Determination of Dopamine in the Presence of Ascorbic Acid by Nafion and Single-Walled Carbon Nanotube Film Modified on Carbon Fiber Microelectrode
Abstract
:1. Introduction
2. Results and Discussion
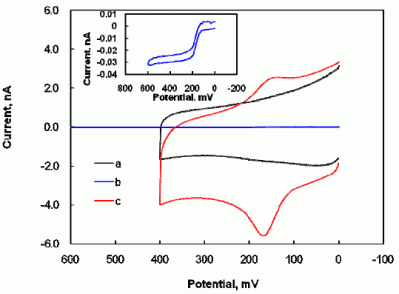
2.1 Electrochemical properties of dopamine at the Nafion-SWNTs/CFME modified microelectrode
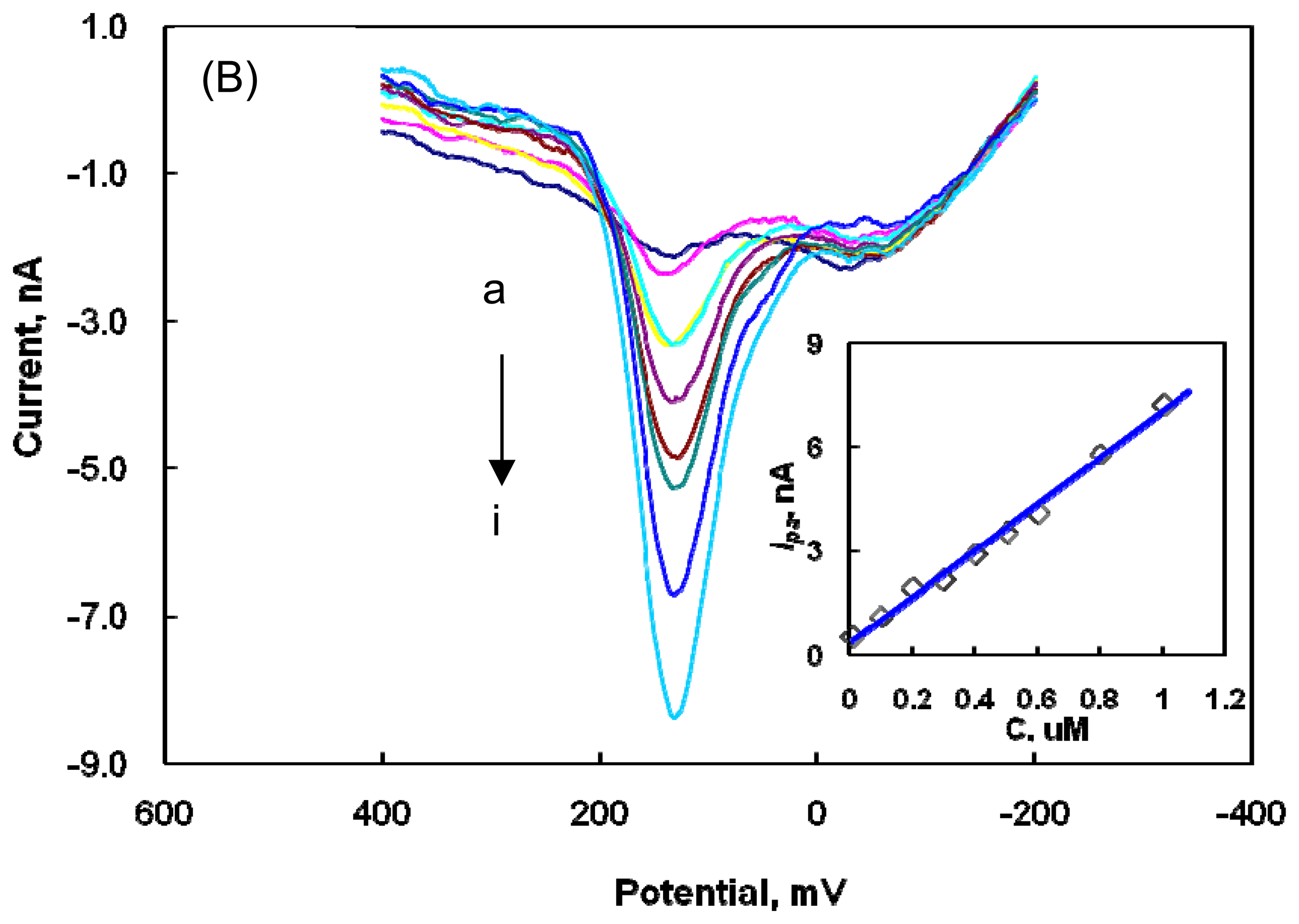
2.2 Determination of DA at the Nafion-SWNTs/CFME modified microelectrode
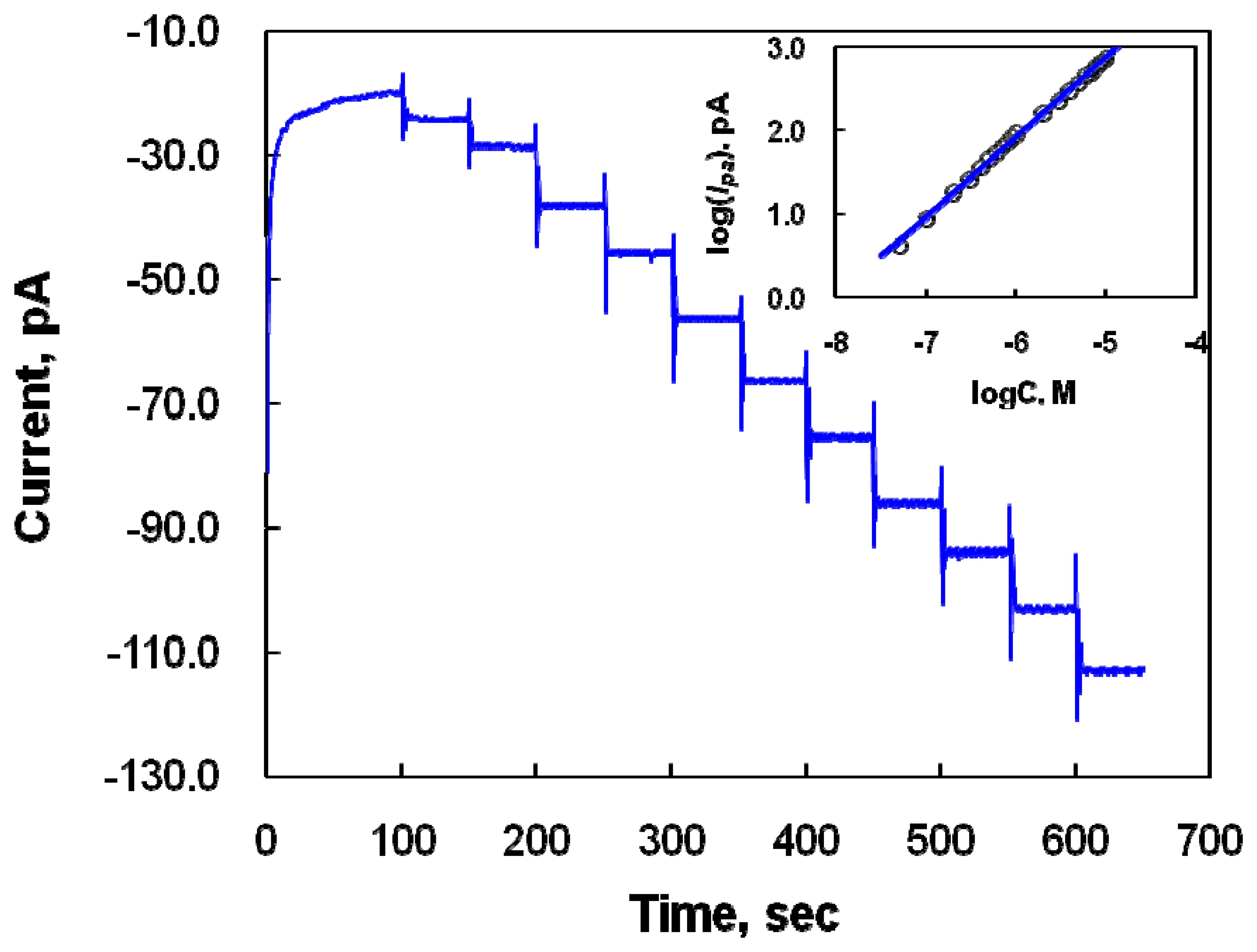
2.3 Determination of DA in the presence of AA at the Nafion-SWNTs/CFME modified microelectrode
3. Experimental Section
3.1 Reagents and electrochemical apparatus
3.2 Preparation of the Nafion-SWCTs film based on CFME
4. Conclusions
Acknowledgments
References and Notes
- Wightman, M.; May, L.J.; Michael, A.C. Detection of dopamine dynamics in the brain. Anal. Chem. 1988, 60, 769A–779A. [Google Scholar]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar]
- Wang, J.; Deo, R.P.; Poulin, P.; Mangey, M. Carbon nanotube fiber microelectrodes. J. Am. Chem. Soc. 2003, 125, 14706–14707. [Google Scholar]
- Chen, R.S.; Huang, W.-H.; Tong, H.; Wang, Z.-L.; Cheng, J.-K. Carbon fiber nanoelectrodes modified by single-walled carbon nanotubes. Anal. Chem. 2003, 75, 6341–6345. [Google Scholar]
- Hočevar, S.B.; Wang, J.; Deo, R.P.; Musameh, M.; Ogorevc, B. Carbon nanotube modified microelectrode for enhanced voltammetric detection of dopamine in the presence of ascorbate. Electroanalysis 2005, 17, 417–422. [Google Scholar]
- Lupu, S.; Parenti, F.; Pigani, L.; Seeber, R.; Zanardi, C. Differential pulse techniques on modified conventional-size and microelectrodes. Electroactivity of poly[4,4′-bis(butylsulfanyl)-2,2′-bithiophene] coating towards dopamine and ascorbic acid oxidation. Electroanalysis 2003, 15, 715–725. [Google Scholar]
- Amatore, C.; Arbault, S.; Guille, M.; Lemaître, F. Electrochemical monitoring of single cell secretion: Vesicular exocytosis and oxidative stress. Chem. Rev. 2008, 108, 2585–2621. [Google Scholar]
