Schiff's Bases and Crown Ethers as Supramolecular Sensing Materials in the Construction of Potentiometric Membrane Sensors
Abstract
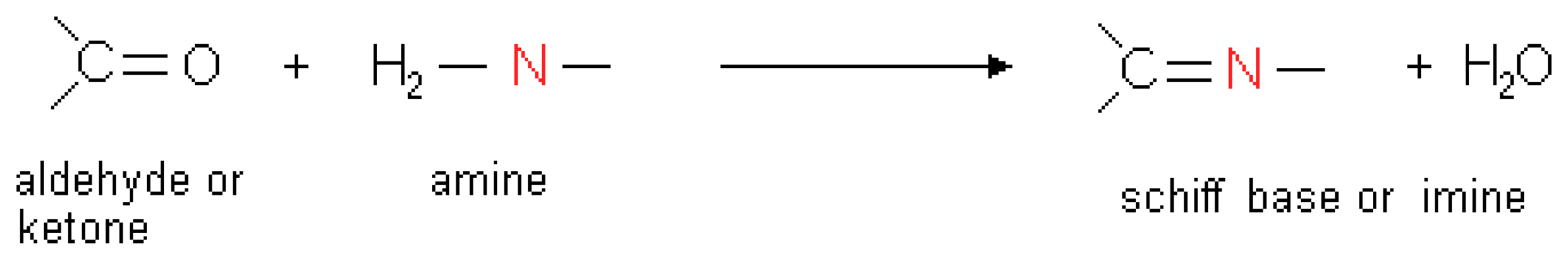
:1. Introduction
2. Principle of Potentiometric Membrane Sensors
2.1. Polymeric membrane
3. Cation-Binding Ionophores
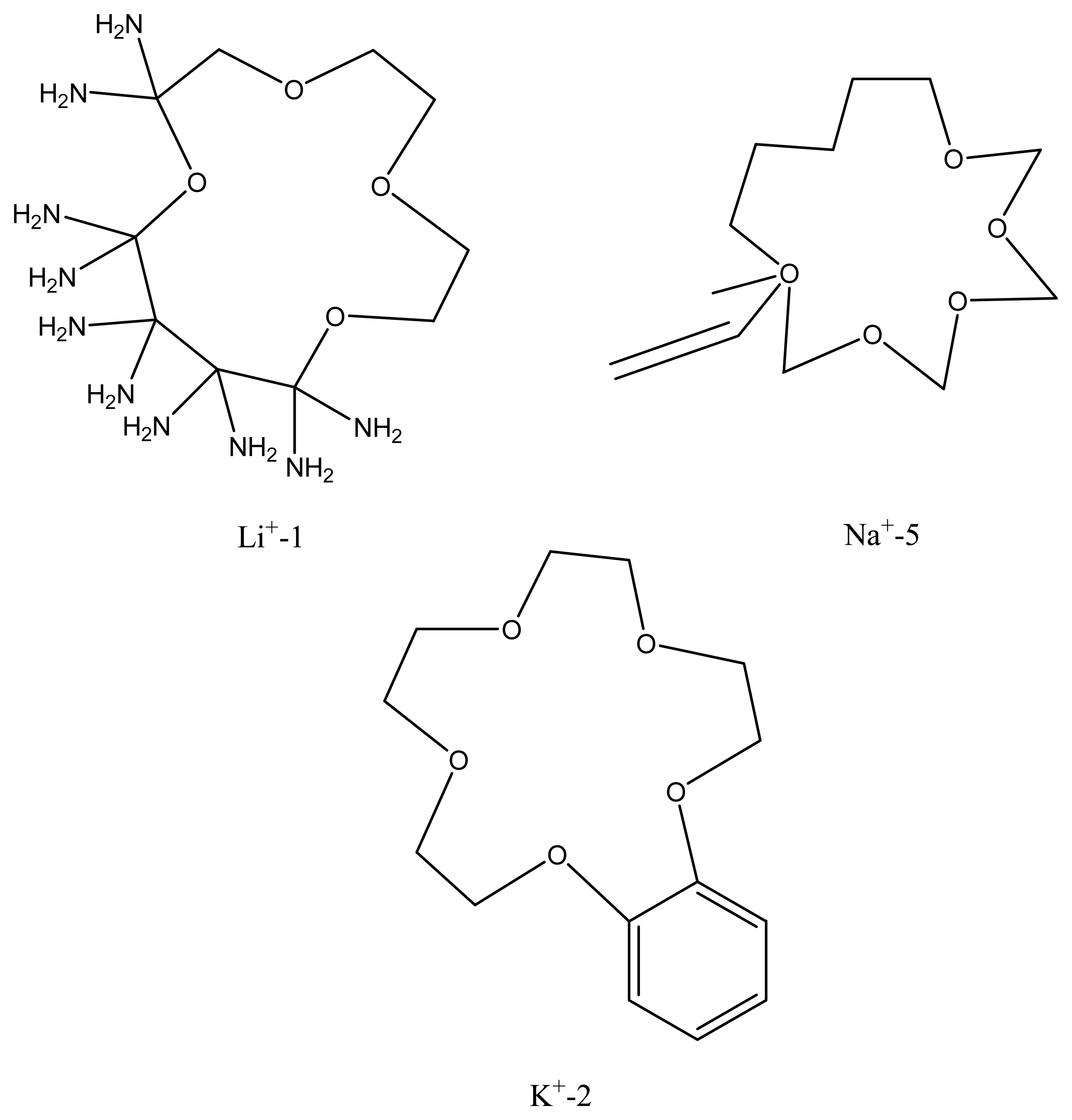
- Cs+ > Ag+ > K+ > NH4+ > Na+ > Li+ > Ca2+ > Pb2+ > Cu2+
- ClO4- > SCN− > I− > C6H4(OH)COO- > NO3- > Br− > NO2- > Cl− > HSO3- > CH3COO- > SO42− > HPO42−
3.1. The principle of the complexation reactions
3.2. Factors effective in complexation
4. Anion-Binding Ionophores
4.1. Metal center-anion interaction
4.2. Anionic sensors with hydrogen bonding
4.3. Anionic sensors with quaternary ammonium or guanidinium sites
4.4. Anionic sensors with trifluoroacetophenone receptors
5. Study of Complexation
5.1. Complexation study by conductometric method
5.2. Complexation study by spectroscopic methods
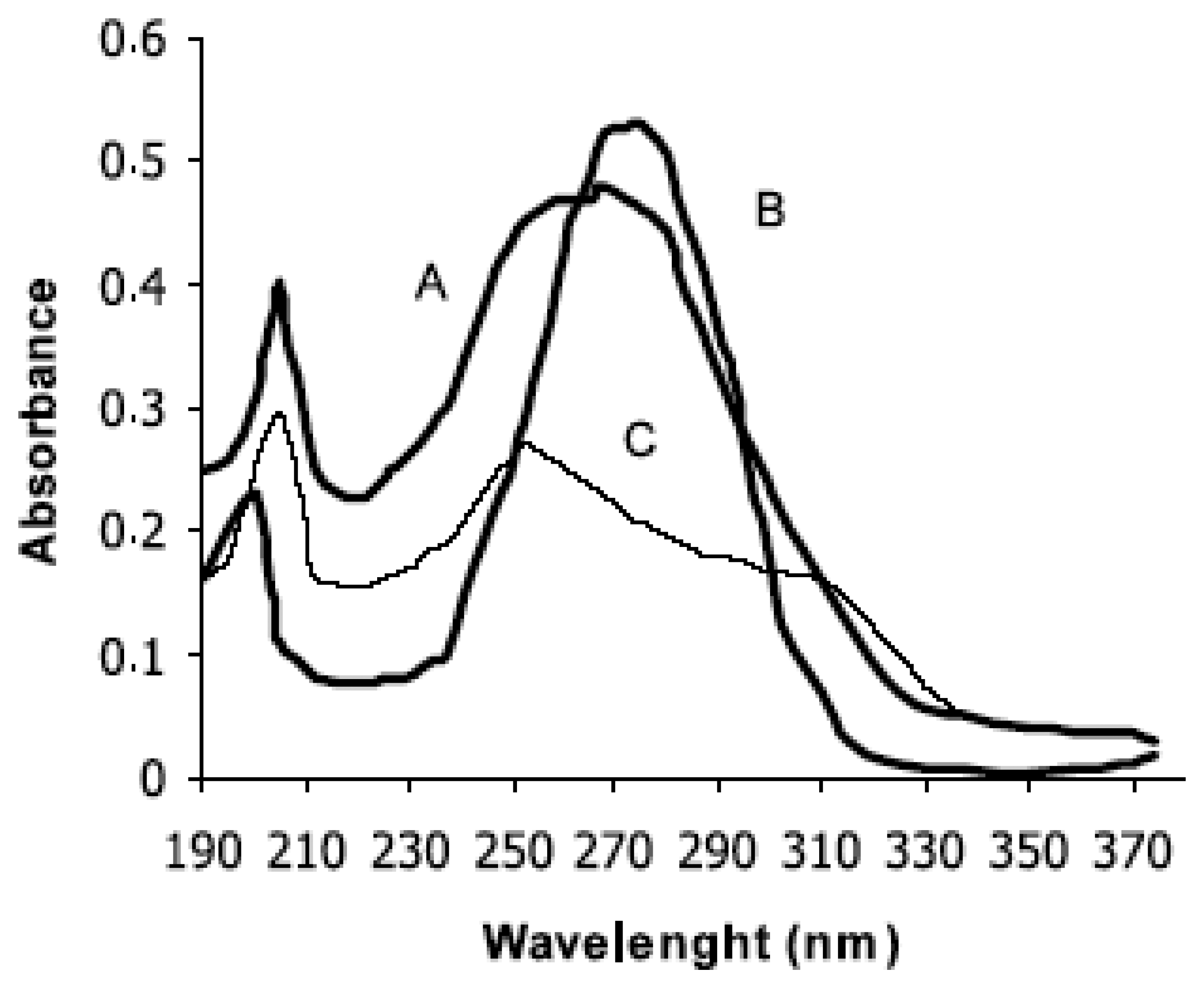
5.2.1. UV-VIS study
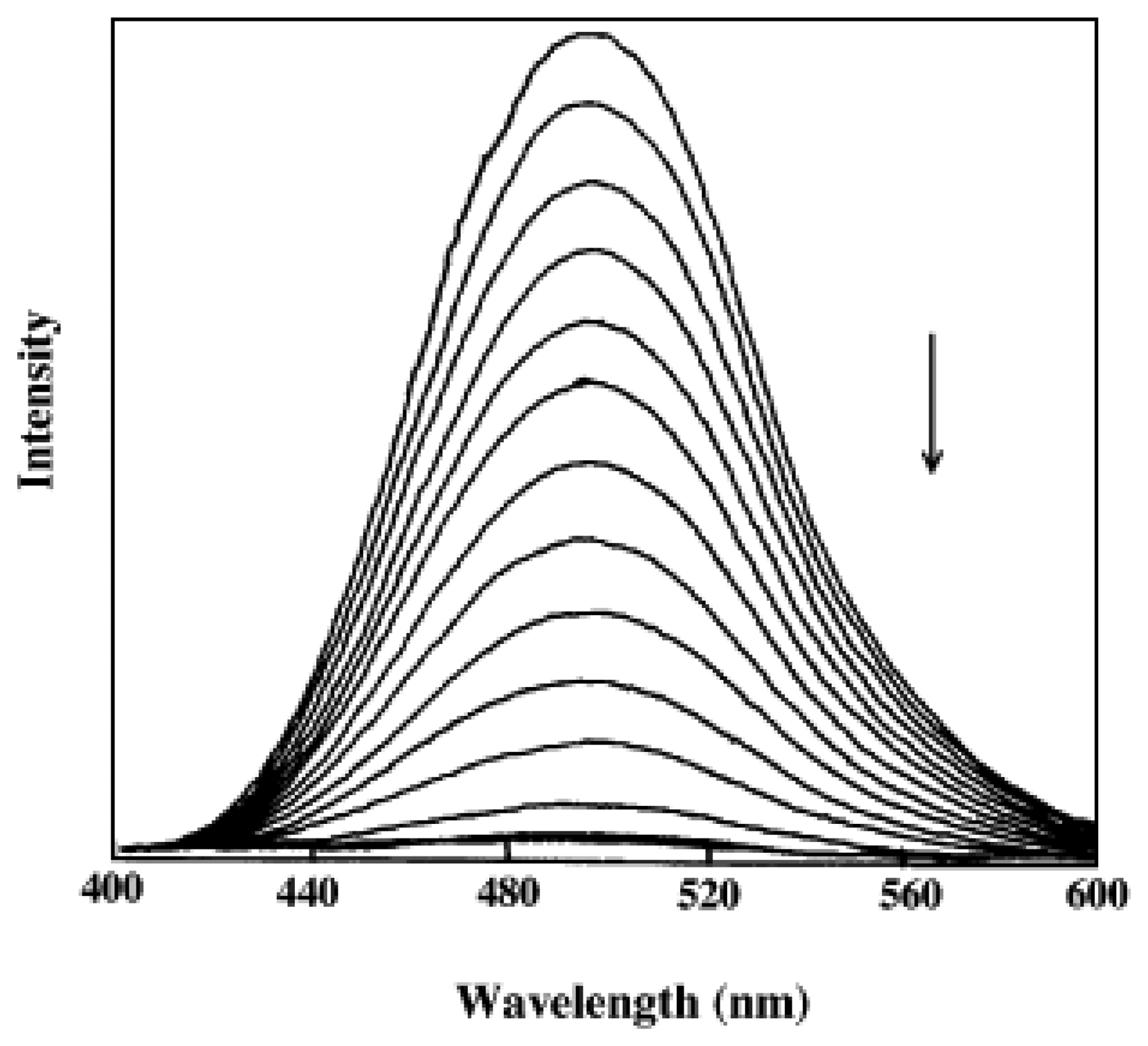
5.2.2. Fluorescence study
5.2.3. NMR study
5.3. Complexation study by electrochemical methods
5.3.1. Polarographic study
- Method 1.
- In the case of the reversible amalgam-forming reduction of labile complexes of relatively high stability, the shift in half-wave (or peak) potential to more negative values upon addition of an excess amount of ligand was found to be in accordance with the Lingane equation:where (E1/2)M and (E1/2)C are the half-wave potentials of the free and complexed metal ion, n is the number of electrons transferred, Kf is the complex formation constant, [L]t is the analytical concentration of the ligand and p is the stoichiometry of the complex. The values of p and log Kf can be obtained from the slopes and intercepts of the linear plots of ΔE1/2/(RT/nF) vs. log [L]t, respectively.
- Method 2.
