, O.P.; Maroto, R. TRPCs as MS channels. Curr. Top. Membr. 2007, 59. in press. [Google Scholar]
- Tsiokas, L.; Arnould, T.; Zhu, C.; Kim, E.; Walz, G.; Sukhatme, V.P. Specific association of the gene product of PKD2 with the TRPC1 channel. Proc. Natl Acad. Sci. USA 1999, 96, 3934–3939. [Google Scholar]
- Delmas, P. Polycystins: from mechanosensation to gene regulation. Cell 2004, 118, 145–148. [Google Scholar]
- Delmas, P. Assembly and gating of TRPC channels in signalling microdomains. In Mammalian TRP Channels as Molecular Targets; John Wiley & Sons, Ltd. Novartis Found. Symp., 2004; Volume 258, pp. 75–97. [Google Scholar]
- Eberl, D.F.; Hardy, R.W.; Kernan, M.J. Genetically similar transduction mechanisms for touch and hearing in Drosophila. J. Neurosci. 2000, 20, 5981–5988. [Google Scholar]
- Walker, R.G.; Willingham, A.T.; Zuker, C.S. A Drosophila mechanosensory transduction channel. Science 2000, 287, 2229–2234. [Google Scholar]
- Sidi, S.; Friedrich, R.W.; Nicolson, T. NompC TRP channel required for vertebrate sensory hair cell mechanotransduction. Science 2003, 301, 96–99. [Google Scholar]
- Shin, J.B.; Adams, D.; Paukert, M.; Siba, M.; Sidi, S.; Levin, M.; Gillespie, P.G.; Gründer, S. Xenopus TRPN1 (NOMPC) localizes to microtubule-based cilia in epithelial cells, including inner-ear hair cells. Proc. Natl Acad. Sci. USA 2005, 102, 12572–12577. [Google Scholar]
- Gopfert, M.C.; Albert, J.T.; Nadrowski, B.; Kamikouchi, A. Specification of auditory sensitivity by Drosophila TRP channels. Nature Neurosci. 2006, 9, 999–1000. [Google Scholar]
- Li, W.; Feng, Z.; Sternberg, P.W.; Xu, X.Z. A C. elegans stretch receptor neuron revealed by a mechanosensitive TRP channel homologue. Nature 2006, 440, 684–687. [Google Scholar]
- Corey, D.P. What is the hair cell transduction channel? J. Physiol. 2006, 576, 23–28. [Google Scholar]
- Corey, D.P.; García-Añoveros, J.; Holt, J.R.; Kwan, K.Y.; Lin, S.Y.; Vollrath, M.A.; Amalfitano, A.; Cheung, E.L.; Derfler, B.H.; Duggan, A.; Geleoc, G.S.; Gray, P.A.; Hoffman, M.P.; Rehm, H.L.; Tamasauskas, D.; Zhang, D.S. TRPA1 is a candidate for the mechanosensitive transduction channel of vertebrate hair cells. Nature 2004, 432, 723–730. [Google Scholar]
- Nagata, K.; Duggan, A.; Kumar, G.; García-Añoveros, J. Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J. Neurosci. 2005, 25, 4052–4061. [Google Scholar]
- Vollrath, M.A.; Kwan, K.Y.; Corey, D.P. The micromachinery of mechanotransduction in hair cells. Annu. Rev. Neurosci. 2007, 30, 339–365. [Google Scholar]
- Bautista, D.M.; Jordt, S.E.; Nikai, T.; Tsuruda, P.R.; Read, A.J.; Poblete, J.; Yamoah, E.N.; Basbaum, A.I.; Julius, D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 2006, 124, 1269–1282. [Google Scholar]
- Kwan, K.Y.; Allchorne, A.J.; Vollrath, M.A.; Christensen, A.P.; Zhang, D.S.; Woolf, C.J.; Corey, D.P. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron 2006, 50, 277–289. [Google Scholar]
- Kindt, K.S.; Viswanath, V.; Macpherson, L.; Quast, K.; Hu, H.; Patapoutian, A.; Schafer, W.R. Caenorhabditis elegans TRPA-1 functions in mechanosensation. Nat. Neurosci. 2007, 10, 568–577. [Google Scholar]
- Story, G.M.; Peier, A.M.; Reeve, A.J.; Eid, S.R.; Mosbacher, J.; Hricik, T.R.; Earley, T.J.; Hergarden, A.C.; Andersson, D.A.; Hwang, S.W.; McIntyre, P.; Jegla, T.; Bevan, S.; Patapoutian, A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 2003, 112, 819–829. [Google Scholar]
- Bautista, D.M.; Movahed, P.; Hinman, A.; Axelsson, H.E.; Sterner, O.; Högestätt, E.D.; Julius, D.; Jordt, S.E.; Zygmunt, P.M. Pungent products from garlic activate the sensory ion channel TRPA1. Proc. Natl Acad. Sci. USA 2005, 102, 12248–12252. [Google Scholar]
© 2007 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Raoux, M.; Rodat-Despoix, L.; Azorin, N.; Giamarchi, A.; Hao, J.; Maingret, F.; Crest, M.; Coste, B.; Delmas, P. Mechanosensor Channels in Mammalian Somatosensory Neurons. Sensors 2007, 7, 1667-1682. https://doi.org/10.3390/s7091667
Raoux M, Rodat-Despoix L, Azorin N, Giamarchi A, Hao J, Maingret F, Crest M, Coste B, Delmas P. Mechanosensor Channels in Mammalian Somatosensory Neurons. Sensors. 2007; 7(9):1667-1682. https://doi.org/10.3390/s7091667
Chicago/Turabian StyleRaoux, Matthieu, Lise Rodat-Despoix, Nathalie Azorin, Aurélie Giamarchi, Jizhe Hao, François Maingret, Marcel Crest, Bertrand Coste, and Patrick Delmas. 2007. "Mechanosensor Channels in Mammalian Somatosensory Neurons" Sensors 7, no. 9: 1667-1682. https://doi.org/10.3390/s7091667