- Boo, H.; Jeong, R.-A.; Park, S.; Kim, K. S.; An, K. H.; Lee, Y. H.; Han, J. H.; Kim, H. C.; Chung, T. D. Electrochemical nanoneedle biosensor based on multiwall carbon nanotube. Anal. Chem. 2006, 78, 617–620. [Google Scholar]
- Valentini, F.; Amine, A.; Orlanducci, S.; Terranova, M. L.; Palleschi, G. Carbon nanotube purification: Preparation and characterization of carbon nanotube paste electrodes. Anal. Chem. 2003, 75, 5413–5421. [Google Scholar]
- Wang, H.-S.; Li, T.-H.; Jia, W.-L.; Xu, H.-Y. Highly selective and sensitive determination of dopamine using a Nafion/carbon nanotubes coated poly(3-methylthiophene) modified electrode. Biosens. Bioelectron. 2006, 22, 664–669. [Google Scholar]
- Zhang, Y.; Cai, Y.; Su, S. Determination of dopamine in the presence of ascorbic acid by poly(styrene sulfonic acid) sodium salt/single-wall carbon nanotube film modified glassy carbon electrode. Anal. Biochem. 2006, 350, 285–291. [Google Scholar]
- Hu, C.; Chen, X.; Hu, S. Water-soluble single-walled carbon nanotubes films: Preparation, characterization and applications as electrochemical sensing films. J. Electroanal. Chem. 2006, 586, 77–85. [Google Scholar]
- Wang, J.; Li, M.; Shi, Z.; Li, N.; Gu, Z. Electrocatalytic oxidation of norepinephrine at a glassy carbon electrode modified with single wall carbon nanotubes. Electroanalysis 2002, 14, 225–230. [Google Scholar]
- Wu, K.; Fei, J.; Hu, S. Simultaneous determination of dopamine and serotonin on a glassy carbon electrode coated with a film of carbon nanotubes. Anal. Biochem. 2003, 318, 100–106. [Google Scholar]
- Wang, Z.-H.; Liang, Q.-L.; Wang, Y.-M.; Luo, G.-A. Carbon nanotube-intercalated graphite electrodes for simultaneous determination of dopamine and serotonin in the presence of ascorbic acid. J. Electroanal. Chem. 2003, 540, 129–134. [Google Scholar]
- Antiochia, R.; Gorton, L. Development of a carbon nanotube paste electrode osmium polymer-mediated biosensor for determination of glucose in alcoholic beverages. Biosens. Bioelectron. 2007, 22, 2611–2617. [Google Scholar]
- Chu, X.; Duan, D.; Shen, G.; Yu, R. Amperometric glucose biosensor based on electrodeposition of platinum nanoparticles onto covalently immobilized carbon nanotube electrode. Talanta 2007, 71, 2040–2047. [Google Scholar]