- Measurement of the half-wave potential of the anodic polarographic signals resulting from the oxidation of mercury to its bivalent state in the presence of the ligands. The complex formation constants can be obtained from the following equation [64]:where E°cell is the formal potential of the Hg/Hg(II) half-cell vs. SCE.
- Method 3.
- Measurement of the positive shift in the reversible cathodic half-wave potential of Hg2+ complexes (as indicator) with increasing concentration of another M2+ cation which can compete with Hg2+ for the ligand. The formation constants for M2+ complexes were obtained from the equation [65]:where (E1/2)HgML and (E1/2)HgL are the cathodic half-wave potentials of the Hg2+L/Hg system in the presence and absence of M2+ ion, respectively, and [M2+] is the analytical concentration of the M2+ ion.
5.3.2. Potentiometric study
5.4. Complexation study by theoretical calculation
6. Schiff's Bases as Supramolecular Sensing Materials in the Construction of Potentiometric Membrane Sensors
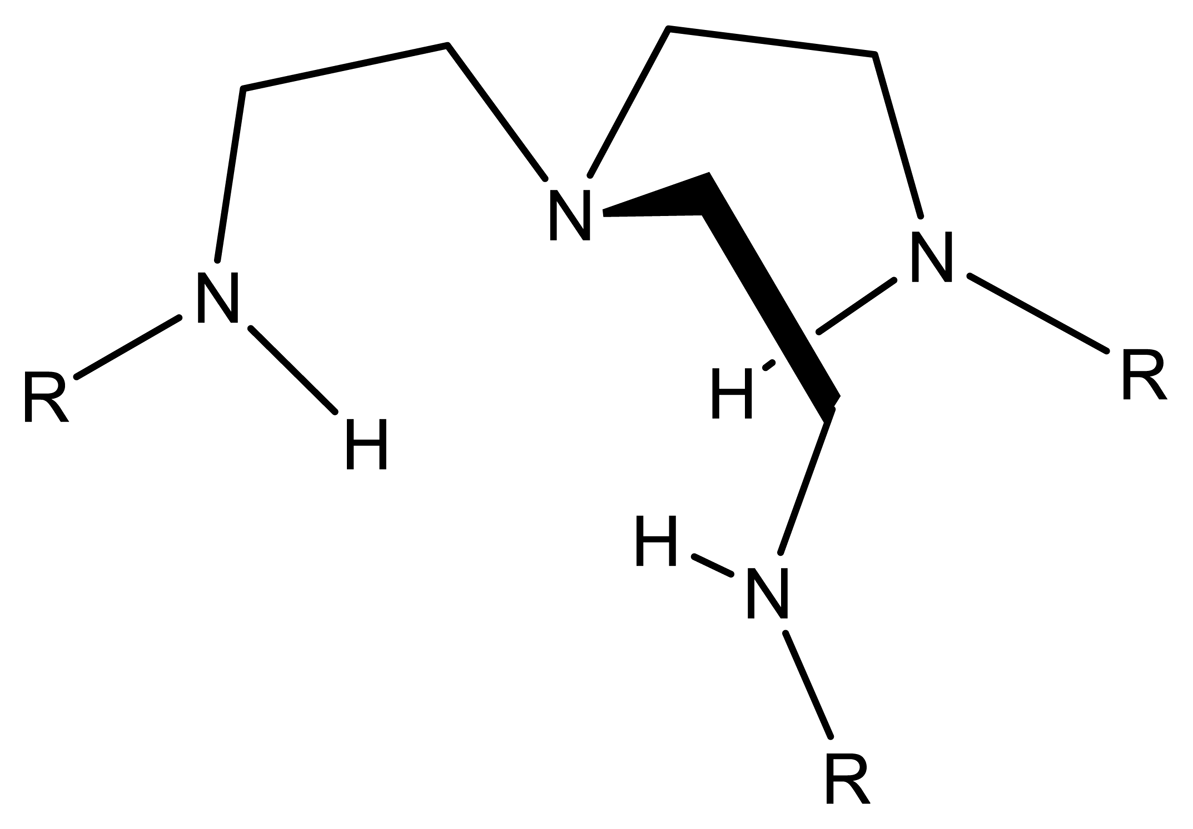
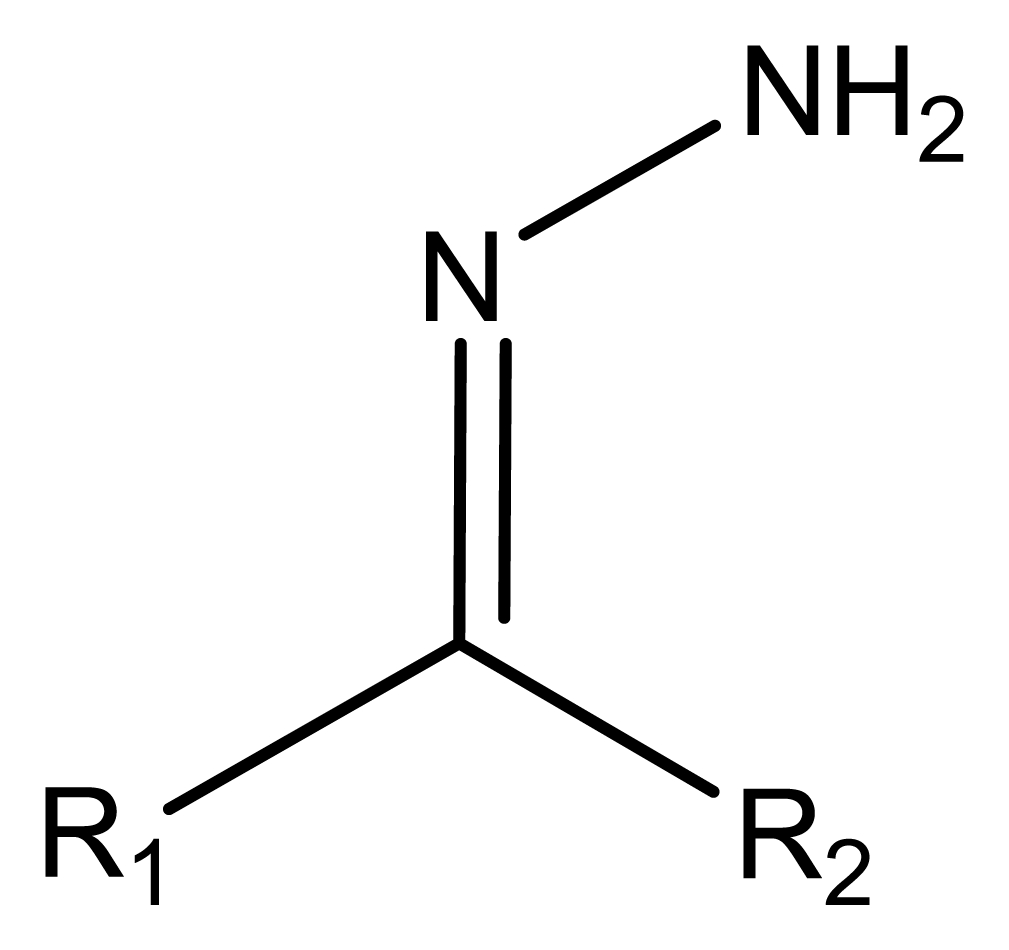
6.1. Structure of Schiff's bases
6.2. Application of Schiff's bases and their complexes
6.3. Different types of Schiff's bases
6.3.1. Schiff's bases and their complexes
6.3.2. Salens
6.3.3. Salophen
6.3.4. Hydrazones
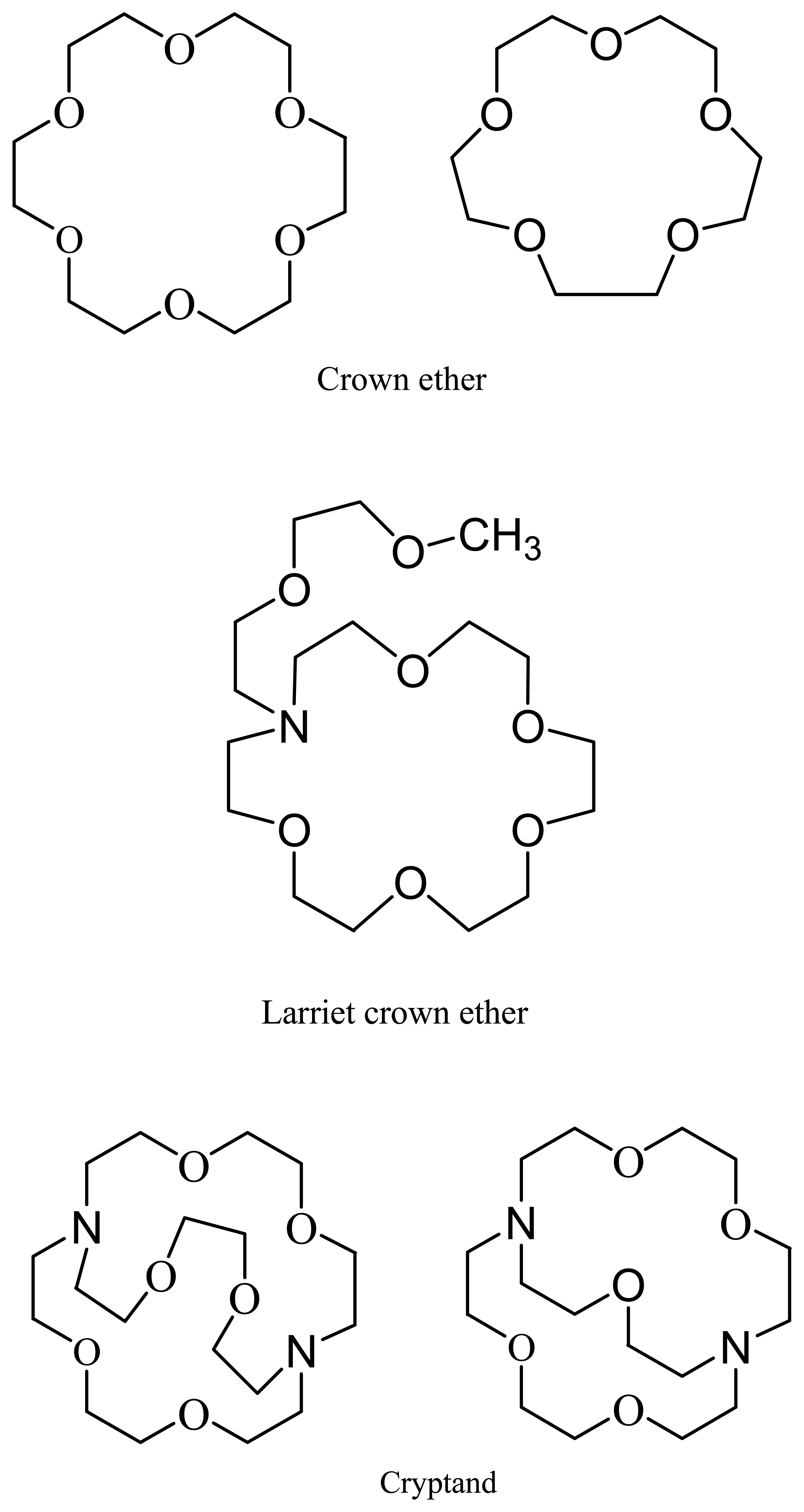
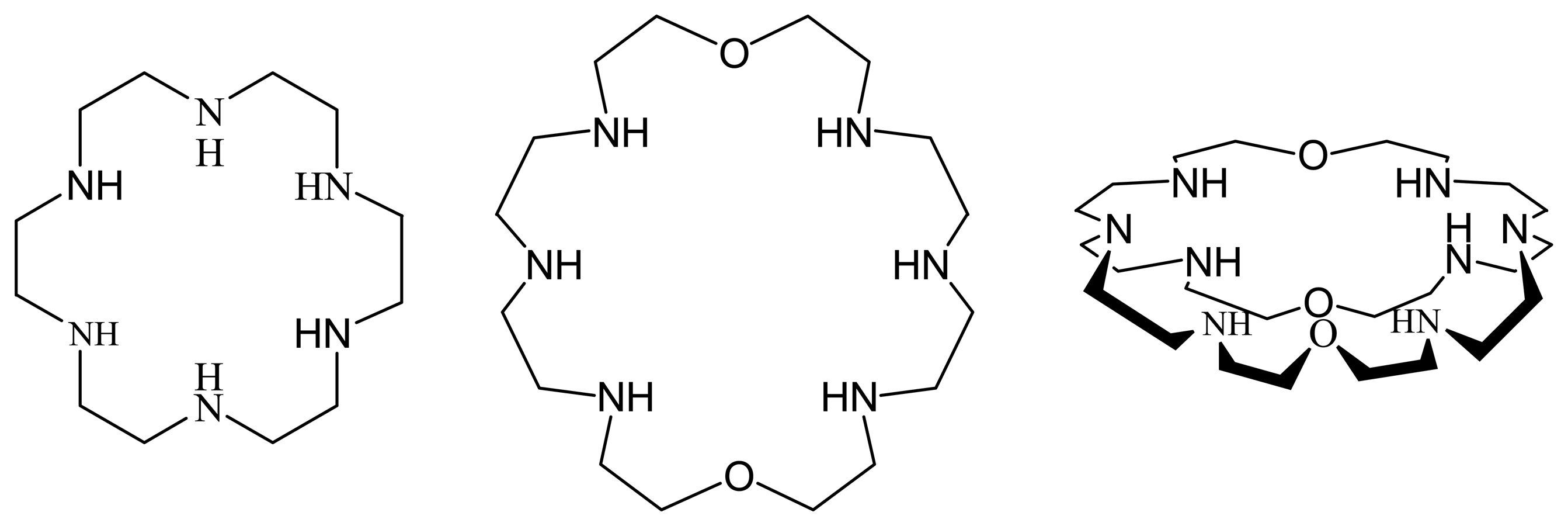
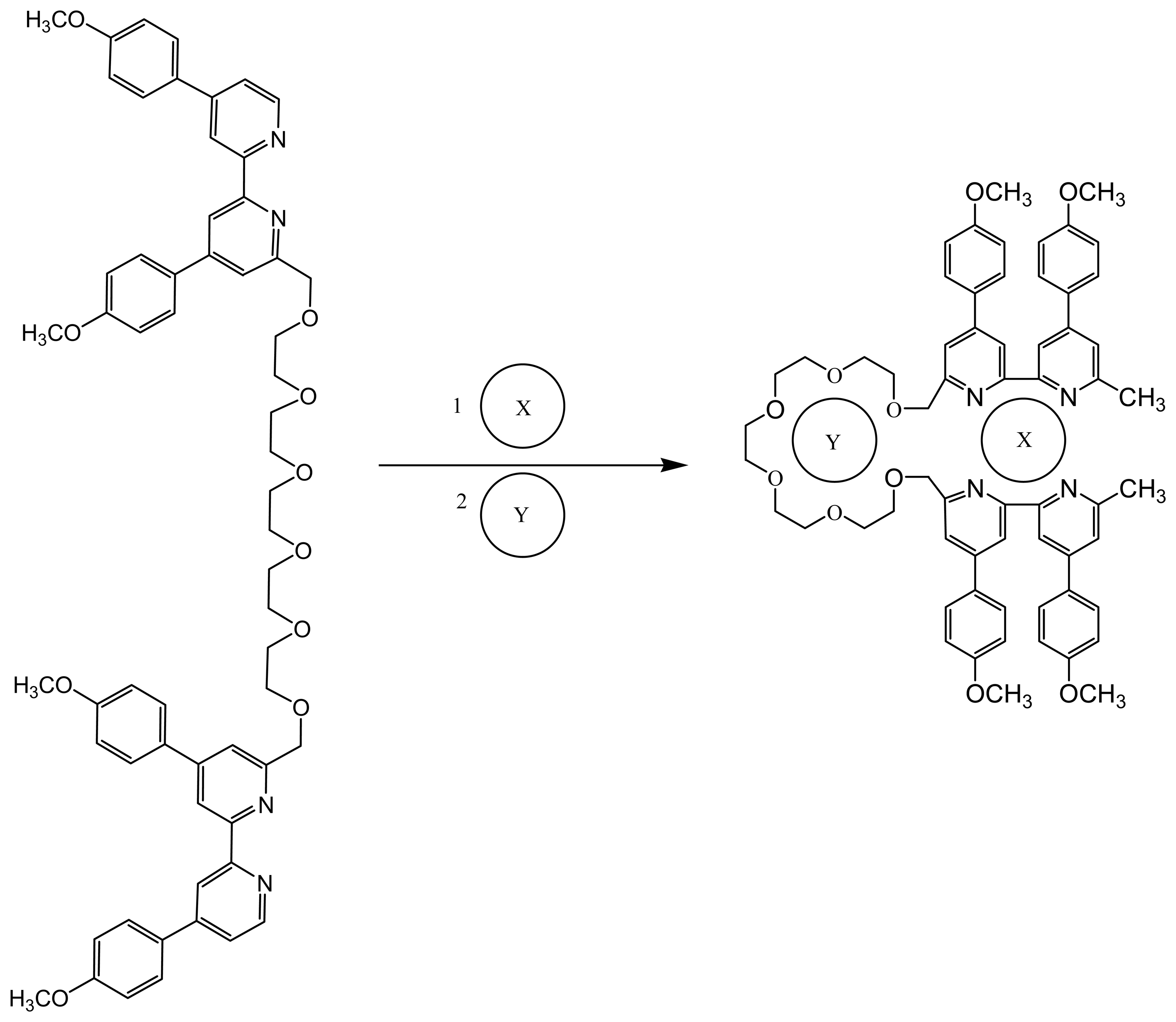
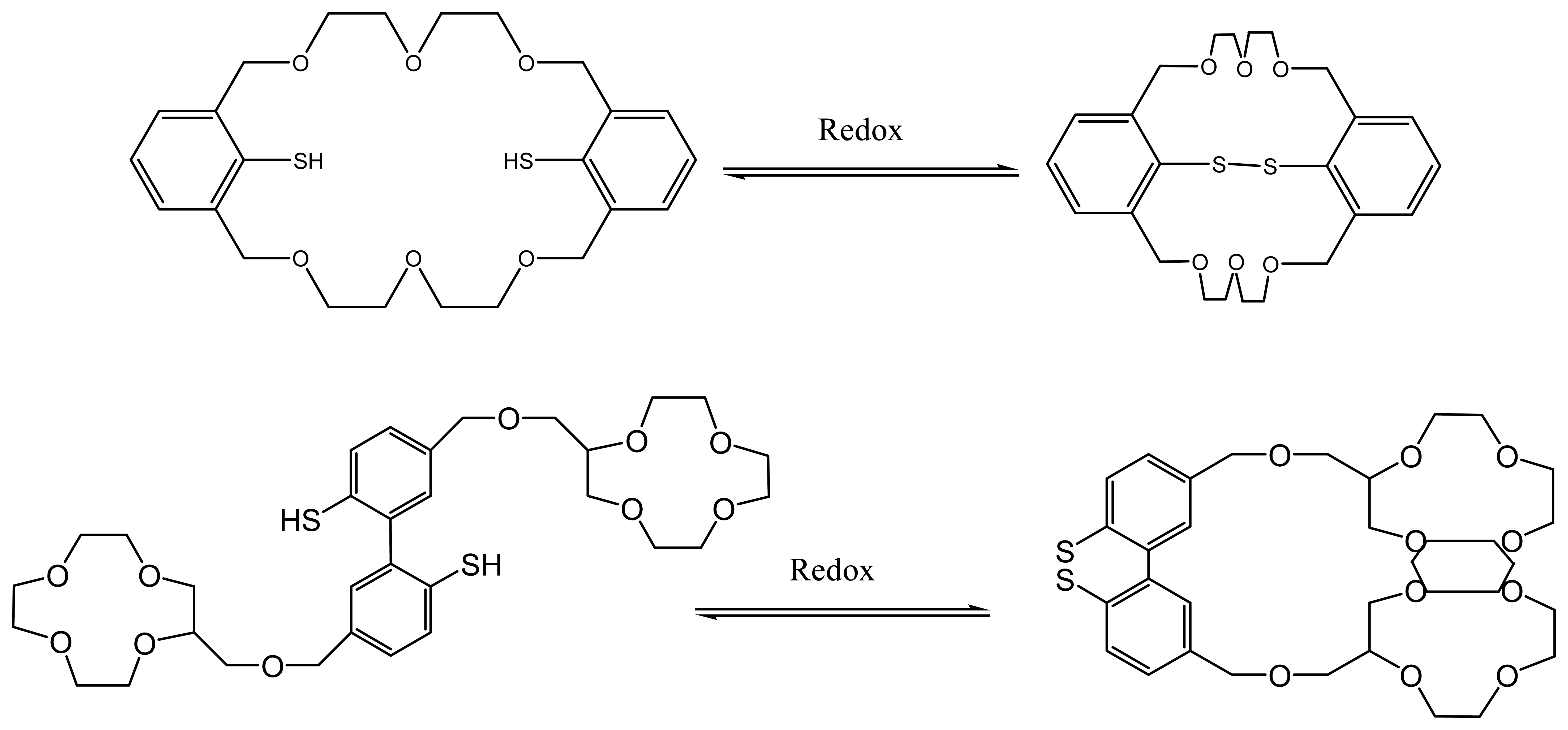
7. Crown Ethers as Supramolecular Sensing Materials in Construction of Membrane Sensors
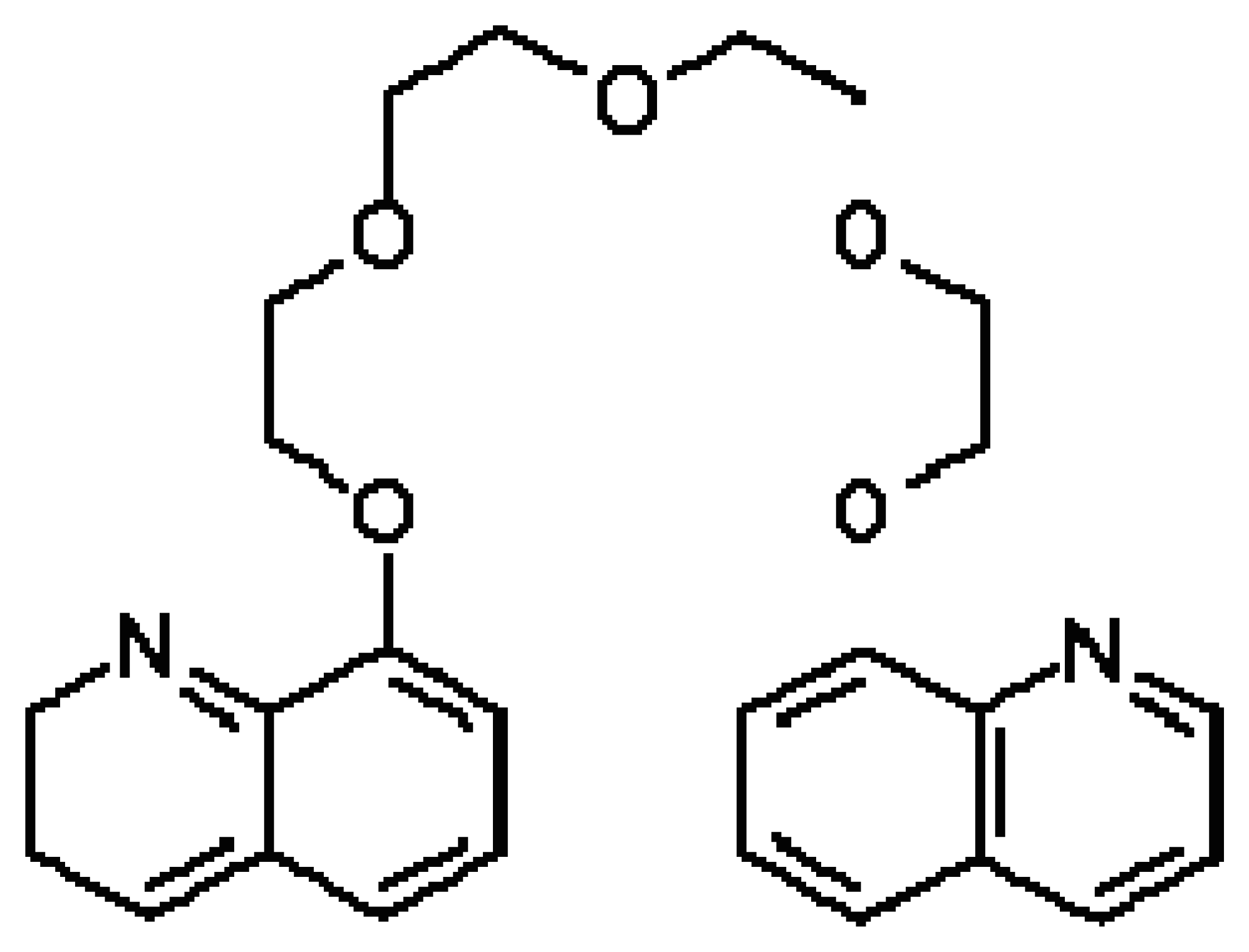
7.1. Classes of Crown Ethers
7.2. Controlling the recognition ability of a crown ethers
8. Conclusions
References
- Werner, A. Coordination Chemistry. Zeitschr. Anorg. Chem. 1893, 3, 267. [Google Scholar]
- Behr, J.-P. (Ed.) The Lock and Key Principle. The State of the Art–100 Years On; Wiley: Chichester, 1994.
- Katsuhiko, A.; Toyoki, K. Supramolecular Chemistry–Fundamentals and Applications Advanced Textbook; Springer-Verlag: Berlin Heidelberg, 2006. [Google Scholar]
- Ganjali, M. R.; Norouzi, P.; Rezapour, M. Encyclopedia of Sensors, Volume 8, Potentiometric ion selective sensors; American Scientific Publishers (ASP): California, 2006. [Google Scholar]
- Pick, J.; Tóth, K.; Vasàk, M.; Pungor, E.; Simon, W. Ion Selective Electrodes.; Pungor, E., Buzás, I., Eds.; Akadémi aiKiadó: Budapest, 1973. [Google Scholar]
- Fiedler, U.; Ruzicka, J. Selectrode. The universal ion selective electrode. Part VII. A valinomycin-based potassium electrode with nonporous polymer membrane and solid-state inner reference system. Anal. Chim. Acta 1973, 67, 179. [Google Scholar]
- Horvai, G.; Graf, E.; Tóth, K.; Pungor, E.; Buck, R.P. Plasticized poly(vinyl chloride) properties and characteristics of valinomycin electrodes. 2. Low-frequency, surface-rate, and Warburg impedance characteristics. Anal. Chem. 1986, 58, 2744. [Google Scholar]
- Van den Berg, A.; Van der Wal, P.D.; Skowronska-Ptasinska, M.; Sudhölter, E.J.R.; Reinhoudt, D.N. Nature of anionic sites in plasticized poly(vinyl chloride) membranes. Anal. Chem. 1987, 59, 2827. [Google Scholar]
- Lindner, E.; Gràf, E.; Nigreisz, Z.; Toth, K.; Pungor, E.; Buck, R.P. Responses of site-controlled, plasticized membrane electrodes. Anal. Chem. 1988, 60, 295. [Google Scholar]
- Bühlmann, P.; Yajima, S.; Tohda, k.; Umezawa, Y. EMF response of neutral-carrier based ion-sensitive field effect transistors with membranes free of ionic sites. Electrochim. Acta 1995, 40, 3021. [Google Scholar]
- Lindner, E.; Cosofret, V.V.; Ufer, S.; Buck, R.P.; Kao, W.J.; Neuman, M.R.; Anderson, J.M. Ion-selective membranes with low plasticizer content–electroanalytical characterization and biocompatibility studies. J. Biomed. Mater. Res. 1994, 28, 591–601. [Google Scholar]
- Wotring, V.J.; Prince, P.K.; Bachas, L.G. Evaluation of poly(vinylidene chloride) as a matrix for polymer membrane ion-selective electrodes. Analyst 1991, 116, 581–584. [Google Scholar]
- Reinhoudt, D.N.; Engbersen, J.F.J.; Bròzka, Z.; Vlekkert, H.H.; Honig, G.W.N.; Holterman, H.A.J.; Verkerk, U.H. Development of durable K+-selective chemically-modified field-effect transistors with functionalized polysiloxane membranes. Anal. Chem. 1994, 66, 3618–3623. [Google Scholar]
- Ganjali, M.R.; Mizani, F.; Salavati-Niasari, M.; Javanbakht, M. Novel potentiometric membrane sensor for the determination of trace amounts of chromium (III) ions. Anal. Sci. 2003, 19, 235–238. [Google Scholar]
- Born, M.Z. Volumes and heats of hydration of ions. Physic 1920, 1, 45–48. [Google Scholar]
- Lindner, E.; Tóth, K.; Pungor, E.; Behm, F.; Oggfnfuss, P.; Welti, D.H.; Ammann, D.; Morf, W.E.; Pretsch, E.; Simon, W. Lead-selective neutral carrier based liquid membrane electrode. Anal. Chem. 1984, 56, 1127. [Google Scholar]
- Morf, W.E.; Ammann, D.; Simon, W. Ion-selective electrodes based on neutral carriers. Chimia 1974, 28, 65–67. [Google Scholar]
- Ammann, D. Ion-Selective Microelectrodes; Springer-Verlag: Berlin, 1986. [Google Scholar]
- Ammann, D.; Pretsch, E.; Simon, W.; Lindner, E.; Bezegh, A.; Pungor, E. Lipophilic salts as membrane additives and their influence on the properties of macro- and micro-electrodes based on neutral carriers. Anal. Chim. Acta 1985, 171, 119. [Google Scholar]
- Mristers, M.; Vandeberg, J.T.; Cassaretto, F.P.; Posvic, H.; Moore, C.E. Studies in the tetraarylborates: Part V. The influence of substituents on the stability of tetraarylborates. Anal. Chim. Acta 1970, 49, 481. [Google Scholar]
- Nishida, H.; Takada, N.; Yoshimura, M.; Sonoda, T.; Kobayashi, H. Agent for Solvent-extraction of Cations. Bull. Chem. Soc. Jpn. 1984, 57, 2600. [Google Scholar]
- Bakker, E.; Bühlmann, P.; Pretsch, E. Carrier-based ion-selective electrodes and bulk optodes. 1. General characteristics. Chem. Rev. 1997, 97, 3083–3132. [Google Scholar]
- Schaller, U.; Bakker, E.; Spichiger, U.E.; Pretsch, E. Nitrite-selective microelectrodes. Talanta 1994, 41, 1001–1005. [Google Scholar]
- Vanysek, P. Electrochemistry on Liquid/Liquid Interfaces; Springer: Berlin, Heidelberg, NewYork, 1985. [Google Scholar]
- Hofmeister, F. On the understanding of the effects of salts, second report. On irregularities in the precipitating effect of salts and their relationship to their physical behavior. Arch. Exp. Pathol. Pharmakol 1888, 24, 247. [Google Scholar]
- Mi, Y.; Bakker, E. Determination of Complex Formation Constants of Lipophilic Neutral Ionophores in Solvent Polymeric Membranes with Segmented Sandwich Membranes. Anal. Chem. 1999, 71, 5279. [Google Scholar]
- Qin, Y.; Mi, Y.; Bakker, E. Determination of complex formation constants of 18 neutral alkali and alkaline earth metal ionophores in poly(vinyl chloride) sensing membranes plasticized with bis(2-ethylhexyl)sebacate and o-nitrophenyloctylether. Anal. Chim. Acta 2000, 421, 207–220. [Google Scholar]
- Morf, W.E. The Principles of Ion-Selective Electrodes and of Membrane Transport; Elsevier: New York, 1981. [Google Scholar]
- Lindner, E.; Tóth, K.; Pungor, E. Dynamic Characteristics of Ion Selective Electrodes.; CRC Press: Boca Raton, FL, 1988. [Google Scholar]
- Lehn, J.M. Cryptates: macropolycyclic inclusion complexes. Pure Appl. Chem. 1977, 49, 857. [Google Scholar]
- Lehn, J.M. Cryptates: inclusion complexes of macropolycyclic receptor molecules. Pure Appl. Chem. 1978, 50, 871. [Google Scholar]
- Poonia, N.S.; Bajaj, A.V. Coordination chemistry of alkali and alkaline earth cations. Chem. Rev. 1979, 79, 389–445. [Google Scholar]
- Izatt, R. Thermodynamic and kinetic data for cation-macrocycle interaction. Chem. Rev. 1985, 85, 271–339. [Google Scholar]
- Tawarah, K.M.; Mizyed, S.A. A conductance study of the association of alkali cations with 1,13-dibenzo-24-crown-8 in acetonitrile. J. Solut. Chem. 1989, 18, 387. [Google Scholar]
- Antonisse, M.M.G.; Reinhoudt, D.N. Potentiometric anion selective sensors. Electroanalysis 1999, 11, 1035–1048. [Google Scholar]
- Marcus, Y. Ion solvation.; Wiley: New York, 1985; p. 166. [Google Scholar]
- Antonisse, M.M.G.; Reinhoudt, D.N. Neutral anion receptors: design and application. Chem. Commun. 1998, 443–448. [Google Scholar]
- Schmidtchen, F.P.; Berger, M. Artificial organic host molecules for anions. Chem. Rev. 1997, 97, 1609–1646. [Google Scholar]
- Ganjali, M.R.; Norouzi, P.; Faridbod, F.; Rezapour, M.; Pourjavid, M.R. One Decade of Research on Ion-Selective Electrodes in Iran (1996-2006). J. Iran. Chem. Soc. 2007, 4, 1–29. [Google Scholar]
- Bühlmann, P.; Pretsch, E.; Bakker, E. Carrier-based ion-selective electrodes and bulk optodes. 2. Ionophores for potentiometric and optical sensors. Chem. Rev. 1998, 98, 1593–1698. [Google Scholar]
- Saeed, Shahrokhian; Ali, Souri. Metalophthalocyanine complexes as ion-carriers in membrane-selective electrodes for detection of thiosalicylic acid. Analytica Chimica Acta 2004, 518, 101–108. [Google Scholar]
- Zamani, H.A.; Rajabzadeh, Gh.; Ganjali, M.R.; Mola Khatami, S. Highly selective and sensitive copper(II) membranes sensors based on 6-methyl-4-(1-phenylmethylidene) amino-3- thioxo-1,2,4-triazin-5-one as a new neutral ionophores. Electroanalysis 2005, 17, 2260–2265. [Google Scholar]
- Debye, P.; Huckel, E. Theory of the Electrolyte. Phys. Z. 1928, 24, 305–325. [Google Scholar]
- Zamani, H.A.; Abedini-Torghabeh, J.; Ganjali, M.R. A highly selective and sensitive barium(II)-selective PVC membrane based on dimethyl 1-acetyl-8-oxo-2,8-dihydro-1hpyrazolo[5,1-a]isoindole-2,3-dicarboxylate. Electroanalysis 2006, 18, 888. [Google Scholar]
- Zamani, H.A.; Rajabzadeh, G.; Ganjali, M.R. Highly selective and sensitive chromium(III) membrane sensors based on 4-amino-3-hydrazino-6-methyl-1,2,4-triazin-5-one as a new neutral ionophores. Sens. Actuators. B 2006, 119, 41–46. [Google Scholar]
- Ganjali, M.R.; Rouhollahi, A.; Moghimi, A.; Shamsipur, M. Conductance study of alkali metal complexes with 4′-carboxy-benzo-24-crown-8 and 4′-amido-benzo-24-crown-8 in nitromethane, acetonitrile and dimethylformamide solutions. Pol. J. Chem. 1996, 70, 1172–1181. [Google Scholar]
- Ganjali, M.R.; Zargazi, M.H.; Mohajeri, A. Thermodynamic study of the interaction between some recently synthesized benzo-substituted macrocyclic diamides with some pyridinium ion derivatives in acetonitrile solution. Pol. J.Chem. 2001, 75, 743–749. [Google Scholar]
- Ganjali, M.R.; Rouhollahi, A.; Mardan, A.R.; Shamsipur, M. Thermodynamic study of the binding of hexathia-18-crown-6-tetraone with some transition and heavy metal ions in dimethyl sulfoxide solution. J. Chem. Soc. Faraday T 1998, 94, 1959–1962. [Google Scholar]
- Rouhollahi, A.; Ganjali, M.R.; Moghimi, A.; Buchanan, G.W.; Shamsipur, M. Synthesis of 4′-carboxybenzo-30-crown-10 and a thermodynamic study of its complexes with thallium and alkali cations in acetonitrile solution. J. Incl. Phenom. Macro. 1999, 33, 361–376. [Google Scholar]
- Pourghobadi, Z.; Seyyed-Majidi, F.; Daghighi-Asli, M.; Parsa, F.; Moghimi, A.; Ganjali, M.R.; Aghabozorg, H.; Shamsipur, M. Synthesis of a new triazine derived macrocycle and a thermodynamic study of its complexes with some transition and heavy metal ions in acetonitrile solution. Pol. J. Chem. 2000, 74, 837–846. [Google Scholar]
- Ganjali, M.R.; Khoshdan, N.; Hashemi, O.R.; Sajjadi, S.A.S. Thermodynamic study of some pyridinium ion derivatives with 18-crown-6, aza-18-crown-6 and 1,10-diaza-18-crown-6 in acetonitrile. Pol. J. Chem. 2000, 74, 1389–1398. [Google Scholar]
- Shamsipur, M.; Ganjali, M.R. Complex formation of some anilinium ion derivatives with 18-crown-6, 1,10-diaza-18-crown-6 and cryptand C222 in acetonitrile, dimethylformamide and their 1:1 mixture. J. Inclus. Phenom. Mol. 1997, 28, 315–323. [Google Scholar]
- Ganjali, M.R.; Akbara, V.; Ghorbania, M.; Norouzia, P.; Ahmadi, A. Fluoride determination in some mouth wash preparations by a novel La(III) graphite coated membrane sensor based on amitraz. Anal. Chim. Acta 2005, 531, 185–191. [Google Scholar]
- Ganjali, M.R.; Norouzi, P.; Hatambeygi, N.; Salavati-Niasari, M. Anion recognition: Fabrication of a highly selective and sensitive HPO4 2-PVC sensor based on a oxo-molybdenum methyl-salen. J. Braz. Chem. Soc. 2006, 17, 859–865. [Google Scholar]
- Ganjali, M.R.; Naji, L.; Poursaberi, T.; Taghizadeh, M.; Pirelahi, H.; Yousefi, M.; Yeganeh-Faal, A.; Shamsipur, M. Novel sulfate ion-selective polymeric membrane electrode based on a derivative of pyrilium perchlorate. Talanta 2002, 58, 359–366. [Google Scholar]
- Jabbari, A.; Shamsipur, M. Spectroscopic study of some alkali and alkaline earth complexes with ben20 crown ethers in ethanol solution. Spectrosc. Lett. 1993, 26, 1715–1724. [Google Scholar]
- Ganjali, M.R.; Naji, L.; Poursaberi, T.; Taghizadeh, M.; Pirelahi, H.; Yousefi, M.; Yeganeh-Faal, A.; Shamsipur, M. Novel sulfate ion-selective polymeric membrane electrode based on a derivative of pyrilium perchlorate. Talanta 2002, 58, 359–366. [Google Scholar]
- de Silva, A.P.; Sandanayake, K.R.A.S. J. Chem. Soc. Chem. Commun. 1989, 1183.
- Ji, H.F.; Dabestani, R.; Brown, G.M.; Sachleben, R.A. A new highly selective calix[4]crown-6 fluorescent caesium probe. Chem. Commun. 2000, 833–834. [Google Scholar]
- Ji, H.F.; Dabestani, R.; Brown, G.M.; Hettich, R.L. Spacer length effect on the photoinduced electron transfer fluorescent probe for alkali metal ions. Photochem. Photobiol. 1999, 69, 513–516. [Google Scholar]
- Karkhaneei, E.; Zebarjadian, M.H.; Shamsipur, M. 23Na NMR Studies of Stoichiometry and Stability of Sodium Ion Complexes with Several Crown Ethers in Binary Acetonitrile– Dimethylformamide Mixtures. J. Incl. Phenom. Macro. 2006, 54, 309–313. [Google Scholar]
- Kolthoff, I.M.; Lingane, J.J. Polarography, 2nd ed.; Interscience: New York, 1952. [Google Scholar]
- Parham, H.; Shamsipur, M. Polarographic study of the interaction between heavy metal ions and some macrocyclic ligands in binary acetonitrile + water mixtures. J. Electroanal. Chem. 1991, 314, 71–80. [Google Scholar]
- Crow, D.R. Polarography of Metal Complexes; Academic Press: New York, 1969. [Google Scholar]
- Hojo, M.; Imai, Y. Bull. Chem. Soc. Jpn. 1983, 56, 1963.
- Shamsipur, M.; Zolgharnein, J. Competitive Potentiometric Study of the Thermodynamics of Complexation of Some Transition and Heavy Metal Ions with Dibenzopyridino-18-crown-6 in Methanol Using Ag+ Ion as a Probe. J. Incl. Phenom. Macro. 2001, 40, 41–44. [Google Scholar]
- Egget, D. J. Computational Methods for the Determination of Formation Constants; Plenum Press: New York, 1985. [Google Scholar]
- Møller, C.; Plesset, M.S. Note on an Approximation Treatment for Many-Electron Systems. Phys. Rev. 1934, 46, 618–622. [Google Scholar]
- Head-Gordon, M.; Pople, J.A.; Frisch, M. MP2 Energy Evaluation of Direct Methods. J Chem. Phys. Lett. 1988, 153, 503. [Google Scholar]
- Sponer, J.; Berger, I.; S¡pac¡kova, N.; Leszczynski, J.; Hobza, P. Aromatic base stacking in DNA: From ab initio calculations to molecular dynamics simulations. J. Biolmol. Struct. Dyn. 2000, 11, 383–407. [Google Scholar]
- Chalasinski, G.; Szczesniak, M.M. Origins of structure and energetics of van-der-waals clusters from ab-initio calculations. Chem. Rev. 1994, 94, 1723–1765. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Zakrzewski, V.G.; Montgomery, J.A.J.; Stratmann, R. E.; Burant, J.C.; Dapprich, S.; Millam, J.M.; Daniels, A.D.; Kudin, K.N.; Strain, M.C.; Farkas, O.; Tomasi, J.; Barone, V.; Cossi, M.; Cammi, R.; Mennucci, B.; Pomelli, C.; Adamo, C.; Clifford, S.; Ochterski, J.; Petersson, G.A.; Ayala, P.Y.; Cui, Q.; Morokuma, K.; Malick, D.K.; Rabuck, A.D.; Raghavachari, K.; Foresman, J.B.; Cioslowski, J.; Ortiz, J.V.; Baboul, A.G.; Stefanov, B.B.; Liu, G.; Liashenko, A.; Piskorz, P.; Komaromi, I.; Gomperts, R.; Martin, R.L.; Fox, D.J.; Keith, T.; AlLaham, M.A.; Peng, C.Y.; Nanayakkara, A.; Gonzalez, C.; Challacombe, M.; Gill, P.M.W.; Johnson, B.; Chen, W.; Wong, M.W.; Andres, J.L.; Gonzalez, C.; Head-Gordon, M.; Replogle, E.S.; Pople, J.A. Gaussian 03, Revision A.7; Gaussian Inc: Pittsburgh, PA, 2003. [Google Scholar]