- Lin, Y.; Lu, F.; Tu, Y.; Ren, Z. Glucose biosensors based on carbon nanotube nanoelectrode ensembles. Nano. Lett. 2004, 4, 191–195. [Google Scholar]
- Zhu, L.; Zhai, J.; Yang, R.; Tian, C.; Guo, L. Electrocatalytic oxidation of NADH with Meldola's blue functionalized carbon nanotubes electrodes. Biosens. Bioelectron. 2007, 22, 2768–2773. [Google Scholar]
- Zhang, M.; Gorski, W. Electrochemical sensing platform based on the carbon nanotubes/redox mediators-biopolymer system. J. Am. Chem. Soc. 2005, 127, 2058–2059. [Google Scholar]
- Zeng, J.; Gao, X.; Wei, W.; Zhai, X.; Yin, J.; Wu, L.; Liu, X.; Liu, K.; Gong, S. Fabrication of carbon nanotubes/poly(1,2-diaminobenzene) nanoporous composite via multipulse chronoamperometric electropolymerization process and its electrocatalytic property toward oxidation of NADH. Sens. Actuat. B: Chem. 2007, 120, 595–602. [Google Scholar]
- Xu, J.-Z.; Zhu, J.-J.; Wu, Q.; Hu, Z.; Chen, H.-Y. An amperometric biosensor based on the coimmobilization of horseradish peroxidase and methylene blue on a carbon nanotubes modified electrode. Electroanalysis 2003, 15, 219–224. [Google Scholar]
- Zou, Y.; Sun, L.; Xu, F. Prussian blue electrodeposited on MWNTs–PANI hybrid composites for H2O2 detection. Talanta 2007, 72, 437–442. [Google Scholar]
- Zhao, Y.-D.; Bi, Y.-H.; Zhang, W.-D.; Luo, Q.-M. The interface behavior of hemoglobin at carbon nanotube and the detection for H2O2. Talanta 2005, 65, 489–494. [Google Scholar]
- Luque, G.L.; Ferreyra, N.F.; Rivas, G.A. Electrochemical sensor for amino acids and albumin based on composites containing carbon nanotubes and copper microparticles. Talanta 2007, 71, 1282–1287. [Google Scholar]
- Sha, Y.; Qian, L.; Ma, Y.; Bai, H.; Yang, X. Multilayer films of carbon nanotubes and redox polymer on screen-printed carbon electrodes for electrocatalysis of ascorbic acid. Talanta 2006, 70, 556–560. [Google Scholar]