- Ganjali, M.R.; Norouzi, P.; Rezapour, M.; Faridbod, F.; Pourjavid, M.R. Supramolecular Based Membrane Sensors. Sensors 2006, 6, 1018–1086. [Google Scholar]
- Cragg, P.J. A Practical Guide to Supramolecular Chemistry.; John Wiley & Sons: Chichester, 2005. [Google Scholar]
- Faridbod, F.; Ganjali, M.R.; Dinarvand, R.; Norouzi, P. Ion recognition: Application of symmetric and asymmetric Schiff bases and their complexes for the fabrication of cationic and anionic membrane sensors to determine ions in real samples. Comb. Chem. High T. Scr. 2007, 10, 527–546. [Google Scholar]
- Gupta, V.K.; Singh, A. K.; Gupta, B. A cerium(III) selective polyvinyl chloride membrane sensor based on a Schiff base complex of N,N′-bis[2-(salicylideneamino)ethyl]ethane-1,2-diamine. Anal. Chim. Acta 2006, 575, 198–204. [Google Scholar]
- Singh, A.K.; Gupta, V.K.; Gupta, B. Chromium(III) selective membrane sensors based on Schiff bases as chelating ionophores. Anal. Chim. Acta 2007, 585, 171–178. [Google Scholar]
- Cozzi, P.G. Metal-Salen Schiff base complexes in catalysis: practical aspects. Chem. Soc. Rev. 2004, 33, 410–421. [Google Scholar]
- Shemirani, F.; Mirroshandel, A.A.; Salavati-Niasari, M.; Kozani, R.R. Silica gel coated with Schiff's base: Synthesis and application as an adsorbent for cadmium, copper, zinc, and nickel determination after preconcentration by flame atomic absorption spectrometry. J. Anal. Chem. 2004, 59, 228–233. [Google Scholar]
- Alizadeh, N.; Ershad, S.; Naeimi, H.; Sharghi, H.; Shamsipur, M. Synthesis of a new naphthol-derivative salen and spectrophotometric study of the thermodynamics and kinetics of its complexation with copper(II) ion in binary dimethylsulfoxide-acetonitrile mixtures. Pol. J. Chem. 1999, 73, 915–925. [Google Scholar]
- Shamsipur, M.; Yousefi, M.; Hoseini, M.; Ganjali, M. R.; Sharghi, H.; Naeimi, H. A schiff base complex of Zn(II) as a neutral carrier for highly selective PVC membrane sensors for the sulfate ion. Anal. Chem. 2001, 73, 2869–2874. [Google Scholar]
- Abbaspour, A.; Esmaeilbeig, A.R.; Jarrahpour, A.A.; Khajeh, B.; Kia, R. Aluminium(III)-selective electrode based on a newly synthesized tetradentate Schiff base. Talanta 2002, 58, 397–403. [Google Scholar]
- Arvand, M.; Moghimi, A.M.; Afshari, A.; Mahmoodi, N. Potentiometric membrane sensor based on 6-(4-nitrophenyl)-2,4-diphenyl-3,5-diaza-bicyclo[3.1.0] hex-2-ene for detection of Sn(II) in real samples. Anal. Chim. Acta 2006, 579, 102–108. [Google Scholar]
- Arida, H.A.; El-Reefy, S.A.; El-Saied, A.M. A new lead (II)-selective PVC-coated graphite rod electrode based on a Schiff base complex. Anal. Sci. 2003, 19, 687–690. [Google Scholar]
- Ardakany, M.M.; Ensafi, A.A.; Naeimi, H.; Dastanpour, A.; Shamlli, A. Highly selective lead(II) coated-wire electrode based on a new Schiff base. Sens. Actuators. B 2003, 96, 441–445. [Google Scholar]
- Ardakany, M.M.; Ensafi, A.A.; Naeimi, H.; Dastanpour, A.; Shamlli, A. Coated, wire-based, new Schiff base potentiometric sensor for lead(II) ion. Russ. J. Electrochem. 2003, 39, 269–273. [Google Scholar]
- Ardakany, M.M.; Pourhakak, P.; Salavati-Niasari, M. Bis(2-hydroxyacetophenone)ethylenediimine as a neutral carrier in a coated-wire membrane electrode for lead(II). Anal. Sci. 2006, 22, 865–870. [Google Scholar]
- Jeong, T.; Jeong, D.C.; Lee, H.K.; Jeon, S. Lead(II)-selective polymeric electrode using a Schiff base complex of N,N′-bis-thiophene-2-ylmethylene-ethane-1,2-diamine as an ion carrier. Bull. Korean Chem. Soc. 2005, 26, 1219–1224. [Google Scholar]
- Jeong, T.; Lee, H.K.; Jeong, D.C.; Jeon, S. A lead (II)-selective PVC membrane based on a Schiff base complex of N,N′-bis(salicylidene)-2,6-pyridinediamine. Talanta 2005, 65, 543–548. [Google Scholar]
- Gupta, V.K.; Jain, A.K.; Kumar, P. PVC-based membranes of N,N′ -dibenzyl-1,4,10,13-tetraoxa-7,16-diazacyclooctadecane as Pb(II)-selective sensor. Sens. Actuators B 2006, 120, 259–265. [Google Scholar]
- Ganjali, M.R.; Daftari, A.; Norouzi, P.; Salavati-Niasari, M. Novel Y (III) PVC based membrane microelectrode based on a new S-N Schiff's base. Anal. Lett. 2003, 36, 1511–1522. [Google Scholar]
- Ganjali, M.R.; Kiani-Anbouhi, R.; Shamsipur, M.; Poursaberi, T.; Salavati-Niasari, M.; Talebpour, Z.; Emami, M. Novel potentiometric PVC-membrane and coated graphite sensors for lanthanum(III). Electroanalysis 2004, 16, 1002–1008. [Google Scholar]
- Ganjali, M.R.; Qomi, M.; Daftari, A.; Norouzi, P.; Salavati-Niasari, M.; Rabbani, M. Novel lanthanum(III) membrane sensor based on a new N-S Schiff's base. Sens. Actuators B 2004, 98, 92. [Google Scholar]
- Gupta, V.K.; Singh, A.K.; Gupta, B. A cerium(III) selective polyvinyl chloride membrane sensor based on a Schiff base complex of N,N′-bis[2-(salicylideneamino)ethyl]ethane-1,2-diamine. Anal. Chim. Acta 2006, 575, 198–204. [Google Scholar]
- Ganjali, M.R.; Emami, M.; Rezapour, M.; Shamsipur, M.; Maddah, B.; Salavati-Niasari, M.; Hosseini, M.; Talebpoui, Z. Novel gadolinium poly(vinyl chloride) membrane sensor based on a new S-N Schiff's base. Anal. Chim. Acta 2003, 495, 51–59. [Google Scholar]
- Ganjali, M.R.; Rezapour, M.; Norouzi, P.; Salavati-Niasari, M. A new pentadentate S-N Schiffs' base as a novel lonophore in construction of a novel Gd(III) membrane sensor. Electroanalysis 2005, 17, 2032–2036. [Google Scholar]
- Ganjali, M.R.; Ghesmi, A.; Hosseini, M.; Pourjavid, M.R.; Rezapour, M.; Shamsipur, M.; Salavati-Niasari, M. Novel terbium(III) sensor based on a new bis-pyrrolidene Schiff's base. Sens. Actuators B 2005, 105, 334–339. [Google Scholar]
- Ganjali, M.R.; Ravanshad, J.; Hosseini, M.; Salavati-Niasari, M.; Pourjavid, M. R.; Baezzat, M.R. Novel Dy(III) sensor based on a new bis-pyrrolidene Schiff's base. Electroanalysis 2004, 16, 1771–1776. [Google Scholar]
- Ganjali, M.R.; Dodangeh, M.; Ghorbani, H.; Norouzi, P.; Adib, M. PPb level monitoring of Dy(III) ions by a highly sensitive and selective Dy(III) sensor based on a new asymmetrical Schiff's base. Anal. Lett. 2006, 39, 495–506. [Google Scholar]
- Ganjali, M.R.; Norouzi, P.; Adib, M.; Ahmadalinezhad, A. A novel Holmium(III) membrane sensor based on N-(1-thien-2-ylmethylene)-1,3-benzothiazol-2-amine. Anal. Lett. 2006, 39, 1075–1086. [Google Scholar]
- Ganjali, M. R.; Tamaddon, A.; Norouzi, P.; Adib, M. Novel Lu(III) membrane sensor based on a new asymmetrically S-N Schiff's base. Sens. Actuators B 2006, 120, 194–199. [Google Scholar]
- Shamsipur, M.; Saeidi, M.; Yari, A.; Yaganeh-Faal, A.; Mashhadizadeh, M. H.; Azimi, G.; Naeimi, H.; Sharghi, H. UO22+ ion-selective membrane electrode based on a naphthol-derivative Schiff's base 2,2′- [1,2-ethandiyl bis(nitriloethylidene)]bis(1-naphthalene). Bull. Korean Chem. Soc. 2004, 25, 629–633. [Google Scholar]
- Ganjali, M.R.; Emami, M.; Salavati-Niasari, M.; Yousefi, M. Determination of trace amounts of Cr(III) in presence of Cr(VI) by a novel potentiometric membrane sensor based on a new tridentate S,N,O Schiff's base. Anal. Lett. 2003, 36, 2735–2747. [Google Scholar]
- Ganjali, M.R.; Mizani, F.; Salavati-Niasari, M.; Javanbakht, M. Novel potentiometric membrane sensor for the determination of trace amounts of chromium(III) ions. Anal. Sci. 2003, 19, 235–238. [Google Scholar]
- Shamsipur, M.; Soleymanpour, A.; Akhond, M.; Sharghi, H.; Sarvari, M.H. Highly selective chromium(III) PVC-membrane electrodes based on some recently synthesized Schiff's bases. Electroanalysis 2005, 17, 776–782. [Google Scholar]
- Ganjali, M.R.; Norouzi, P.; Faridbod, F.; Ghorbani, M.; Adib, M. Highly selective and sensitive chromium(III) membrane sensors based on a new tridentate Schiff's base. Anal. Chim. Acta 2006, 569, 35–41. [Google Scholar]
- Singh, A.K.; Gupta, V.K.; Gupta, B. Chromium(III) selective membrane sensors based on Schiff bases as chelating ionophores. Anal. Chim. Acta 2007, 585, 171–178. [Google Scholar]
- Gupta, V.K.; Jain, A. K.; Maheshwari, G. Manganese (II) selective PVC based membrane sensor using a Schiff base. Talanta 2007, 72, 49–53. [Google Scholar]
- Mashhadizadeh, M.H.; Shoaei, I. S.; Monadi, N. A novel ion selective membrane potentiometric sensor for direct determination of Fe(III) in the presence of Fe(II). Talanta 2004, 64, 1048–1052. [Google Scholar]
- Mashhadizadeh, M.H.; Sheikhshoaie, I. Co2+-selective membrane electrode based on the Schiff base NADS. Anal. Bioanal. Chem. 2003, 375, 708–712. [Google Scholar]
- Kumar, K.G.; Poduval, R.; Augustine, P.; John, S.; Saraswathyamma, B. A PVC plasticized sensor for Ni (II) ion based on a simple ethylenediamine derivative. Anal. Sci. 2006, 22, 1333–1337. [Google Scholar]
- Mashhadizadeh, M.H.; Sheikhshoaie, I.; Saeid-Nia, S. Nickel(II)-selective membrane potentiometric sensor using a recently synthesized Schiff base as neutral carrier. Sens. Actuators B 2003, 94, 241–246. [Google Scholar]
- Jain, A.K.; Gupta, V.K.; Ganeshpure, P.A.; Raisoni, J.R. Ni(II)-selective ion sensors of salen type Schiff base chelates. Anal. Chim. Acta 2005, 553, 177–184. [Google Scholar]
- Kumar, K.G.; Poduval, R.; John, S.; Augustine, P. A PVC plasticized membrane sensor for nickel ions. Mikrochim. Acta 2006, 156, 283–287. [Google Scholar]
- Pleniceanu, M.; Isvoranu, M.; Spinu, C. Liquid membrane ion-selective electrodes for potentiometric dosage of coper and nickel. J. Serb. Chem. Soc. 2005, 70, 269–276. [Google Scholar]
- Pleniceanu, M.; Isvoranu, M.; Spinu, C. Electroanalytical applications of some mixed complex combinations with Schiff base. Asian J. Chem. 2005, 17, 2129–2136. [Google Scholar]
- Pleniceanu, M.; Isvoranu, M.; Spinu, C. New electrochemical sensors used for potentiometric determination of copper and nickel. J. Indian Chem. Soc. 2002, 79, 884–886. [Google Scholar]
- Alizadeh, N.; Ershad, S.; Naeimi, H.; Sharghi, H.; Shamsipur, M. Copper (II)-selective membrane electrode based on a recently synthesized naphthol-derivative Schiff's base. Fresenius J. Anal. Chem. 1999, 365, 511–515. [Google Scholar]
- Ganjali, M.R.; Poursaberi, T.; Babaei, L.H.A.; Rouhani, S.; Yousefi, M.; Kargar-Razi, M.; Moghimi, A.; Aghabozorg, H.; Shamsipur, M. Highly selective and sensitive copper(II) membrane coated graphite electrode based on a recently synthesized Schiff's base. Anal. Chim. Acta 2001, 440, 81–87. [Google Scholar]
- Poursaberi, T.; Hajiagha-Babaei, L.; Yousefi, M.; Rouhani, S.; Shamsipur, M.; Kargar-Razi, M.; Moghimi, A.; Aghabozorg, H.; Ganjali, M.R. The synthesis of a new thiophene-derivative Schiff's base and its use in preparation of copper-ion selective electrodes. Electroanalysis 2001, 13, 1513–1517. [Google Scholar]
- Ganjali, M.R.; Emami, M.; Salavati-Niasari, M. Novel copper(II)-selective sensor based on a new hexadentates Schiff's base. Bull. Korean Chem. Soc. 2002, 23, 1394–1398. [Google Scholar]
- Sadeghi, S.; Eslahi, M.; Naseri, M. A.; Naeimi, H.; Sharghi, H.; Shameli, A. Copper ion selective membrane electrodes based on some Schiff base derivatives. Electroanalysis 2003, 15, 1327–1333. [Google Scholar]
- Fakhari, A.R.; Raji, T.A.; Naeimi, H. Copper-selective PVC membrane electrodes based on salens as carriers. Sens. Actuators B 2005, 104, 317–323. [Google Scholar]
- Jeong, D.C.; Lee, H.K.; Jeon, S. Highly copper(II)-selective PVC membrane based on a Schiff base complex of N,N′ -bis-pyridin-2-ylmethylene-naphthalene-1,8-diamine as an ionophore. Bull. Korean Chem. Soc. 2006, 27, 1593–1596. [Google Scholar]
- Xie, N.; Chen, Y. Design and synthesis of a selective chemosensor for Zn2+. Chin. J. Chem. 2006, 24, 1800–1803. [Google Scholar]
- Gholivand, M.B.; Rahimi-Nasrabadi, M.; Ganjali, M.R.; Salavati-Niasari, M. Highly selective and sensitive copper membrane electrode based on a new synthesized Schiff base. Talanta 2007, 73, 553–560. [Google Scholar]
- Ganjali, M.R.; Golmohammadi, M.; Yousefi, M.; Norouzi, P.; Salavati-Niasari, M.; Javanbakht, M. Novel PVC-based copper(II) membrane sensor based on 2-(1′-(4′-(1′-hydroxy-2″-naphthyl)-methyleneamino)butyl iminomethyl)-1-naphthol. Anal. Sci. 2003, 19, 223–227. [Google Scholar]
- Singh, L.P.; Bhatnagar, J.M. Copper (II) selective electrochemical sensor based on Schiff Base complexes. Talanta 2004, 64, 313–319. [Google Scholar]
- Norouzi, P.; Ganjali, M.R.; Faridbod, F.; Salavati-Niasari, M. Determination of copper in black, red pepper and the waste water samples by a highly selective sensitive Cu(II) microelectrode based on a new hexadentates Schiff,s base. Bull. Korean Chem. Soc. 2006, 27, 1439. [Google Scholar]
- Gupta, K.C.; D'Arc, M.J. Performance evaluation of copper ion selective electrode based on cyanocopolymers. Sens. Actuators B 2000, 62, 171–176. [Google Scholar]
- Alizadeh, N.; Ershad, S.; Naeimi, H.; Sharghi, H.; Shamsipur, M. Copper(II)-selective membrane electrode based on a recently synthesized naphthol-derivative Schiff's base. Fresenius J. Anal. Chem. 1999, 365, 511–515. [Google Scholar]
- Gupta, V.K.; Agarwal, S.; Jakob, A.; Lang, H. A zinc-selective electrode based on N,N′ -bis(acetylacetone)ethylenediimine. Sens. Actuators B 2006, 114, 812–818. [Google Scholar]
- Mahajan, R.K.; Kumar, M.; Sharma, V.; Kaur, I. Silver (I) ion-selective membrane based on Schiff base-p-tert-butylcalix[4]sqb;arene. Analyst 2001, 126, 505–507. [Google Scholar]
- Ueda, M.; Sakaki, N.; Moriuchi-Kawakami, T.; Shibutani, Y. Potentiometric performance of a dioxime-type Schiff base as a silver-ion recognizable material. Bunseki Kagaku 2001, 50, 619–625. [Google Scholar]
- Mahajan, R.K.; Kaur, I.; Sharma, V.; Kumar, M. Sensor for silver (I) ion based on Schiff-base-p-tertbutylcalix[4] arene. Sensors 2002, 2, 417–423. [Google Scholar]
- Demirel, A.; Dogan, A.; Akkus, G.; Yilmaz, M.; Kilic, E. Silver(I)-selective PVC membrane potentiometric sensor based on a recently synthesized calix[4]arene. Electroanalysis 2006, 18, 1019–1027. [Google Scholar]
- Mahajan, R.K.; Kaur, I.; Kumar, M. Silver ion-selective electrodes employing Schiff base p-tertbutyl calix[4]arene derivatives as neutral carriers. Sens. Actuators B 2003, 91, 26–31. [Google Scholar]
- Mashhadizadeh, M.H.; Mostafavi, A.; Allah-Abadi, H.; Sheikhshoai, I. New Schiff base modified carbon paste and coated wire PVC membrane electrode for silver ion. Sens. Actuators B 2006, 113, 930–936. [Google Scholar]
- Demirel, A.; Dogan, A.; Akkus, G.; Yilmaz, M.; Kilic, E. Silver(I)-selective PVC membrane potentiometric sensor based on a recently synthesized calix[4]arene. Electroanalysis 2006, 18, 1019–1027. [Google Scholar]
- Singh, A.K.; Saxena, P. Silver(I)-selective electrode based on [Bz(2)Oxo(4)(18)dieneS(4)] tetrathia macrocyclic carrier. Anal. Bioanal. Chem. 2006, 385, 90–95. [Google Scholar]
- Mashhadizadeh, M.H.; Sheikhshoaie, I.; Saeid-Nia, S. Asymmetrical Schiff bases as carriers in PVC membrane electrodes for cadmium (II) ions. Electroanalysis 2005, 17, 648–654. [Google Scholar]
- Gupta, V.K.; Singh, A.K.; Gupta, B. Schiff bases as cadmium(II) selective ionophores in polymeric membrane electrodes. Anal. Chim. Acta 2007, 583, 340–348. [Google Scholar]
- Gismera, M.J.; Hueso, D.; Procopio, J.R.; Sevilla, A.T. Ion-selective carbon paste electrode based on tetraethyl thiuram disulfide for copper(II) and mercury(II). Anal. Chim. Acta 2004, 524, 347–353. [Google Scholar]
- Xu, L.; Yuan, R.; Chai, Y.Q. Mercury(II) ion potentiometric sensor based on a sulfur Schiff's base 1-(2-hydroxy-1,2-diphenylethylidene)thiosemicarbazide as ionophore. Chem. Lett. 2005, 34, 440–441. [Google Scholar]
- Mashhadizadeh, M.H.; Sheikhshoaie, I. Mercury(II) ion-selective polymeric membrane sensor based on a recently synthesized Schiff base. Talanta 2003, 60, 73–80. [Google Scholar]
- Saleh, M.B.; Soliman, E.M.; Gaber, A.A.A.; Ahmed, S.A. A novel Hg(II) PVC membrane sensor based on simple ionophore ethylenediamine bis-thiophenecarboxaldehyde. Anal. Lett. 2006, 39, 659–673. [Google Scholar]
- Mashhadizadeh, M.H.; Talakesh, M.; Peste, M.; Momeni, A.; Hamidian, H.; Majum, M. A novel modified carbon paste electrode for potentiometric determination of mercury(II) ion. Electroanalysis 2006, 18, 2174–2179. [Google Scholar]
- Ganjali, M.R.; Norouzi, P.; Alizadeh, T.; Salavati-Niasari, M. Synthesis of a new hexadendates Schiff's base and its application in the fabrication of a highly selective mercury(II) sensor. Bull. Korean Chem. Soc. 2007, 28, 68–72. [Google Scholar]
- Dai, J.Y.; Chai, Y.Q.; Yuan, R.; An, L.X.; Liu, Y.; Zhong, X.; Tang, D.P. Tricoordinate Schiff base copper(II) complex as neutral carrier for highly selective thiocyanate electrode. Anal. Lett. 2005, 38, 389–400. [Google Scholar]
- Bühlmann, P.; Yahya, L.; Enderes, R. Ion-selective electrodes for thiocyanate based on the dinuclear Zinc(II) complex of a Bis-N,O-bidentate Schiff base. Electroanalysis 2004, 16, 973–978. [Google Scholar]
- Jyo, A.; Egawa, H. Effect of membrane matrices on performances of a thiocyanate ion-selective electrode based on the (5,10,15,20-tetraphenyl-porphyrinato)manganese(III) anion carrier. Anal. Sci. 1992, 8, 823–827. [Google Scholar]
- Ganjali, M.R.; Poursaberi, T.; Basiripour, F.; Salavati-Niasari, M.; Yousefi, M.; Shamsipur, M. Highly selective thiocyanate poly(vinyl chloride) membrane electrode based on a cadmium-Schiff's base complex. Fresenius J. Anal. Chem. 2001, 370, 1091–1095. [Google Scholar]
- Ardakani, M.M.; Salvati-Niassari, M.; Sadeghi, A. Novel selective thiocyanate PVC membrane electrode based on new Schiff base complex of 2.2-[(1,3-dimethyl-1,3-propanediylidene)dinitrilo]bis-benzenethiolato cadmium(II). New J. Chem. 2004, 28, 595–599. [Google Scholar]
- Ardakani, M.M.; Sadeghi, A.; Salavati-Niasari, M. Highly selective thiocyanate membrane electrode based on butane-2,3-dione bis(salicylhydrazonato)zinc(II) complex. Talanta 2005, 66, 837–843. [Google Scholar]
- Shamsipur, M.; Yousefi, M.; Hosseini, M.; Ganjali, M.R.; Sharghi, H.; Naeimi, H. A schiff base complex of Zn(II) as a neutral carrier for highly selective PVC membrane sensors for the sulfate ion. Anal. Chem. 2001, 73, 2869–2874. [Google Scholar]
- Ganjali, M.R.; Norouzi, P.; Golmohammadi, M.; Mizani, F.; Poursaberi, T.; Salavati-Niasari, M.; Shamsipur, M.; Hosseini, M.; Javanbakht, M. Ann. Chim (Rome); 2003; Volume 93, p. 679. [Google Scholar]
- Ganjali, M.R.; Rezapour, M.; Pourjavid, M.R.; Salavati-Niasari, M.; Poursaberi, T. A novel potentiometric membrane sensor for quick determination of trace amount of SO42- based on a zinc-Schiff's base. Anal. Lett. 2003, 36, 881–894. [Google Scholar]
- Soleymanpour, A.; Asl, E.H.; Nasseri, M.A. Chemically modified carbon paste electrode for determination of sulfate ion by potentiometric method. Electroanalysis 2006, 18, 1598–1604. [Google Scholar]
- Mitchell-Koch, J.T.; Malinowska, E.; Meyerhoff, M.E. Gallium(III)-Schiff base complexes as novel ionophores for fluoride selective polymeric membrane electrodes. Electroanalysis 2005, 17, 1347–1353. [Google Scholar]
- Ganjali, M.R.; Pourjavid, M.R.; Rezapour, M.; Poursaberi, T.; Daftari, A.; Salavati-Niasari, M. Ruthenium(III) Schiff's base complex as novel chloride selective membrane sensor. Electroanalysis 2004, 16, 922–927. [Google Scholar]
- Kumar, K.G.; John, K.S.; Indira, C.J. A chloride ion-selective potentiometric sensor based on a polymeric schiff base complex. Indian J. Chem. Technol. 2006, 13, 13–16. [Google Scholar]
- Shamsipur, M.; Soleymanpour, A.; Akhond, M.; Sharghi, H.; Naseri, M.A. Iodide-selective carbon paste electrodes based on recently synthesized Schiff base complexes of Fe(III). Anal. Chim. Acta 2001, 450, 37–44. [Google Scholar]
- Farhadi, K.; Maleki, R.; Yamchi, R. H.; Sharghi, H.; Shamsipur, M. [Tetrakis(4-N,N-dimethylaminobenzene)porphyrinato]-manganese(III) acetate as a novel carrier for a selective iodide PVC membrane electrode. Anal. Sci. 2004, 20, 805–809. [Google Scholar]
- Ganjali, M.R.; Emami, M.; Javanbakht, M.; Salavati-Niasari, M.; Shamsipur, M.; Yousefi, M. Novel triiodide ion-selective polymeric membrane sensor based on mercury-salen. Sens. Actuators B 2005, 105, 127–131. [Google Scholar]
- Sadeghi, S.; Fathi, F.; Ali Esmaeili, A.; Naeimi, H. Novel triiodide ion-selective polymeric membrane electrodes based on some transition metal-Schiff base complexes. Sens. Actuators B 2006, 114, 928–935. [Google Scholar]
- Ganjali, M.R.; Norouzi, P.; Qomi, M.; Salavati-Niasari, M. Charge-transfer complex between iodine and a new Schiff's base as anion-carrier in construction of a highly selective triiodide PVC-based membrane electrode. Can. J. Anal. Sci. Spect. 2006, 51, 108–116. [Google Scholar]
- Ganjali, M.R.; Moghaddamb, M.R.; Norouzi, P.; Shirvani-Arani, S.; Daneshgar, P.; Adib, M.; Sobhi, H.R. Highly selective and sensitive triiodide PVC-based membrane electrode based on a new charge transfer complex of 2-(((2-(((E)-1-(2-hydroxyphenyl) methylidine) amino) phenyl) imino) methyl) phenol for nano-level monitoring of triiodide. Anal. Lett. 2006, 39, 683–695. [Google Scholar]
- Sadeghi, S.; Gafarzadeh, A.; Naseri, M.A.; Sharghi, H. Triiodide-selective polymeric membrane electrodes based on Schiff base complexes of Cu(II) and Fe(III). Sens. Actuators B 2004, 98, 174–179. [Google Scholar]
- Sadeghi, S.; Fathi, F.; Ali Esmaeili, A.; Naeimi, H. Novel triiodide ion-selective polymeric membrane electrodes based on some transition metal-Schiff base complexes. Sens. Actuators B 2006, 114, 928–935. [Google Scholar]
- Chai, Y.Q.; Jiang, F.; Yuan, R.; Xu, L.; Xu, W.J. A highly selective salicylate electrode based on Schiff base complexes of cobalt(III). Anal. Lett. 2003, 36, 2379–2392. [Google Scholar]
- Ardakani, M.M.; Jamshidpoor, M.; Naeimi, H.; Heidarnezhad, A. Determination of salicylate by selective poly(vinylchloride) membrane electrode based on N,N′ -1,4-butylene bis(3-methyl salicylidene iminato) copper(II). Bull. Korean Chem. Soc. 2006, 27, 1127–1132. [Google Scholar]
- Amini, M.K.; Khorasani, J.H.; Khaloo, S.S.; Tangestaninejad, S. Cobalt(II) salophen-modified carbon-paste electrode for potentiometric and voltammetric determination of cysteine. Anal. Biochem. 2003, 320, 32–38. [Google Scholar]
- Larrow, J.F.; Jacobsen, E.N. (R,R)-N,N′-Bis(3,5-Di-tert-Butylsalicylidene)-1,2-Cyclohexane diamino Manganese(III) Chloride, A Highly Enantioselective Epoxidation Catalyst. Org. Synth. Coll. 2004, 10, 96. [Google Scholar]
- Ganjali, M.R.; Rezapour, M.; Pourjavid, M.R.; Salavati-Niasari, M. Highly selective PVC-membrane electrodes based on Co(II)-Salen for determination of nitrite ion. Anal. Sci. 2003, 19, 1127–1131. [Google Scholar]
- Ganjali, M.R.; Mizani, F.; Emami, M.; Salavati-Niasari, M.; Shamsipur, M.; Yousefi, M.; Javanbakht, M. Novel liquid membrane electrode for selective determination of monohydrogenphosphate. Electroanalysis 2003, 15, 139–144. [Google Scholar]
- Ganjali, M.R.; Norouzi, P.; Hatambeygi, N.; Salavati-Niasari, M. Anion recognition: fabrication of a highly selective and sensitive HPO42- PVC sensor based on a oxo-molybdenum methyl-salen. J. Braz. Chem. Soc. 2006, 17, 859–865. [Google Scholar]
- Ganjali, M.R.; Norouzi, P.; Faridbod, F.; Ghorbani, M.; Adib, M. Highly selective and sensitive chromium(III) membrane sensors based on a new tridentate Schiff's base. Anal. Chim. Acta 2006, 569, 35–41. [Google Scholar]
- Ganjali, M.R.; Norouzi, P.; Golmohammadi, M.; Rezapour, M.; Salavati- Niasari, M. Novel bromide PVC-based membrane sensor based on iron(III)-salen. Electroanalysis 2004, 16, 910–914. [Google Scholar]
- Shamsipur, M.; Sadeghi, S.; Naeimi, H.; Sharghi, H. Iodide ion-selective PVC membrane electrode based on a recently synthesized salen-Mn(II) complex. Pol. J. Chem. 2000, 74, 231–238. [Google Scholar]
- Ganjali, M.R.; Poursaberi, T.; Hosseini, M.; Salavati-Niasari, M.; Yousefi, M.; Shamsipur, M. Highly selective iodide membrane electrode based on a cerium salen. Anal. Sci. 2002, 18, 289–292. [Google Scholar]
- Ganjali, M.R.; Emami, M.; Javanbakht, M.; Salavati-Niasari, M.; Shamsipur, M.; Yousefi, M. Novel triiodide ion-selective polymeric membrane sensor based on mercury-salen. Sens. Actuators B 2005, 105, 127–131. [Google Scholar]
- Gholivand, M.B.; Ahmadi, F.; Rafiee, E. A novel Al(III)-selective electrochemical sensor based on N,N′-bis(salicylidene)-1,2-phenylenediamine complexes. Electroanalysis 2006, 18, 1620–1626. [Google Scholar]
- Ganjali, M.R.; Shirvani-Arani, S.; Norouzi, P.; Rezapour, M.; Salavati-Niasari, M. Novel nitrite membrane sensor based on cobalt(II) salophen for selective monitoring of nitrite ions in biological samples. Mikrochim. Acta 2004, 146, 35–41. [Google Scholar]
- Wroblewski, W.; Brzozka, Z.; Rudkevich, D.M.; Reinhoudt, D.N. Nitrite-selective ISE based on uranyl salophen derivatives. Sens. Actuators B 1996, 37, 151–155. [Google Scholar]
- Wroblewski, W.; Wojciechowski, K.; Dybko, A.; Brzozka, Z.; Egberink, R.J.M.; Snellink-Ruel, B.H.M.; Reinhoudt, D.N. Uranyl salophenes as ionophores for phosphate-selective electrodes. Sens. Actuators B 2000, 68, 313–318. [Google Scholar]
- Ganjali, M.R.; Mizani, F.; Salavati-Niasari, M. Novel monohydrogenphosphate sensor based on vanadyl salophen. Anal. Chim. Acta 2003, 481, 85–90. [Google Scholar]
- Ion, I.; Ion, A.C.; Barbu, L. Potentiometric determination of fluoride in groundwaters. Rev. Roum. Chim. 2005, 50, 407–412. [Google Scholar]
- Zare, H.R.; Memarzadeh, F.; Gorji, A.; Ardakani, M.M. Iodide-selective membrane electrode based on salophen complex of cobalt (III). J. Braz. Chem. Soc. 2005, 16, 571–577. [Google Scholar]
- Shamsipur, M.; Ershad, S.; Samadi, N.; Esmaeilbeig, A.R.; Kia, R.; Abdolmaleki, A. Polymeric membrane lanthanum(III)-selective electrode based on N,N′-adipylbis(5-plenylazo salicylaldehyde hydrazone). Electroanalysis 2005, 17, 1828–1834. [Google Scholar]
- Ganjali, M.R.; Matloobi, P.; Ghorbani, M.; Norouzi, P.; Salavati-Niasari, M. La (III) selective membrane sensor based on a new N-N Schiff's base. Bull. Korean Chem. Soc. 2005, 26, 38–42. [Google Scholar]
- Ganjali, M. R.; Gholivand, M.B.; Maddah, B.; Salavati-Niasari, M.; Ahmadi, F. Synthesis of a new octadentates Schiff's base and its application in construction of a highly selective and sensitive lanthanum (III) membrane sensor. Sensor Lett. 2006, 4, 356–363. [Google Scholar]
- Ganjali, M.R.; Norouzi, P.; Nasrin Yousefian, N.; Faridbod, F.; Adib, M. Sub-micro molar monitoring of La3+ by a novel lanthanum PVC-based membrane sensor based on 3-hydroxy-N′ -(pyridin-2-ylmethylene)-2-naphthohydrazide. Bull. Korean Chem. Soc. 2006, 27, 1581–1586. [Google Scholar]
- Ganjali, M.R.; Norouzi, P.; Alizadeh, T.; Adib, M. Application of 8-amino-N-(2-hydroxybenzylidene)naphthyl amine as a neutral ionophore in the construction of a lanthanum ion-selective sensor. Anal. Chim. Acta 2006, 576, 275–282. [Google Scholar]
- Zamani, H.A.; Ganjali, M.R.; Norouzi, P.; Adib, M.; Aceedy, M. Synthesis of N′-(1-pyridin-2-ylmethylene)-2-furohydrazide and its application in construction of a highly selective PVC-based membrane sensor for La(III) ions. Anal. Sci. 2006, 22, 943–948. [Google Scholar]
- Ganjali, M.R.; Mirnaghi, F.S.; Norouzi, P.; Adib, M. Novel Pr(III)-selective membrane sensor based on a new hydrazide derivative. Sens. Actuators B 2006, 115, 374–378. [Google Scholar]
- Norouzi, P.; Ganjali, M.R.; Ahmadalinezhad, A.; Adib, M. Novel neodymium(III) membrane sensor based on N-(2-furylmethylene)pyridine-2,6-diamine. J. Braz. Chem. Soc. 2006, 17, 1309–1315. [Google Scholar]
- Ganjali, M.R.; Ahmadalinezhad, A.; Norouzi, P.; Adib, M. Nd(III)-PVC membrane sensor based on 2-{[(6-aminopyridin-2-yl)imino]methyl}-phenol. J. Appl. Electrochem. 2006, 36, 931–936. [Google Scholar]