- Wu, K.; Hu, S.; Fei, J.; Bai, W. Mercury-free simultaneous determination of cadmium and lead at a glassy carbon electrode modified with multi-wall carbon nanotubes. Anal. Chim. Acta 2003, 489, 215–221. [Google Scholar]
- Nagy, G.; Gerhardt, G.A.; Oke, A.F.; Rice, M.E.; Adams, R.N.; Moore, R.B., III; Szentirmay, M.N.; Martin, C.R. Ion exchange and transport of neurotransmitters in Nafion films on conventional and microelectrode surfaces. J. Electroanal. Chem. 1985, 189, 85–94. [Google Scholar]
- Chen, Y.; Tan, T.C. Dopamine sensing and selectivity of Nafion-coated plant tissue powder sensors. Talanta 1995, 42, 1181–1188. [Google Scholar]
- Dávila, M.M.; Elizalde, M. P.; Mattusch, J.; Wennrich, R. Study of the composite electrodes carbon–polyvinyl chloride and carbon–polyvinyl chloride/Nafion by ex situ and in situ methods. Electrochim. Acta 2001, 46, 3189–3197. [Google Scholar]
- Yuan, S.; Hu, S. Characterization and electrochemical studies of Nafion/nano-TiO2 film modified electrodes. Electrochim. Acta 2004, 49, 4287–4293. [Google Scholar]
- Zhao, Y.; Gao, Y.; Zhan, D.; Liu, H.; Zhao, Q.; kou, Y.; Shao, Y.; Li, M.; Zhuang, Q.; Zhu, Z. Selective detection of dopamine in the presence of ascorbic acid and uric acid by a carbon nanotubes-ionic liquid gel modified electrode. Talanta 2005, 66, 51–57. [Google Scholar]





© 2008 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the CreativeCommons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jeong, H.; Jeon, S. Determination of Dopamine in the Presence of Ascorbic Acid by Nafion and Single-Walled Carbon Nanotube Film Modified on Carbon Fiber Microelectrode. Sensors 2008, 8, 6924-6935. https://doi.org/10.3390/s8116924
Jeong H, Jeon S. Determination of Dopamine in the Presence of Ascorbic Acid by Nafion and Single-Walled Carbon Nanotube Film Modified on Carbon Fiber Microelectrode. Sensors. 2008; 8(11):6924-6935. https://doi.org/10.3390/s8116924
Chicago/Turabian StyleJeong, Haesang, and Seungwon Jeon. 2008. "Determination of Dopamine in the Presence of Ascorbic Acid by Nafion and Single-Walled Carbon Nanotube Film Modified on Carbon Fiber Microelectrode" Sensors 8, no. 11: 6924-6935. https://doi.org/10.3390/s8116924