- Zamani, H.A.; Ganjali, M.R.; Adib, M. Fabrication of a new samarium(III) ion-selective electrode based on 3-{[2-oxo-1(2H)-acenaphthylenyliden]amino}-2-thioxo-1,3-thiazolidin-4-one. J. Braz. Chem. Soc. 2007, 18, 215–222. [Google Scholar]
- Ganjali, M.R.; Norouzi, P.; Daftari, A.; Faridbod, F.; Salavati-Niasari, M. Fabrication of a highly selective Eu(III) membrane sensor based on a new S-N hexadentates schiff's base. Sens. Actuators B 2007, 120, 673–678. [Google Scholar]
- Ganjali, M.R.; Rezapour, M.; Rasoolipour, S.; Norouzi, P.; Adib, M. Application of Pyridine-2-carbaldehyde-2-(4-methyl-1,3-benzo thiazol-2-yl)hydrazone as a Neutral Ionophore in the Construction of a Novel Er(III) Sensor. J. Braz. Chem. Soc. 2007, 18, 352–358. [Google Scholar]
- Ganjali, M.R.; Faridbod, F.; Norouzi, P.; Adib, M. A novel Er(III) sensor based on a new hydrazone for the monitoring of Er(III) ions. Sen. Actuators B 2006, 120, 119–124. [Google Scholar]
- Faridbod, F.; Gangali, M.R.; Larijani, B.; Norouzi, P.; Riahi, S.; Mirnaghi, F.S. Lanthanide Recognition: an Asymetric Erbium Microsensor Based on a Hydrazone Derivative. Sensors 2007, 7, 3119. [Google Scholar]
- Ganjali, M.R.; Rasoolipour, S.; Rezapour, M.; Norouzi, P.; Adib, M. Synthesis of thiophene-2-carbaldehyde-(7-methyl-1,3-benzothiazol-2-yl)hydrazone and its application as an ionophore in the construction of a novel thulium (III) selective membrane sensor. Electrochem. Commun. 2005, 7, 989–994. [Google Scholar]
- Ganjali, M. R.; Norouzi, P.; Tamaddon, A.; Adib, M. Nano-level monitoring of ytterbium(III) by a novel ytterbium(III) membrane sensor based on 3-hydroxy-N′ - [(2-hydroxyphenyl) methylene]-2-naphthohydrazide. Sens. Actuators B 2006, 114, 855–860. [Google Scholar]
- Ganjali, M. R.; Norouzi, P.; Alizadeh, T.; Salavati-Niasari, M. Synthesis of a new hexadendates Schiff's base and its application in the fabrication of a highly selective mercury(II) sensor. Bull. Korean Chem. Soc. 2007, 28, 68–72. [Google Scholar]
- Ganjali, M.R.; Norouzi, P.; Shirvani-Arani, S.; Salavati-Niasari, M. Novel triiodide PVC-based membrane sensor based on a charge transfer complex of iodine and bis(2-hydroxyacetophenone)butane-2,3-dihydrazone. Bull. Korean Chem. Soc. 2005, 26, 1738–1742. [Google Scholar]
- Ganjali, M.R.; Shirvani-Arani, S.; Bidhendi, G.N.; Norouzi, P.; Salavati-Niasari, M. Highly selective and sensitive triiodide PVC-membrane electrode based on a new charge-transfer complex of bis(2,4-dimethoxybenzaidehyde)butane-2,3-dihydrazone with iodine. J. Chin. Chem. Soc. 2006, 53, 275–281. [Google Scholar]
- Ganjali, M.R.; Norouzi, P.; Shirvani-Arani, S.; Salavati-Niasari, M. Novel triiodide PVC-based membrane sensor based on a charge transfer complex of iodine and bis(2-hydroxyacetophenone)butane-2,3-dihydrazone. Bull. Korean Chem. Soc. 2005, 26, 1738–1742. [Google Scholar]
- Weber, E. Supramolecular Chemistry II-Host Design and Molecular Recognition; Springer-Verlag: Berlin Heidelberg, 1995. [Google Scholar]
- Lehn, J. M.; Sauvage, J. P. Cation selectivities of alkali and alkaline-earth cryptates. Chem. Commun. 1971, 440. [Google Scholar]
- Kobiro, K.; Tobe, Y.; Watanabe, K.; Yamada, H.; Suzuki, K. Highly selective lithium ion electrode based on decalino-14-Crown-4. Anal. Lett. 1993, 26, 49–54. [Google Scholar]
- Jung, S.O.; Park, S.S.; Kim, B.G.; Kim, J.S. Lithium ion-selective electrode based on TNF-based 16-Crown-4 derivatives. Bull. Korean Chem. Soc. 1995, 16, 197–199. [Google Scholar]
- Cretin, M.; Fabry, P. Detection and selectivity properties of Li+-ion-selective electrodes based on Nasicon-type ceramics. Anal. Chim. Acta 1997, 354, 291–299. [Google Scholar]
- Kang, Y.R.; Lee, K.M.; Nam, H.; Cha, G.S.; Jung, S.O.; Kim, J.S. Lithium ion-selective electrodes employing tetrahydrofuran-based 16-crown-4 derivatives as neutral carriers. Analyst 1997, 122, 1445–1450. [Google Scholar]
- Shibata, Y.; Maruizumi, T.; MiyagiI, H. Characteristics of sodium ion- selective electrodes based on neutral carriers and their application to analysis of physiological fluids. Nippon Kagaku Kaishi 1992, 9, 961–967. [Google Scholar]
- Lukyanenko, N.G.; Titova, N.Y.; Karpinchik, O.S.; Melnik, O.T. Sodium- Selective Electrodes Based On PVC Membranes Containing Bis[(3N+1)-Crown-N]. Ether Derivatives. Anal. Chim. Acta 1992, 259, 145–148. [Google Scholar]
- Yamamoto, H.; Ueda, K.; Suenaga, H.; Sakaki, T.; Shinkai, S. Exploitation of Na+-selective electrodes for protein solutions from crown-bridged calix[4]quinones. Chem. Lett. 1996, 1, 39–40. [Google Scholar]
- Tavakkoli, N. Sodium ion-selective membrane electrode based on dibenzopyridino-18-crown-6. Bull. Korean Chem. Soc. 2004, 25, 1474–1476. [Google Scholar]
- Chandra, S.; Lang, H. A new sodium ion selective electrode based on novel silacrown ether. Sens. Actuators B 2006, 114, 849–854. [Google Scholar]
- Ganjali, M.R.; Moghimi, A.; Buchanan, G.W.; Shamsipur, M. The synthesis of styrene/4′-vinylbenzo-24-crown-8 copolymer and its use in potassium-ion selective electrodes. J. Inclus. Phenom. Mol. Rec. Chem. 1998, 30, 29–43. [Google Scholar]
- Xia, Z.R.; Badr, I.H.A.; Plummer, S.L.; Cullen, L.; Bachas, L.G. Synthesis and evaluation of a bis(crown ether) ionophore with a conformationally constrained bridge in ion-selective electrodes. Anal. Sci. 1998, 14, 169–173. [Google Scholar]
- Hyun, M.H.; Piao, M.H.; Cho, Y.J.; Shim, Y.B. Ionophores in rubidium ion-selective membrane electrodes. Electroanalysis 2004, 16, 1785–1790. [Google Scholar]
- Shamsipur, M.; Alizadeh, K.; Hosseini, M.; Mousavi, M.F.; Ganjali, M.R. PVC membrane and coated graphite potentiometric sensors based on dibenzo-21-crown-7 for selective determination of rubidium ions. Anal. Lett. 2005, 38, 573–588. [Google Scholar]
- Kang, Y.R.; Oh, H.J.; Lee, K.M.; Cha, G.S.; Nam, H.; Paek, K.; Ihm, H. Potentiometric characteristics of ion-selective electrodes based on upper-rim calix[4]crown neutral carrier. Bull. Korean Chem. Soc. 1998, 19, 207–211. [Google Scholar]
- Kim, J.S.; Ohki, A.; Ueki, R.; Ishizuka, T.; Shimotashiro, T.; Maeda, S. Cesium- ion selective electrodes based on calix[4]arene dibenzocrown ethers. Talanta 1999, 48, 705–710. [Google Scholar]
- Choi, Y.; Kim, H.; Lee, J.K.; Lee, S.H.; Lim, H.B.; Kim, J.S. Cesium ion-selective electrodes based on 1,3-alternate thiacalix[4]biscrown-6,6. Talanta 2004, 64, 975–980. [Google Scholar]
- Ganjali, M.R.; Moghimi, A.; Shamsipur, M. Beryllium-selective membrane electrode based on benzo-9-crown-3. Anal. Chem. 1998, 70, 5259–5263. [Google Scholar]
- Ganjali, M.R.; Daftari, A.; Rastegar, M.F.; Moghimi, A. Novel beryllium membrane sensor based on 2,4-dinitrophenylhydrazinebenzo-9-crown-3 coated graphite. Anal. Lett. 2003, 36, 317–328. [Google Scholar]
- Ganjali, M.R.; Daftari, A.; Faal-Rastegar, M.; Moghimi, A. Novel potentiometric sensor for monitoring beryllium based on naphto-9-crown-3. Anal. Sci. 2003, 19, 353–356. [Google Scholar]
- Ganjali, M.R.; Ghorbani, M.; Norouzi, P.; Daftari, A.; Faal-Rastegar, M.; Moghimi, A. Nano levels detection of beryllium by a novel beryllium PVC-based membrane sensor based on 2,3,5,6,8,9-hexahydro-1,4,7,10-benzotetraoxacyclododecine-12-carbaldehyde-12-(2,4-dinitrophenyl) hy. Sens. Actuators B 2004, 100, 315–319. [Google Scholar]
- Ganjali, M.R.; Rahimi-Nasrabadi, M.; Maddah, B.; Moghimi, A.; Faal-Rastegar, M.; Borhany, S.; Namazian, M. Sub-micro level monitoring of Beryllium ions with a novel beryllium sensor based on 2,6-diphenyl-4-benzo-9-crown-3- pyridine. Talanta 2004, 63, 899–906. [Google Scholar]
- Ganjali, M.R.; Norouzi, P.; Faridbod, F.; Sepehrifard, A.; Ghandi, M.; Moghimi, A. Monitoring of beryllium(II) ions by use of a novel beryllium(II) membrane sensor, based on 2, 3, 5, 6, 8, 9-hexahydronaphto [2,3-b] [1, 4, 7, 10] tetraoxacyclo dodecine. Can. J. Anal. Sci. Spect. 2007, 52, 46–56. [Google Scholar]
- Ganjali, M.R.; Daftari, A.; Pourjavid, M.R.; Rastegar, M.F.; Moghimi, A. Novel Be(II)membrane electrode-based on a derivative of benzo-9-crown-3. Main group met. chem. 2002, 25, 669–676. [Google Scholar]
- Ganjali, M.R.; Akbar, V.; Norouzi, P.; Moghimi, A. Sepehrifard A, Be(II) graphite coated membrane sensor based on a recently synthesized benzo-9-crown-3 derivative. Electroanalysis 2005, 17, 895–900. [Google Scholar]
- Gupta, V.K.; Chandra, S.; Mangla, R. Magnesium-selective electrodes. Sens. Actuators B 2002, 86, 235–241. [Google Scholar]
- Gupta, V.K.; Jain, A.K.; Khurana, U.; Singh, L.P. PVC-based neutral carrier and organic exchanger membranes as sensors for the determination of Ba2+ and Sr2+. Sens. Actuators B 1999, 55, 201–211. [Google Scholar]
- Shamsipur, M.; Rouhani, S.; Shaghi, H.; Ganjali, M.R.; Eshghi, H. Strontium-selective membrane electrodes based on some recently synthesized benzo-substituted macrocyclic diamides. Anal. Chem. 1999, 71, 4938–4943. [Google Scholar]
- Abdulla, N.I.; Al-Haider, A.M.; Al-Joboury, M.I.; Nassory, N.S. Construction and characterization of indium liquid ion selective electrodes based on crown ethers in a PVC matrix membrane. Turish J. Chem. 2005, 29, 687–696. [Google Scholar]
- Khayatian, G.; Shariati, S.; Salimi, A. Thallium (I)-selective membrane potentiometric sensor based on dibenzyldiaza-18-crown-6. Bull. Korean Chem. Soc. 2003, 24, 421–425. [Google Scholar]
- Aghaie, H.; Giahi, A.; Monajjemi, M.; Arvand, A.; Nafissi, G.H.; Aghaie, M. Tin(II)-selective membrane potentiometric sensor using a crown ether as neutral carrier. Sens. Actuators B 2005, 107, 756–761. [Google Scholar]
- Sheen, S.R.; Shih, J.S. Lead (II) ion-selective electrodes based on crown ethers. Analyst 1992, 117, 1691–1695. [Google Scholar]
- Pouretedal, H.R.; Keshavarz, M.H. Lead (H)-selective electrode based on dibenzodiaza-15-crown-4. Asian J. Chem. 2004, 16, 1319–1326. [Google Scholar]
- Ganjali, M.R.; Roubollahi, A.; Mardan, A.R.; Hamzeloo, M.; Mogimi, A.; Shamsipur, M. Lead ion-selective electrode based on 4′ -vinylbenzo-l5-crown-5 homopolymer. Microchim. J. 1998, 60, 122–133. [Google Scholar]
- Yang, X.H.; Kumar, N.; Chi, H.; Hibbert, D.B.; Alexander, P.W. Lead-selective membrane electrodes based on dithiophenediazacrown ether derivatives. Electroanalysis 1997, 9, 549–553. [Google Scholar]
- Ardakani, M.M.; Kashani, M.K.; Salavati-Niasari, M.; Ensafi, A.A. Lead ion- selective electrode prepared by sol-gel and PVC membrane techniques. Sens. Actuators B 2005, 107, 438–445. [Google Scholar]
- Shamsipur, M.; Ganjali, M.R.; Rouhollahi, A. Lead-selective membrane potentiometric sensor based on an 18-membered thiacrown derivative. Anal. Sci. 2001, 17, 935–938. [Google Scholar]
- Ganjali, M.R.; Hosseini, M.; Basiripour, F.; Javanbakht, M.; Hashemi, O.R.; Rastegar, M.F.; Shamsipur, M.; Buchanen, G.W. Novel coated-graphite membrane sensor based on N,N′-dimethylcyanodiaza-18-crown-6 for the determination of ultra-trace amounts of lead. anal. Chim. Acta 2002, 464, 181–186. [Google Scholar]
- Gupta, V.K.; Jain, S.; Chandra, S. Chemical sensor for lanthanum (III) determination using aza-crown as ionophore in poly(vinyl chloride) matrix. Anal. Chim. Acta 2003, 486, 199–207. [Google Scholar]
- Shamsipur, M.; Yousefi, M.; Hosseini, M.; Ganjali, M.R. PVC-based 1,3,5-trithiane coated graphit electrode for determination of ceriume(III) ions. Anal. Lett. 2001, 34, 2249–2261. [Google Scholar]
- Akl, M.A.; Ghoneim, A.K.; Abd El-Aziz, M.H. Novel plastic chromium (III)-ion selective electrodes based on different ionophoric species and plasticizing solvent mediators. Electroanalysis 2006, 18, 299–306. [Google Scholar]
- Ekmekci, G.; Uzun, D.; Somer, G.; Kalayci, S. A novel iron (III) selective membrane electrode based on benzo-18-crown-6 crown ether and its applications. J. Membr. Sci. 2007, 288, 36–40. [Google Scholar]
- Shamsipur, M.; Poursaberi, T.; Rouhani, S.; Niknam, K.; Sharghi, H.; Ganjali, M.R. obalt(II)-selective membrane electrode based on a recently synthesized benzo-substituted macrocyclic diamide. Anal. Sci. 2001, 17, 1049–1054. [Google Scholar]
- Shamsipur, M.; Rouhani, S.; Poursaberi, T.; Ganjali, M.R.; Sharghi, H.; Niknam, K. Cobalt(II)-selective coated graphite PVC-membrane electrode based on a recently synthesized dibenzopyridino-substituted macrocyclic diamide. Electroanalysis 2002, 14, 729–735. [Google Scholar]
- Shamsipur, M.; Kazemi, S.Y. A PVC-based dibenzodiaza-15-crown-4 membrane potentiometric sensor for Ni (II). Electroanalysis 2000, 12, 1472–1475. [Google Scholar]
- Shamsipur, M.; Javanbakht, M.; Mousavi, M.F.; Ganjali, M.R.; Lippolis, V.; Garau, A.; Tei, L. Copper(II)-selective membrane electrodes based on some recently synthesized mixed aza-thioether crowns containing a 1,10-phenanthroline sub-unit. Talanta 2001, 55, 1047–1054. [Google Scholar]
- Shamsipur, M.; Rouhani, S.; Ganjali, M.R.; Eshghi, H.; Sharghi, H. Copper(II)-selective membrane electrode based on a recently synthesized macrocyclic diamide. Microchim. J. 1999, 63, 202–210. [Google Scholar]
- Park, S.S.; Jung, S.O.; Kim, S.M.; Kim, J.S. Lipophilic pyrrole-based tetraazacrown ether as neutral carrier for silver ion-selective electrode. Bull. Korean Chem. Soc. 1996, 17, 405–407. [Google Scholar]
- Goldcamp, M.J.; Ashley, K.; Edison, S.E.; Pretty, J.; Shumaker, J. A bis-oxime derivative of diaza-18-crown-6 as an ionophore for silver ion. Electroanalysis 2005, 17, 1015–1018. [Google Scholar]
- Mashhadizadeh, M.H.; Shamsipur, M. Silver(I)-selective membrane electrode based on hexathia-18-crown-6. Anal. Chim. Acta 1999, 381, 111–116. [Google Scholar]
- Shamsipur, M.; Javanbakht, M.; Ganjali, M.R.; Mousavi, M.F.; Lippolis, V.; Garau, A. Mixed aza-thioether crowns containing a 1,10-phenanthroline sub-unit as neutral ionophores for silver ion. Electroanalysis 2002, 14, 1691–1698. [Google Scholar]
- Chen, L.X.; He, X.W.; Zhang, H.Y.; Liu, Y.; Hu, X.B.; Sheng, Y. Selective electrode for silver based on polymer membranes containing exocyclic chalcogen atoms calix[4]arene and crown ether. Anal. Lett. 2001, 34, 2237–2248. [Google Scholar]
- Demirel, A.; Dogan, A.; Akkus, G.; Yilmaz, M.; Kilic, E. Silver(I)-selective PVC membrane potentiometric sensor based on a recently synthesized calix[4]arene. Electroanalysis 2006, 18, 1019–1027. [Google Scholar]
- Shamsipur, M.; Javanbakht, M.; Ganjali, M.R.; Mousavi, M.F.; Lippolis, V.; Garau, A. Mixed aza-thioether crowns containing a 1,10-phenanthroline sub-unit as neutral ionophores for silver ion. Electroanalysis 2002, 14, 1691–1698. [Google Scholar]
- Pouretedal, H.R.; Shamsipur, M. A PVC-based cryptand C2(B)22 membrane potentiometric sensor for zinc(II). Fresenius J. Anal. Chem. 1998, 362, 415–418. [Google Scholar]
- Gupta, V.K.; Al Khayat, M.; Minocha, A.; Kumar, P. Zinc(II)-selective sensors based on dibenzo-24-crown-8 in PVC matrix. Anal. Chim. Acta 2005, 532, 153–158. [Google Scholar]
- Shamsipur, M.; Rouhani, S.; Ganjali, M.R.; Sharghi, H.; Eshghi, H. Zinc-selective membrane potentiometric sensor based on a recently synthesized benzo-substituted macrocyclic diamide. Sens. Actuators B 1999, 59, 30–34. [Google Scholar]
- Gupta, V.K.; Jain, A.K.; Kumar, P. PVC-based membranes of dicyclohexano-24-crown-8 as Cd(II) selective sensor. Electrochim. Acta 2006, 52, 736–741. [Google Scholar]
- Abbas, M.N.; Zahran, E. Novel solid-state cadmium ion-selective electrodes based on its tetraiodo- and tetrabromo-ion pairs with cetylpyridinium. J. Electroanal. Chem. 2005, 576, 205–213. [Google Scholar]
- Singh, A.K.; Saxena, P.; Singh, R. New cadmium(II)-selective electrode based on a tetraazacyclohexadeca macrocyclic ionophore. Anal. Sci. 2005, 21, 179–181. [Google Scholar]
- Gupta, K.C.; D'Arc, M.J. Cadmium ion-selective electrode based on cyanocopolymer. Electroanalysis 2000, 12, 1408–1413. [Google Scholar]
- Mahajan, R.K.; Kaur, R.; Kaur, I.; Sharma, V.; Kumar, M. Mercury(II) ion- selective electrodes based on p-tert-butyl calix[4]crowns with imine units. Anal. Sci. 2004, 20, 811–814. [Google Scholar]
- Gupta, V.K.; Jain, S.; Khurana, U. A PVC-based pentathia-15-crown-5 membrane potentiometric sensor for mercury(II). Electroanalysis 1997, 9, 478–480. [Google Scholar]
- Javanbakht, M.; Ganjali, M.R.; Eshghi, H.; Sharghi, H.; Shamsipur, M. Mercury(II) ion-selective electrode based on dibenzodiazathia-18-crown-6-dione. Electroanalysis 1999, 11, 81–84. [Google Scholar]
- Fakhari, A.R.; Ganjali, M.R.; Shamsipur, M. PVC-based hexathia-18-crown-6-tetraone sensor for mercury(II) ions. Anal Chem. 1997, 69, 3693–3696. [Google Scholar]
- Sadeghi, S.; Dashti, G.R. Triiodide PVC membrane electrodes based on charge-transfer complexes. Anal. Chem. 2002, 74, 2591–2595. [Google Scholar]
- Poursaberi, T.; Salavati-Niassari, M.; Khodabakhsh, S.; Hajiagha-Babaei, L.; Shamsipur, M.; Yousefi, M.; Rouhani, S.; Ganjali, M.R. A selective membrane electrode for thiocyanate ion based on a copper-1,8-dimethyl-1,3,6,8,10,13-azacyclotetradecane complex as ionophore. Anal. Lett. 2001, 34, 2621–2632. [Google Scholar]
- Ganjali, M.R.; Yousefi, M.; Javanbakht, M.; Poursaberi, T.; Salavati-Niasari, M.; Hajiagha-Babaei, L.; Latifi, L.; Shamsipur, M. Determination of SCN- in urine and saliva of smokers and non-smokers by SCN--selective polymeric membrane containing a nickel(II)-azamacrocycle complex coated on a graphite electrode. Anal. Sci. 2002, 18, 887–892. [Google Scholar]
- Shamsipur, M.; Yousefi, M.; Ganjali, M.R.; Poursaberi, T.; Faal-Rastgar, Majid. Highly selective sulfate PVC-membrane electrode based on 2,5-diphenyl-1,2,4,5-tetraaza-bicyclo[2.2.1]heptane as a neutral carrier. Sens. Actuators B 2002, 82, 105–110. [Google Scholar]



| Cation | Ionophore | Slope (mV decade-1) | Linear Range (M) | Most Important Interfering ions (log Ksel> -2) | Ref. |
|---|---|---|---|---|---|
| Al+3-1 | bis(5-phenyl azo salicylaldehyde)-2,3-naphthalene diimine (5PHAZOSALNPHN) | 19.3 | 5.0× 10-6-1.0 × 10-2 | Li+,NH4+,Ag+ | 82 |
| Sn2+-1 | 6-(4-nitrophenyl)-2,4-diphenyl-3,5-diazabicyclo-[3.1.0]-hex-2-ene (NDDBH) | 28.8 ± 1.1 | 1.0 ×10-5-1.0 × 10-1 | Tl+, Mn2+, Ba2+,Cs+ | 83 |
| Pb2+-1 | bis(acetylacetone)-p-phenylenediamine-lead(II) [LPb(NO3)2]H2O complex ionophore | 30.0 ± 0.2 | 1×10-5-1× 10-1 | - | 84 |
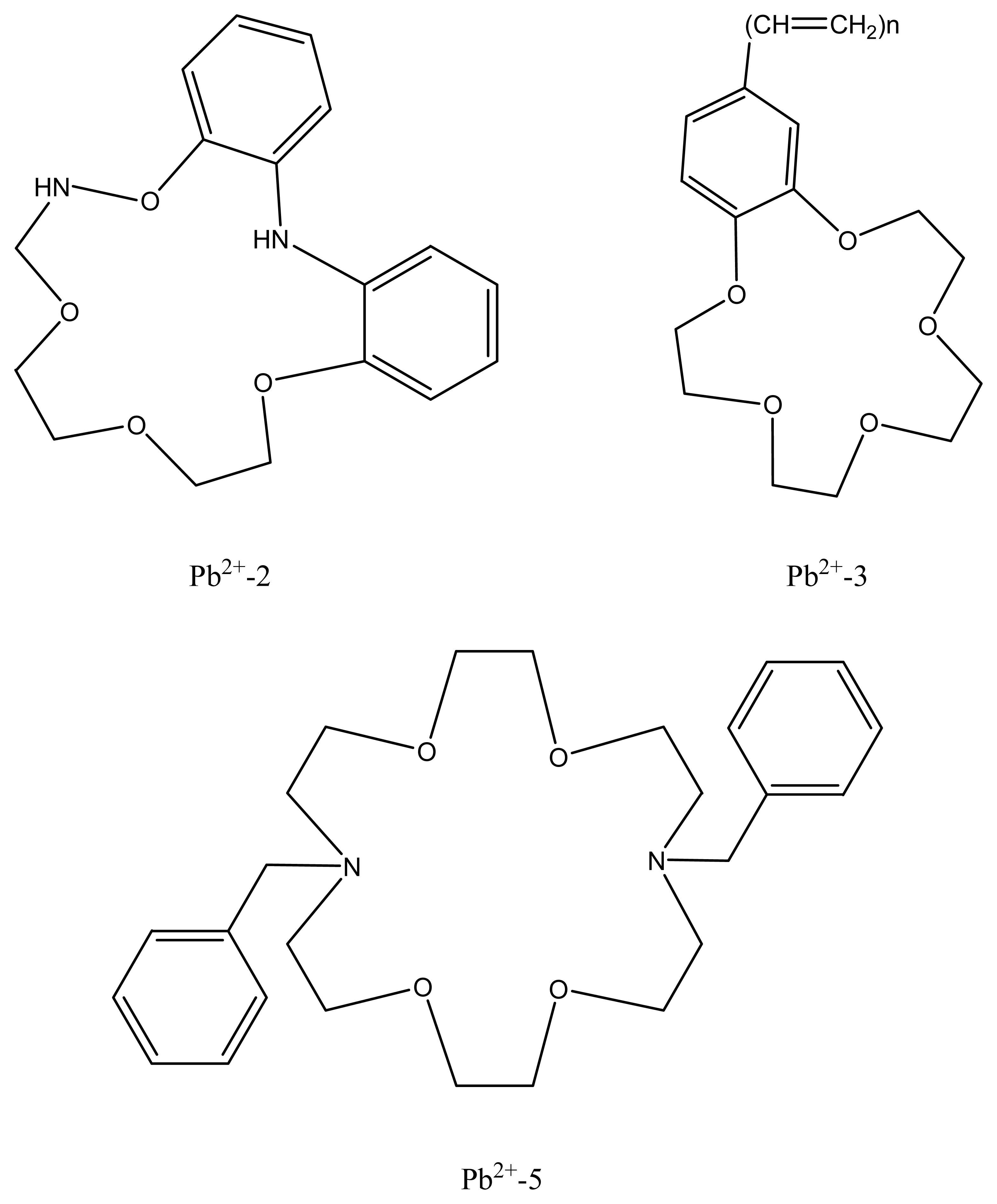
| Pb2+-2 | N,N′-bis(5-methylsalicylidene)-p-diphenylenemethane diamine | 29.4 | 5.0 × 10-6-0.10 | Zn2+, Fe3+, K+, NH4+ | 85 |
| Pb2+-3 | N,N′-bis(3-methylsalicylidine)-p-phenylmethane diamine | 30.3 ± 0.6 | 2.0×10-5-0.10 | Na+, K+, NH4+ | 86 |
| Pb2+-4 | Schiff base as a neutral carrier | 29.4± 0.5 | 1.0× 10-5-1.0 × 10-1 | Na+, K+, Cu2+ | 87 |
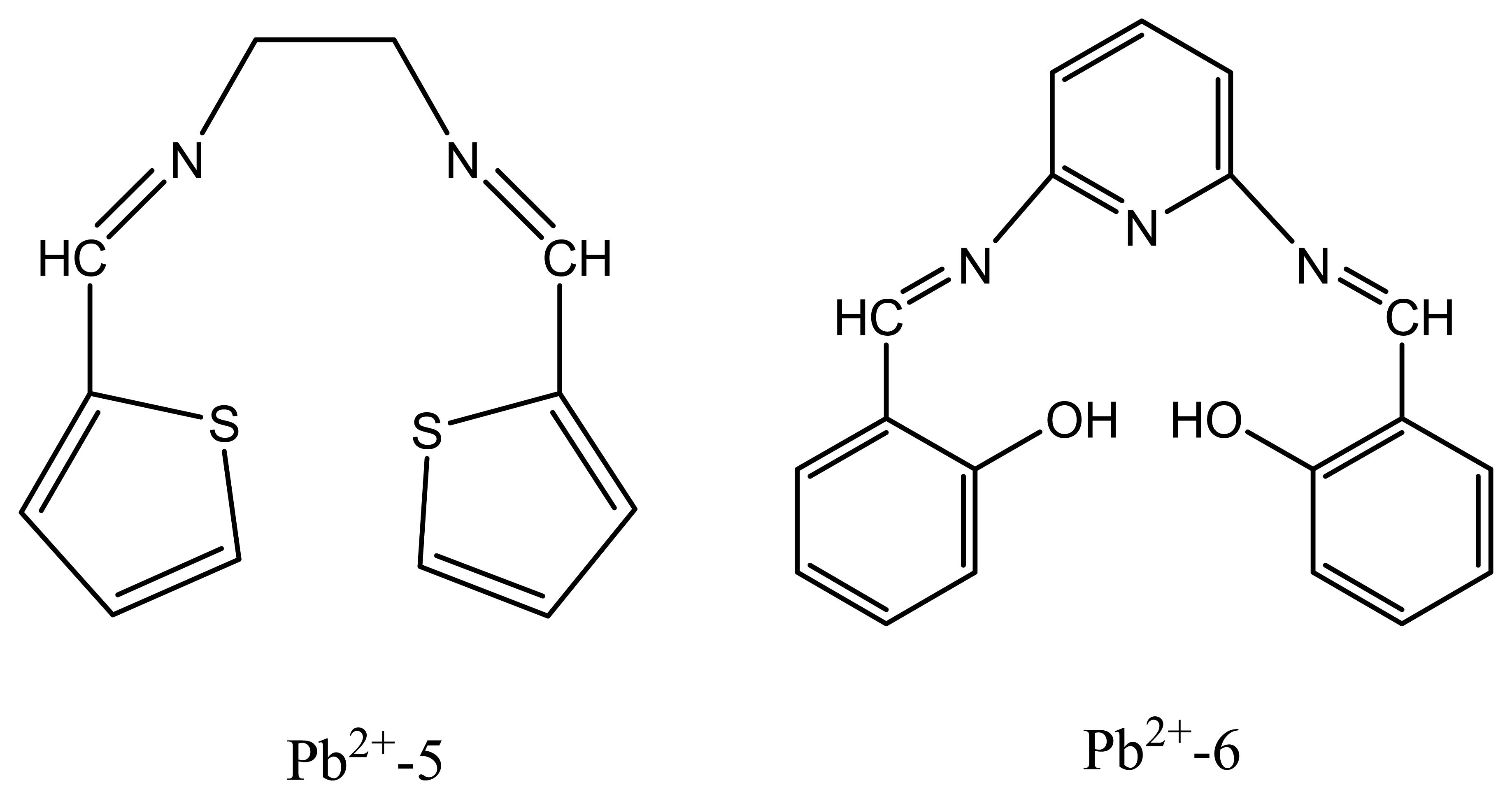
| Pb2+-5 | N,N′-bis-thiophene-2-yl-methyleneethane -1,2-diamine | 29.79 | 1.0× 10-5-1.0 × 10-1 | - | 88 |
| Pb2+-6 | N,N′-bis(salicylidene)-2,6-pyridinediamine | 29.4 | 1.0×10-6-1.0×10-1 | K+, Ag+ | 89 |
| Pb2+-7 | N,N′-dibenzyl-1,4,10,13-tetraoxa-7,16-diazacyclooctadecane (I) | 30.0± 0.1 | 8.2 × 10-6-1.0 ×10-1 | Cd2+, Zn2+, Ag+ | 90 |
| Y3+-1 | A new Schiff's base with sulfur and nitrogen donor atoms (2-({(E)1,2-diphenyl-2-[(2-2-sulfanylphenyl)imino]ethylidene} amino)-1-benzenethiol | 19.2 | 1.0×10-7-1.0×10-2 | Sc3+ | 91 |
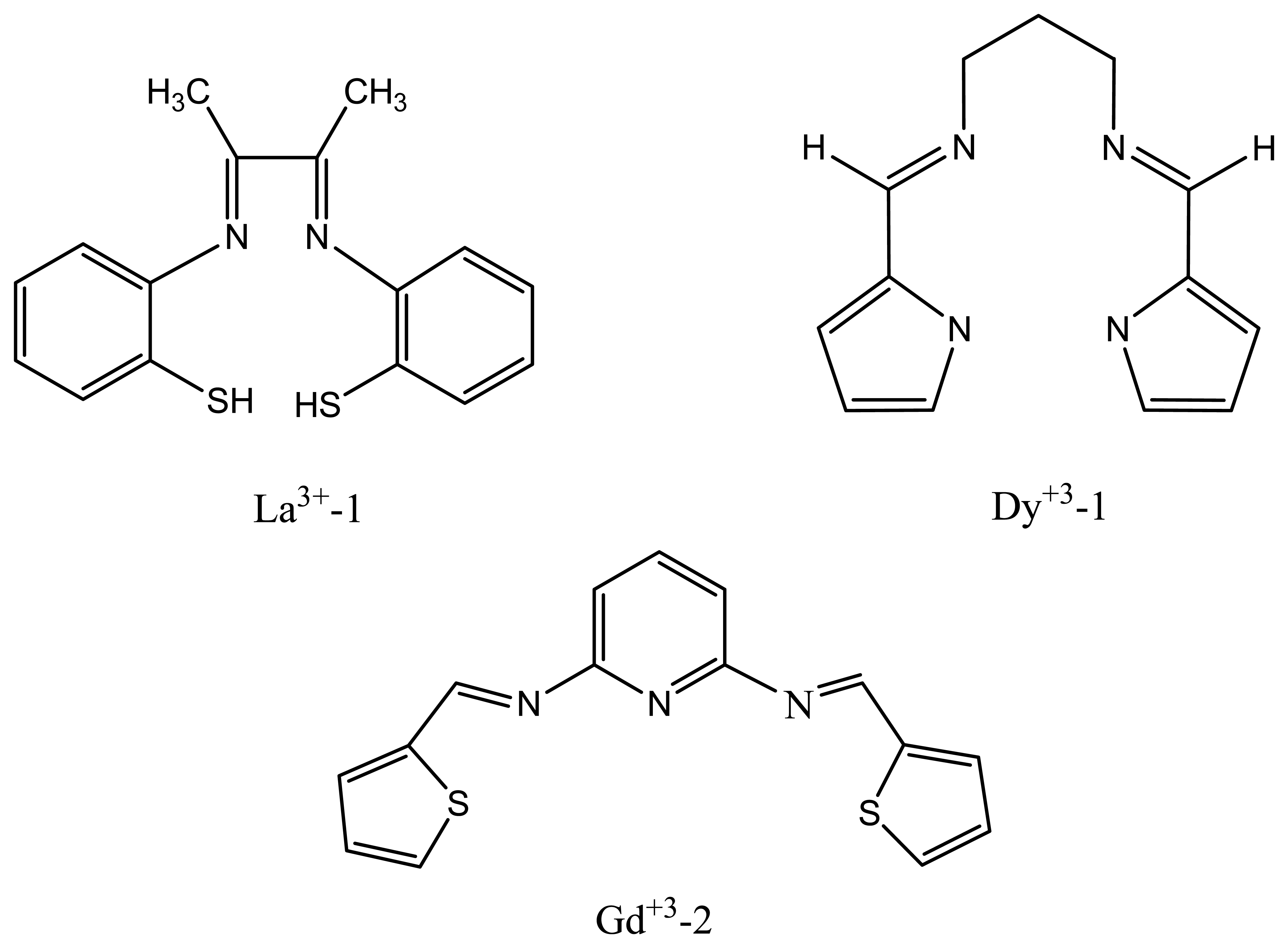
| La3+-1 | bis (2-mercaptoanil) diacetyl (BMDA) | 19.7 | 10-5-10-1 and 10-6-10-1 | Ce3+, Gd3+ | 92 |
| La3+-2 | bis(thiophenal)phenylen-1,3-diamine (TPD) | 19.6 | 1.0 ×10-7-1.0 × 10-1 | Sm3+, Ce3+ | 93 |
| Ce3+-1 | N,N-bis[2-(salicylideneamino)ethyl]ethane-1,2-diamine | 20 | 1.41×10-7- 1.0×10-2 | La3+ | 94 |
| Gd3+-1 | S-N Schiff s base (2-[{3-[(2-sulfanylphenyl)imino)- 1-methylbutylidene}amino]phenyl hydrosulfide (SMPH) | 19.8 ± 0.3 | 1.0 × 10-1-1.0× 10-5 | Tb3+, Dy3+, Eu3+ | 95 |
| Gd+3-2 | bis(thiophenal) pyridine-2,6-diamine (BPD) | 19.4 ± 0.4 | 1.0×10-6 -1.0×10-1 | La3+, Mg2+, Hg2+ | 96 |
| Tb+3-1 | N,N-bis(pyrrolidene) benzene-1,2-diamine | 19.8 | 1.0×10-5-1.0 × 10-1 | La3+, Yb3+, Dy3+ | 97 |
| Dy+3-1 | N,N-bis(pyrrolidene) benzene-1,2-diamine | 20.6±0.2 | 1.0×10-5-1.0 × 10-1 | Ce3+, La3+ | 98 |
| Dy+3-2 | a new asymmetrical Schiff's base [(E)-N-(2-hydroxybenzylidene)-benzohydraide] or BBH | 20.1± 0.8 | 1.0×10-2-1.0×10-6 | Yb3+, Tm3+, Na+ | 99 |
| Ho+3-1 | N-(1-thien-2-yl-methylene)-1,3-benzothiazol-2-amine | 19.7 | 10-5-10-2 | Lu3+, Dy3+, Gd3+ | 100 |
| Lu+3-1 | a new asymmetrically S-N Schiff's base, N-(thien-2-ylmethylene)-pyridine-2,6-diamine (TPD) | 20.5 ± 0.4 | 1.0×10-2 -1.0×10-6 | Nd3+, Dy3+, Gd3+ | 101 |
| UO22+-1 | 2,2′- [1,2-ethanediyl bis (nitriloethylidene)]bis(1-naphthalene) | 28.5± 0.8 | 10-1-10-7 | Mg2+, Cu2+ | 102 |
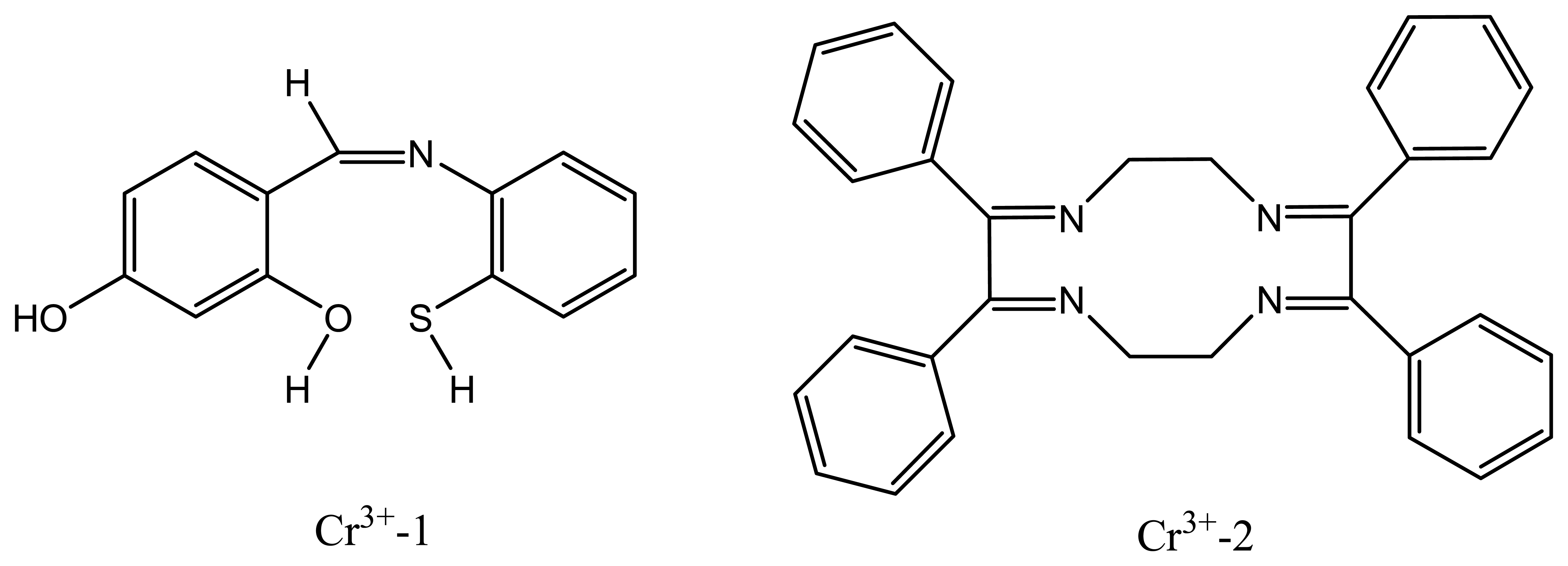
| Cr3+-1 | a new tridentate S,N,O Schiff's base 4-hydroxysalicylade-2-mercaptoanil (TSNO) | 20.2 | 3.0×10-6 -1.0 ×10-1 | Na+, La3+, Pb2+ | 103 |
| Cr3+-2 | 2,3,8,9-tetraphenyl-1,4,7,10-tetraazacyclododeca-1,3,7,9-tetraene (TTCT) | 19.5 | 1.0×10-6 -1.0 ×10-1 | Ag+ | 104 |
| Cr3+-3 | 2-hydroxybenzaldehyde-O,O′-(1,2-dioxetane-1,2-diyl) oxime | 19.6 and 19.2 | 1.5×10-6 − 8.0×10-3 M for PME and 4.0×10-7 − 3.0×10-3 M for CGCE | Ce3+, Cu2+ | 105 |
| Cr3+-4 | N-(1-thien-2-ylethylidene)-benzene-1,2-diamine (SNS) | 19.9± 0.3 | 1.0× 10-6- 1.0×10-1 | Fe3+, Eu3+, Mg2+ | 106 |
| Cr3+-5 | Schiff bases, N-(acetoacetanilide)-1,2-diaminoethane (L-1) and N,N′-bis (acetoacetanilide)-triethylene-tetrammine (L-2) | 19.8 | 8.9× 10-8 -1.0×10-1 | - | 107 |
| Mn2+-1 | N,N′,N″,N‴-1,5,8,12-tetraazadodecane-bis(salicylaldiminato) (H2L) | 30 | 5.0× 10-6-1.0 × 10-1 | Cd2+, Fe3+, Ni2+ | 108 |
| Fe3+-1 | 2-[(2-hydroxy-1-propenyl-buta-1,3-dienylimino)-methyl]-4-p-tolylazo-phen ol [HPDTP] | 28.5± 0.5 | 3.5× 10-6-4.0 × 10-2 | Fe2+, Cr3+ | 109 |
| Co2+-1 | 5-((4-nitrophenyl)azo)-N-(2′,4′-dimethoxyphenyl)salicylaldimine | 29 | 9.0×10-7-1.0×10-2 | K+, Ag+ | 110 |
| Ni2+-1 | thiophene-derivative Schiff base | 29.5± 1.0 | 5.0×10-6-1.0×10-1 | - | 111 |
| Ni2+-2 | N,N′-bis-(4-dimethylaminobenzylidene)-benzene-1,2-diamine | 30 | 1.0× 10-2-2.0× 10-7 | Tl+, Ag+ | 112 |
| Ni2+-3 | Schiff bases, N-(2-hydroxybenzyl)-N′-(2-hydroxybenzylidene)-ethylenediamine and N-(2-hydroxybenzylidene)-Al'-(2-picolyl)ethylenediatmine (II) | 30 and 29 | 6.3×10-6 to 5.0 × 10-1 and 3.2×10-6 to 5.0 × 10-1 | Ag+, Co2+, Hg2+ | 113 |
| Ni2+-4 | N1,N2-bis((naphthalen-1-ymethylene)ethane-1,2-diamine | 29.9 | 1.0 ×10-1 -5.0 ×10-6 | Na+, K+, Ba2+, Co2+, Ag+, Zn2+ | 114 |
| Cu2, Ni2+-1 | two Schiff base ligands: N-[2-thienylmethylidene]-2-propanolamine (TNAIIP) and N-[-2-thienylmethylidene]aminopropane (TNAP) | 29 | 1.0×10-6-1.0×10-2 | Cd2+, Fe3+ | 115 |
| Cu2, Ni2+-2 | Electrodes 1 and 2 are based on mixed complexes of Cu(II) and Ni(II) with N-[2-thienylmethylidene]-2-aminopyridine as ligand and electrodes 3 and 4 are based on the mixed complexes with N,N′- [2,2′-bis-thienylmethylidene]tolylene | 29 | 1.0×10-5-1.0×10-2 | Co2+, Fe3+ | 116 |
| Cu2, Ni2+-3 | N-[2-thienylmethilidene]-2-aminoethanol (TNAHE) | 29 and 29 | 10-6-10-1 and 10-5-10-1 | - | 117 |
| Cu2+-1 | naphthol-derivative Schiff's base | 29± 1 | 5.0×10-6-5.0×10-2 | Na+, Hg2+, Ni2+ | 118 |
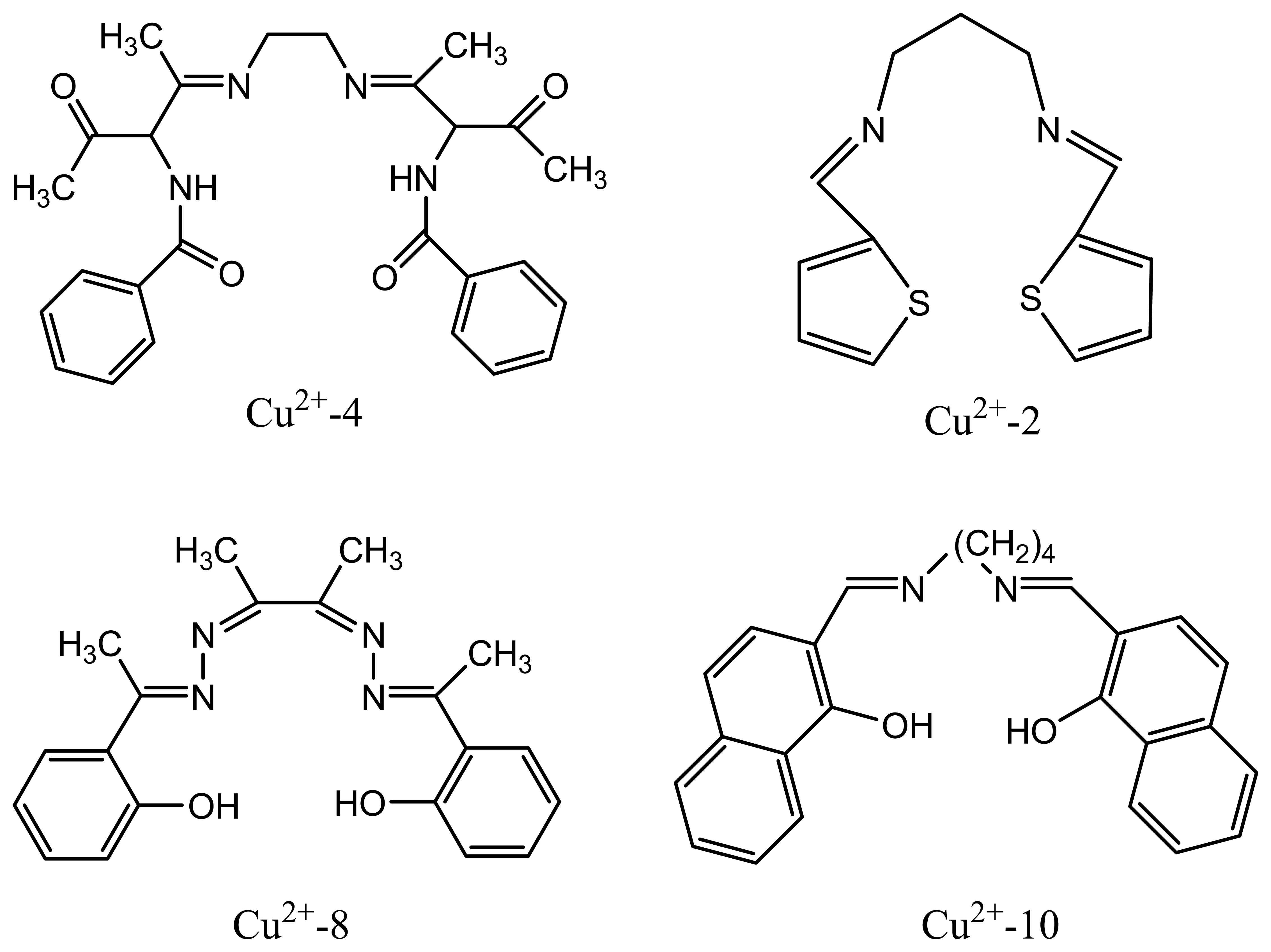
| Cu2+-2 | bis-2-thiophenal propanediamine (TPDA) | 29.1 | 1.0×10-1- 6.0×10-8 | Ag+ | 119 |
| Cu2+-3 | new thiophene-derivative Schiffs base | 29.3± 0.7 | 6.0×10-8- 1.0×10-1 | Zn2+, Hg2+ | 120 |
| Cu2+-4 | diphenylisocyanate bis(acetylacetone) ethylenedinnine (DIBAE) | 29.8 | 1.0×10-1 -1.0 ×10-6 | - | 121 |
| Cu2+-5 | 2,2′- [4,4′diphenyl-methanebis-(nitrilomethylidyne)]-bisphenol | 29 | 8.0× 10-6-1.0× 10-1 | Hg2+ | 122 |
| Cu2+-6 | 2,2-[1,2-ethandiyl-bis(nitrilomethylidine)-bis] m-cresol-(l),2,2-[1,2-ethandiyl-bis(nitrilomethylidine)-bis] p-cresol(II) and 2,2′- [1,2-ethandiyl-bis(nitritomethylidine)-bis]ortho cresole(III) | 29.2± 0.3, 29.7 ± 0.3 and 28.2 ± 0.3 | 1.0×10-5-1.0 ×10-1 | Ni2+, Co2+, Pb2+ | 123 |
| Cu2+-7 | N,N′-bis-pyridin-2-ylmethylene-naphthalene-1,8-diamine | 29.6 | 10-6 - 10-2 | Cu2+ | 124 |
| Cu2+-8 | Bis(2-hydroxyacetophenone)-butane-2,3-dihydrazone (BHAB) | 29.6 | 5.0×10-8-1.0×10-2 | - | 125 |
| Cu2+-9 | Copper (II) complex of 2,4-dimethyl-1,5,9,12-tetraazacyclopentadeca-1,4-diene | 29.9 | 1.1×10-6 -1.0×10-1 | Co2+, Mn2+, Ni2+ | 126 |
| Cu2+-10 | 2-(1′-(4′-(1″-hydroxy-2′-naphthyl)-methyleneamino)butyI imino-methyl)-1-naphthol (BHNB) as a novel Schiff base | 29 | 10-6-10-1 | Tl+ | 127 |
| Cu2+-11 | Schiff Base complexes, derived from 2,3-diaminopyridine and omicron-vanilin | 29.6 | 5.0×10-6 to 1.0×10-1 | Hg2+, Ag+ | 128 |
| Cu2+-12 (micro) | a symmetrical hexadentate Schiffs base 2-{1-(E)-2-((Z)-2-{(E)-2-[(Z)-1-(2-hydroxyphenyl)ethylidene] hydrazono)-1-methylpropylidene)-hydrazono]ethyl)phenol (HDNOS) | 25.9 ± 0.3 | 1.0×10-11-1.0×10-5 | Ni2+, Pb2+, Cd2+ | 129 |
| Cu2+-13 | copper(II) salicylaniline Schiffs base | 30 | 10-6-10-2 | Li+,Na+,Cs+,k+ | 130 |
| Cu2+-14 | naphthol-derivative Schiff's base | 29.5 | 5.0×10-6-5.0×10-2 | Hg2+,Ni2+,Na+ | 131 |
| Zn2+-1 | N,N′-bis(acetylacetone)ethylenediimine | 30.0 | 1.0× 10-6 -1.0×10-1 | Cu2+, Al3+, Cd2+ | 132 |
| Ag+-1 | Schiff base-p-tert-butylcalix[4]arene | 59.7 | 1.0×10-5-.0×10-1 | Hg2+ | 133 |
| Ag+-2 | a dioxime-type Schiff base, N,N′-bis(2′-hydroxyimino-1′-phenyl-propylidene)-1,3-propanediamine, PHO3, derived from α-isonitrosopropiophenone and 1,3-diaminopropane | 59 | 10-6..3 -10-1.1 | Hg2+ | 134 |
| Ag+-3 | Schiff base p-tert-butyl calix-[4]arene derivatives containing N and O as binding sites | 59 | 1.0×10-5-.0×10-1 | Hg2+ | 135 |
| Ag+-4 | calix[4]arene compound of 5,11,17,23-tetra-tert-butyl-25,27-dihydroxy-calix[4]arene-thiacrown-4 | 53.8 ± 1.6 | 1.0×10-6-1.0×10-2 | Hg2+ | 136 |
| Ag+-5 | calix[4] arene derivative | 58.9 | 1.0×10-5-1.0× 10-1 | K+, NH4+, Na+ | 137 |
| Ag+-6 | [bis 5-(4-nitrophenyl azo)salisylaldimine] 1,8-diamino, 3,6-dioxooctane (BNSAO) | 56.2 and 58.4 | 1.9 × 10−6 − 2.7 × 10−2 and 9.0 × 10−7 − 3.1 × 10−2 | K+, NH4+ | 138 |
| Ag+-7 | 5,11,17,23-tetra-tert-butyl-25,27-dihydroxy-calix[4]arene-thiacrown-4 | 53.8 ± 1.6 | 1.0×10-6-1.0×10-2 | Hg2+ | 139 |
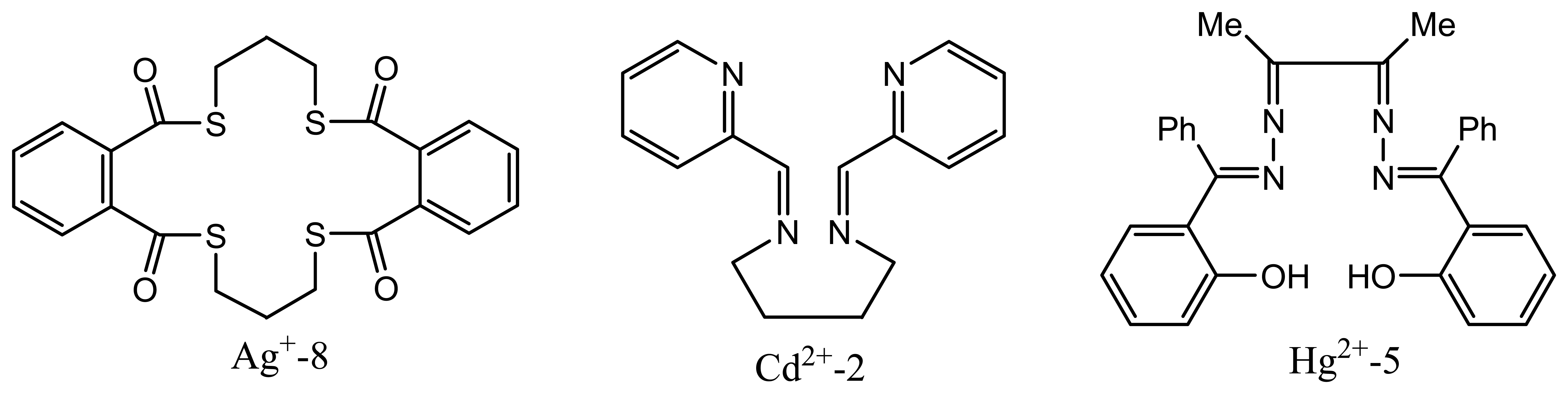
| Ag+-8 | 7,8: 16,17-dibenzo- 6,9,15,18-tetraoxo- 1,5,10,14-tetrathiacyclooctadeca-7,16- diene [Bz(2)Oxo(4)(18) dieneS(4) | 58.4 | 1.26×10-6 -1.0×10-1 | Tl+, Hg2+, Pb2+ | 140 |
| Cd2+-1 | 5-[((4-Methyl phenyl) azo)-N-(6-amino-2-pyridin) salicylaldimine] (S-1), and 5-[((4-methyl phenyl) azo)-N-(2-diamino-2-cyano-1-ethyl cyanide) salicylaldehyde] (S-2) | 28 and 22 | 1.5×10-1-7.5×10-7 and 2.0×10-1-4.0×10-7 | Pb2+, Ni2+ | 141 |
| Cd2+-2 | N,N′- [bis(pyridm-2-yl)-formylidene] butane- 1,4-diamine (SI) and N-(2-pyridinylmethylene)-1,2-benzenediamine (S-2) | 29.5 | 7.9 ×10-8-1.0 × 10-1 | Cu2+NH4+Cr3+ | 142 |
| Hg+2,Cu+2 -1 | Tetraethylthiuram disulfide was chosen as a chemical modifier | 79.4 and 43.1 | 10-7-10-3and 10−7.18- 10−3.67 | Mg2+, Al3+ | 143 |
| Hg2+-1 | sulfur Schiff's base 1-(2-hydroxy-1,2-diphenylethylidene)thiosemicarbazide (HDPET) | 29 | 1.0×10-6 -2.0×10-2 | Ag+ | 144 |
| Hg2+-2 | bis[5-((4-nitrophenyl)azo salicylaldehyde)] (BNAS) | 30 | 5.0 ×10-2 -7.0× 10-7 | - | 145 |
| Hg2+-3 | ethylenediamine bisthiophene-carboxaldehyde (EDBT) | 30.0 ± 0.4 | 10-7-10-2 | Ag+ | 146 |
| Hg2+-4 | Ethyl-2-(benzoylamino)-3-(2-hydroxy-4-methoxyphenyl)-2-propenoate (EBHMP) | 48.5 ± 1.0 | 3.0 × 10-7-3.1× 10-2 | Cu2+,Cd2+, Pb2+ | 147 |
| Hg2+-5 | bis(2-hydroxybenzophenone) butane-2,3-dihydrazone (HBBD) | 29.7 | 1.0 × 10-6-1.0× 10-1 | Fe3+,Cd2+, La3+ | 148 |
| SCN--1 | tricoordinate Schiff base copper(II) complex | -57.6 | 2.6×10-6 -1.0×10-1 | - | 149 |
| SCN--2 | two zinc(II) ions and two molecules of the bis-N,O-bidentate Schiff base 2,2′- [methylenebis(4,1-phenylenenitrilomethylidyne)]bisphenol | -57.5 | 10-3.5 - 10-2 | N3-, NO2- | 150 |
| SCN--3 | cadmium-Schiff's base complex | -57 | 1.0×10-5- 1.0×10-1 | 151 | |
| SCN--4 | (N,N′-bis-salicylidene-1,2-ethylenediamine) | -59.1 | 10-6-10-1 | MnO4-, I- | 152 |
| SCN--5 | 2.2-[(1,3-dimethyl-1,3-propanediylidene)dinitrilo]bis-benzenethiolato cadmium(II) | -58.9 | 10-6-10-1 | MnO4-, ClO4-, Br- | 153 |
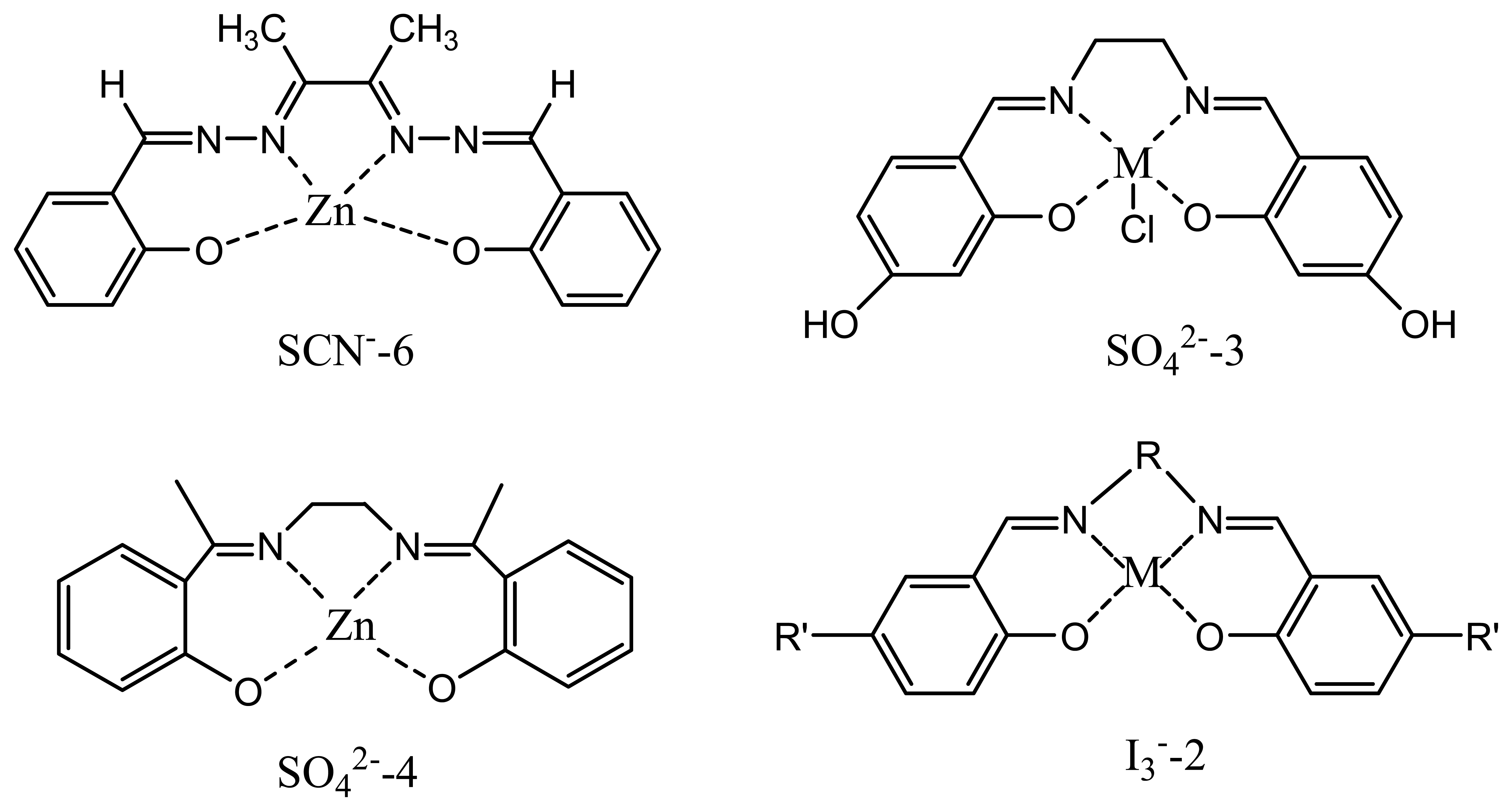
| SCN--6 | butane-2,3-dione bis(salicylhydrazonato) zinc(II) | -56.5 | 10-6-10-1 | ClO4- | 154 |
| SO42--1 | Schiff base complex of Zn(II) | -29.7 and -29.3 | 5.0×10-5-10-1 | SCN-, ClO4- | 155 |
| SO42--2 | strontium Schiff's base complex (SS) | -29.2 | 10-2-10-6 | SO32-, CO32-, Cl- | 156 |
| SO42--3 | zinc-Schiff base | -29.2 | 10-2-10-6 | - | 157 |
| SO42--4 | N,N′-ethylenebis(5-hydroxysalicylideneiminato) chromium(III) Chloride | -28.9 | 1.5 × 10-6-4.8× 10-2 | I-, HPO4- | 158 |
| F--1 | Gallium(III)-Schiff base | -61.1 | 1.0×10-5-1.0×10-1 | SCN- | 159 |
| Cl--1 | ruthenium(III) Schiff's base | -54.5 | 1.0×10-1-3.0×10-6 | - | 160 |
| Cl--2 | Schiff base complex of cobalt(II) | -59 | 2.5×10-5-0.5×10-1 | Br- | 161 |
| I--1 | Schiff base complex of Fe(III) | -71.0 | 1.0×10-6 -5.0 × 10-1 | SCN-, F-, NO2- | 162 |
| I--2 | [5,10,15,20-tetrakis(4-N,N-dimethylaminobenzene)porphyrinato]Mn(III) acetate | -59.4 | 1.0 ×10-2-7.5 × 10-6 | Salicylate, SCN-, ClO4- | 163 |
| I3--1 | bis (salicylaldehyde) ethylenediamine mercury(II) complex MS) | -59.0 | 5.0 × 10-8-10 × 10-2 | - | 164 |
| I3--2 | N,N′-1,2-propylene-bis-(5-methyl salicylidene iminato) copper(H) | -61.4 | 4.0 ×10-5 -0.7× 10-1 | I-, SCN-, ClO4- | 165 |
| I3--3 | a Charge-Transfer Complex of (1,3-diphenyldihydro-1H-Imidazole-4,5-dione dioxide with Iodide | -59.3 | 10-7-10-1 | - | 166 |
| I3--4 | 2-(((2(((E)-1-(2-hydroxyphenyl) methylidine)amino)phenyl) imino) methyl)phenol with iodine (CTC) | -59 | 5.0 × 10-8-1.0× 10-2 | - | 167 |
| I3--5 | Schiff base 2,2′ [4,4′-diphenyl-methane bis (nitromethylidyne)] bisphenol, L, with copper (II) and schiff base 2,2′ [4,4′-diphenyl-methane bis (nitromethylidyne)] bisphenol, L, with iron (III) | -60 | 6.0×10-6- 8.0×10-1 and 5.0×10-5 -1.0×10-1 | SCN-, Salicylate | 168 |
| I3--6 | bis-N,O-bidentate Schiff base | -61.4 | 4.0×10-5- 0.7×10-1 | I- | 169 |
| Salycilate | Schiff base complexes of Co(III) | -57.2 | 1.6 ×10-6-1.0 × 10-1 | SCN- | 170 |
| Salycilate | the complex N,N′-1,4-butylene bis(3-methyl salicylidene iminato) copper(II) | -59.1 | 1.0 × 10-6-1 | - | 171 |
| Cysteine | N,N′-bis(salicylidene)-1,2-phenylenediaminocobalt(II) | -59 | 2.0 ×10-6-1.0 × 10-2 | - | 172 |
| Cation | Ionophore | Slope (mV decade-1) | Linear Range (M) | Most Important Interfering ions (log Ksel> -2) | Ref. |
|---|---|---|---|---|---|
| NO2--1 | Co(II)-Salen | -58.2 | 10-6-10-1 | - | 174 |
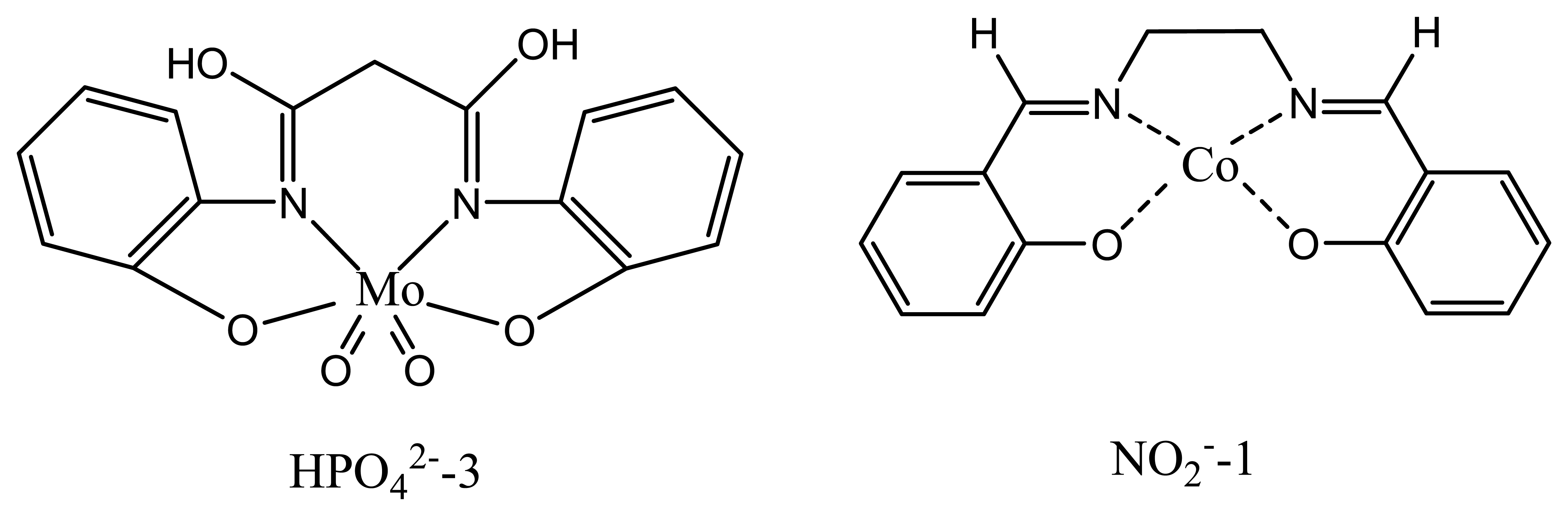
| HPO42--1 | Vanadyl salen | -28.8 | 1.0×10-1-5.0×10-6 | - | 175 |
| HPO42--2 | oxo-molybdenum methyl-salen | -28.6 | 1.0×10-1 - 4.0×10-7 | - | 176 |
| HPO42--3 | oxo-molybdenum methyl-salen (MS) | -28.6 | 1.0×10-1-4.0×10-7 | - | 177 |
| Br--1 | iron(III)-salen (IS) | -59.0 | 7.0×10-6-1.0×10-1 | SCN-, I-, Cl- | 178 |
| I--1 | salen-Mn(II) | -59.0 | 1.0×10-5-3.4 ×10-1 | CN- | 179 |
| I--2 | Cerium-salen | -57.5 | 5.0×10-2 -8.0×10-6 | SCN- | 180 |
| I3--1 | Mercury-salen | -59.0±0.5 | 5.0×10−8−1.0×10−2 | - | 181 |
| Cation | Ionophore | Slope (mV decade-1) | Linear Range (M) | Most Important Interfering ions (log Ksel> -2) | Ref. |
|---|---|---|---|---|---|
| Al+3-1 | NM-bis(salicylidene)-1,2-phenylenediamine (salophen) | 20.1 | 8.0 ×10-7 -3.0× 10-2 | Cu2+, Cr3+ | 182 |
| NO2--1 | Co(II)-salophen | -59.8 | 10-6-10-1 | - | 183 |
| NO2--2 | Uranyl salophen | 56.2 | 10-3 -10-1 | - | 184 |
| H2PO4--1 | Uranyl salophenes | -59 | 10-4-10-1 | - | 185 |
| HPO42--2 | vanadyl salophen | -24.3 | 10-1-10-6 | - | 186 |
| F--1 | uranyl salophen derivatives | -56 | 1.0×10-4-1.0×10-1 | - | 187 |
| I--1 | cobalt-salophen | - 58.9 | 5.0 ×10-7-1.0×10-1 | - | 188 |
| Cation | Ionophore | Slope (mV decade-1) | Linear Range (M) | Most Important Interfering ions (log Ksel > -2) | Ref. |
|---|---|---|---|---|---|
| La3+-1 | N,N′-adipylbis(5-phenylazosalicylaldehyde hydrazone) | 19.4 | 10-6 -10-2 | Yb3+ | 189 |
| La3+-2 | Bis(2-methylbenzaldehyde)-butane-2,3-dihydrazone (TDSB) | 19.8 | 1.0 × 10-5-1.0 × 10-1 | Cu2+, Ce3+, Pr3+ | 190 |
| La3+-3 | N-[hexahydrocyclopenta-pyrol-2((1H)yl)amino]-carbonyl]-4-methylbenzene sulfonamide | 20.1 | 10−6-10−1 | Sm3+, Ce3+ | 191 |
| La3+-4 | 3-hydroxy-N′-(pyridin-2-ylmethylene)-2-naphthohydrazide (HPMN) | 19.2 | 10−7-10−2 | Yb3+, Ce3+ | 192 |
| La3+-5 | 8-amino-N-(2-hydroxybenzylidene)naphthylamine (AIP) | 20.3 ± 0.3 | 1.0 × 10-7-1.0 × 10-1 | Pr3+ | 193 |
| La3+-6 | N′-(1-pyridin-2-ylmethylene)-2-furohydrazide (NPYFH) | 19.2 ± 0.6 | 1.0 × 10-6-1.0 × 10-1 | Sm3+, Nd3+ | 194 |
| Pr+3-1 | N-(pyridin-2-ylmethylene)benzohydrazide | 21.1 | 10−2-10−6 | Sm3+, Er3+ | 195 |
| Nd+3-1 | N-(2-furylmethylene) pyridine-2,6-diamine | 19.6 | 10-5-10-2 | La3+, Gd3+, Sm3+ | 196 |
| Nd+3-2 | 2-{[(6-aminopyridin-2-yl)imino]methyl}phenol | 19.6 | 10-5-10-2 | La3+, Sm3+ | 197 |
| Sm3+-1 | 3-{[2-oxo-1(2H)-acenaphthylenyliden]amino} -2-thioxo-1,3-thiazolidin-4-one | 19.3 | 10-6-10-1 | - | 198 |
| Eu3+-1 | bis(thiophenol)butane2,3-dihydrazone | 19.8 | 1.0 × 10-5-1.0 × 10-2 | La3+, Gd3+, Sm3+ | 199 |
| Er+3-1 | Pyridine-2-carbaldehyde-2-(4-methyl-1,3-benzothiazol-2-yl)hydrazone | 21.8 | 10-5-10-2 | Sm3+, Tm3+,Gd3+ | 200 |
| Er+3-2 | N′-(2-Hydroxy-1,2-diphenyl-ethylidene) benzohydrazide (HDEBH) | 21.0 | 10-7-10-2 | Ho3+ | 201 |
| Er+3-3 | N′-(2-Hydroxy-1,2-diphenyl- ethylidene) benzohydrazide (HDEBH) | 17.5 (microelectrode) | 1.0×10-3-3.0×10-10 | - | 202 |
| Tm+3-1 | thiophene-2-carbaldehyde-(7-methyl-1,3-benzothiazol-2-yl)hydrazone | 19.5 | 10-5–10-2 | Er3+ | 203 |
| Yb+3-1 | 3-hydroxy-N′- [(2-hydroxyphenyl)methylene]-2-naphthohydrazide | 19.2 | 10−7-10−2 | Nd3+, Pb2+,Gd3+ | 204 |
| Hg2+-1 | bis(2-hydroxybenzophenone) butane-2,3-dihydrazone (HBBD) | 29.7 | 1.0 × 10-6-1.0 × 10-1 | Fe3+,Cd2+, La3+ | 205 |
| I3--1 | complex of iodine and bis(2-hydroxyacetophenone)-butane-2,3-dihydrazone.” | -58. 99 ± 0.3 | 1.0×10-2 - 5.0×10-7 | - | 206 |
| I3--2 | bis(2,4-dimethoxybenzaldehyde)butane-2,3-dihydrazone with iodine | -60.6 | 10-7-10-2 | - | 207 |
| I3--3 | bis(2-hydroxyacetophenone)butane-2,3-dihydrazone. | -58.99 | 1.0×10-2 - 5.0×10-7 | - | 208 |
| Compound | Size of the Cavity (Å) |
|---|---|
| 15-crown-5 | 1.7-2.2 |
| 18-crown-6 | 2.6-3.2 |
| 21-crown-7 | 3.4-4.3 |
| Cryptand 111 | 1.0 |
| Cryptand 211 | 1.6 |
| Cryptand 221 | 2.2 |
| Cryptand 222 | 2.8 |
| Cryptand 322 | 3.6 |
| Cryptand 332 | 4.2 |
| Cryptand 333 | 4.8 |
| Cation | Ionophore | Slope (mV decade-1) | Linear Range (M) | Most Important Interfering ions (log Ksel> -2) | Ref. |
|---|---|---|---|---|---|
| Li+-1 | Decalino-14-Crown-4 | 58 | 1×10-6 − 1 | Na+ | 211 |
| Li+-2 | Tnf-Based 16-Crown-4 Derivatives | 58.2 | 1×10-5 -1×10-1 | Na+,NH4+ | 212 |
| Li+-3 | Lipophilic Crown-4 Derivatives | 58 | 10-4-10-1 | Na+, K+,NH4+ | 213 |
| Li+-4 | 16-crown-4 derivatives | 59.7 | 7×10-4 - 1.5×10-3 | - | 214 |
| Na+-1 | Bis[(12-crown-4)methyl] methyl(dodecyl)malonate [bis(12-crown-4)] and polyether-amide compounds (ETH 157, ETH 2120) | 54 | 1.0×10-4-1.0×10-1 | K+ | 215 |
| Na+-2 | Bis(10-crown-3)-hexamethylenebis(3,6,10-trioxacycloundecane) | 54 | 1×10-5-1 | K+ | 216 |
| Na+-3 | Crown-bridged calix[4]quinones | 54 | 1.0×10-4-1.0×10-1 | K+ | 217 |
| Na+-4 | dibenzopyridino-18-crown-6 | 51.2 | 1.0×10-4-1.0×10-1 | Pb2+, NH4+, Cs+ | 218 |
| Na+-5 | 1-methyl-1-vinyl-14-crown-5 | 55.0 | 3.16×10-6 -1.0×10-1 | K+ | 219 |
| K+-1 | styrene/4(-vinylbenzo-24-crown-8) copolymer | 58 | 1.0×10-6 1.0×10-1 | - | 220 |
| K+-2 | Bis(crown ether) ionophore containing two benzo-15-crown-5 moieties | 57 | 5.0×10-6 1.0×10-1 | NH4+, Na+, Cs+ | 221 |
| Rb+-1 | crown ethers incorporating anthraquinone, benzoquinone, and 1,4-dimethoxybenzene | 54.7 | 1.0×10-5 -1.0×10-1 | Na+,K+,Mg2+,NH4+,Li+ | 222 |
| Rb+-2 | dibenzo-21-crown-7 (DB21C7) | 57.8 | 1.0×10-5 -5.0 ×10-2 | - | 223 |
| Cs+-1 | upper-rim calix[4]crown | 48 | 1×10-6 -1×10-1 | Rb+ | 224 |
| Cs+-2 | calix[4]arene dibenzocrown ether | 58.5 | 1×10-6 -1 × 10-1 | Na+,K+ | 225 |
| Cs+-3 | 1,3-alternate thiacalix[4]biscrown-6,6 | 57.6 | 1.0×10-6 to 3.2×10-2 | K+ | 226 |
| Be2+-1 | benzo-9-crown-3 | 28 | 2.5×10-6-4.0×10-3 | - | 227 |
| Be2+-2 | 2,4-dinitrophenylhydrazineben zo-9-crown-3 | 29.5 | 1.0×10-1- 4.0× 10-7 | - | 228 |
| Be2+-3 | naphto-9-crown-3 | 29.5 | 1.0×10-1- 8.0×10−6 | - | 229 |
| Be2+-4 | 2,3,5,6,8,9-hexahydro-1,4,7,10-benzotetra oxacyclododecine-12-carbaldehyde-12-(2,4-dinitrophenyl)hy | 29.9 | 1.0×10-7-1.0 × 10-1 | Na+, Ca+2, Li+, | 230 |
| Be2+-5 | 2,6-diphenyl-4-benzo-9-crown-3-pyridine | 29.6 | 1.0×10−7-1.0×10−1 | Mg+2,Ca+2,K+,Na+ | 231 |
| Be2+-6 | 2,3,5,6,8,9-hexahydronaphto [2,3-b]- [1,4,7,10] tetraoxacyclododecine | 29.8 | 1.0×10−8-1.0×10−2 | - | 232 |
| Be2+-7 | a derivative of benzo-9-crown-3 | 29.6 | 1.0×10−6-1.0×10−1 | - | 233 |
| Be2+-8 | a derivative of benzo-9-crown-3 | 29.5 | 1.0×10−7-1.0×10−1 | - | 234 |
| Mg2+-1 | benzo-15-crown-5 | 31.0 | 1.0×10-5 -1.0×10-1 | K+, Cd2+. Mg2+, | 235 |
| Sr+2-1 | dibenzo-24-crown-8(I) and 4-tert-butylcalix[8]arene | 30 | 1.4×10-5-1.0×10-1 | Na+ | 236 |
| Sr+2-2 | benzo-substituted macrocyclic diamides | 30 | 3.2×10-5-1.0×10-1 | - | 237 |
| In3+-1 | 15-crown-5 (15C5), dicyclohexano-18-crown-6 (DCH18C6) | 20.1 | 3.8×10-5 -5.0×10-2 | - | 238 |
| Tl3+-1 | dibenzyldiaza-18-crown-6 | 56.9 | 1.0×10-5-1.0×10-1 | Cd2+, Hg2+, Ag+ | 239 |
| Sn2+-1 | dibenzo-18-crown-6(DB18C6) | 27.5 | 1.0×10-6 -1.0×10-2 | - | 240 |
| Pb2+-1 | monobenzo-15-crown-5 (MB15C5), MB15C5-phosphotungstic acid (PW) and MB15C5-phosphomolybdic acid (PMo) | 30 | 1×10-1 -1×10-5 | Ag2+, Hg2+ | 241 |
| Pb2+-2 | dibenzodiaza-15-crown-4 | 29 | 5.0×10-6-1.0×10-2 | - | 242 |
| Pb2+-3 | 4′-vinylbenzo-15-crown-5 | 59 | 4.0 ×10-3- 1.0 ×10-6 | K+ | 243 |
| Pb2+-4 | dithiophenediazacrown ether derivatives. | 29.2 | 10 5.0- 10 2.7 | Hg2+ | 244 |
| Pb2+-5 | 1,10-dibenzyl-1,10-diaza-18-crown-6 | 29.1and 28.9 | 5.0× 10-6-10-1 | Cd2+, Cu2+ | 245 |
| Pb2+-6 | 18-membered thiacrown derivative | 29.0 | 1.0× 10-6-8.0× 10-3 | - | 246 |
| Pb2+-7 | N,N′-dimethylcyanodiaza-18-crown-6 | 29 | 10-7-10-2 | - | 247 |
| La3+-1 | monoaza-12-crown-4 | 20.5 ± 1.0 | 3.16 ×10-5-1.0×10-1 | Pb2+,Mg2+,Ca2+, Cu2+,Zn2+,Cd2+, Cr3+,Ce3+,Eu3+ | 248 |
| Ce3+-1 | 1,3,5-trithiane | 19.2 | 4.7 × 10-4-2.5 ×10-8 | La3+ | 249 |
| Cr3+-1 | Different ionophoric species, viz.: 18-crown-6 (18C6), dibenzo-18-crown-6 (DB18C6) and calix[6]arene (CAX) | 18.5and 20 | 1.0×10-5 - 1.0×10-1 | Pb2+ and Na+ | 250 |
| Fe3+-1 | benzo-18-crown-6 crown ether | 15.7±1 | 1×10-6-1.0×10-1 | - | 251 |
| Co2+-1 | benzo-substituted macrocyclic diamide | 29 | 2.0×10-6-1.2×10-2 | - | 252 |
| Co2+-2 | dibenzopyridino-substituted macrocyclic diamide | 29 | 7.0×10-7-1.0×10-2 | - | 253 |
| Ni2+-1 | dibenzodiaza-15-crown-4 | 28.6 | 7.1×10-7-1.2×10-2 | Ag+, Pd2+ | 254 |
| Cu2+-1 | Aza-thioether crowns containing a 1,10-phenanthroline sub-unit | 30 | 1×10−5-2×10−1 | La+3 | 255 |
| Cu2+-2 | 23-member macrocyclic diamide | 30 | 3.2×10−5-1.0×10−1 | - | 256 |
| Ag+-1 | Lipophilic pyrrole-based tetraazacrown ether | 55 | 1.0×10-5-1.0×10-1 | Hg2+ | 257 |
| Ag+-2 | diaza-18-crown-6, containing two oxime donor groups | 59.5 | 1.0×10-5 -1.0×10-2 | - | 258 |
| Ag+-3 | hexathia-18-crown-6 | 59 | 6.0×10-6-3.2×10-3 | - | 259 |
| Ag+-4 | Aza-thioether crowns containing a 1,10-phenanthroline sub-unit | 59.4 and 59.1 | 1.0×10-5-1.0×10-1 and 5.0×10-8-4.0×10-2 | Cu2+, Tl+ | 260 |
| Ag+-5 | exocyclic sulfur and selenium ligands based on calix[4]arenes and crown ethers | 56.0 | 1.0×10-5-1.0×10-2 | Hg2+ | 261 |
| Ag+-6 | 5,11,17,23-tetra-tert-butyl-25,27-dihydroxy-calix[4]arene-thiacrown-4 | 53.8 ± 1.6 | 1.0×10-6- 1.0×10-2 | Hg2+ | 262 |
| Ag+-7 | 1,10-phenanthroline subunit | 56 | 1.0×10-5- 1.0×10-1 | Hg2+ | 263 |
| Zn2+-1 | cryptand C2(B)22 | 24 | 5.0×10-5-5.0×10-2 | Na+ | 264 |
| Zn2+-2 | dibenzo-24-crown-8 | 29.0±0.5 | 9.2×10-5-1.0×10-1 | - | 265 |
| Zn2+-3 | benzo-substituted macrocyclic diamide | 29.0 | 9.0×10-5-1.0×10-1 | - | 266 |
| Cd2+-1 | dicyclohexano-24-crown-8 | 30.0 ± 1.0 | 3.0×10-5 -1.0×10-1 | Fe3+, Cr3+, Ce3+ | 267 |
| Cd2+-2 | dicyclohexano-18-crown-6 | 29.0 ±1.0 | 2.5×10-5-1.0×10-1 | - | 268 |
| Cd2+-3 | tetrathia-12-crown-4 | 29.0 ±1.0 | 4×10-7-1.0×10-1 | - | 269 |
| Cd2+-4 | monoaza-18-crown-6 | 29 | 1.0×10-5-1.0×10-1 | Cu2+,Na+, Ca2+ | 270 |
| Hg2+-1 | p-tert-butyl calix[4]crown with imine units | 27.3 | 5.0×10-5-1.0×10-1 | - | 271 |
| Hg2+-2 | Pentathia-15-crown-5 | 32.1 | 2.51×10-7-1.0×10-1 | 272 | |
| Hg2+-3 | dibenzodiazathia-18-crown-6-dione | 29 | 8.0×10-6-1.0×10-2 | Cd2+, Pb2+, K+ | 273 |
| Hg2+-4 | hexathia-18-crown-6-tetraone | 29.0 ±0.3 | 4.0×10-6-1.0×10-3 | - | 274 |
| I3--1 | Two different charge-transfer complexes and amino crown ether | -59 | 1.0×10-5 - 1.0×10-1 | - | 275 |
| SCN--1 | Cu(II)-1,8-dimethyl-1,3,6,8,10,13-hexaazacyclotetradecane complex | -57.2 | 7.0×10-6 - 1.0×10-1 | ClO4- | 276 |
| SCN--2 | Ni(II)-azamacrocycle complex | -57.8 | 1.0×10-7 - 1.0×10-1 | - | 277 |
| SO42--1 | 2,5-diphenyl-1,2,4,5-tetraaza-bicyclo[2.2.1]heptane | -28.8 | 9.0×10-6 - 1.0×10-1 | - | 278 |
© 2008 by MDPI Reproduction is permitted for noncommercial purposes.
Share and Cite
Faridbod, F.; Ganjali, M.R.; Dinarvand, R.; Norouzi, P.; Riahi, S. Schiff's Bases and Crown Ethers as Supramolecular Sensing Materials in the Construction of Potentiometric Membrane Sensors. Sensors 2008, 8, 1645-1703. https://doi.org/10.3390/s8031645
Faridbod F, Ganjali MR, Dinarvand R, Norouzi P, Riahi S. Schiff's Bases and Crown Ethers as Supramolecular Sensing Materials in the Construction of Potentiometric Membrane Sensors. Sensors. 2008; 8(3):1645-1703. https://doi.org/10.3390/s8031645
Chicago/Turabian StyleFaridbod, Farnoush, Mohammad Reza Ganjali, Rassoul Dinarvand, Parviz Norouzi, and Siavash Riahi. 2008. "Schiff's Bases and Crown Ethers as Supramolecular Sensing Materials in the Construction of Potentiometric Membrane Sensors" Sensors 8, no. 3: 1645-1703. https://doi.org/10.3390/s8031645




