SAW RFID-Tags for Mass-Sensitive Detection of Humidity and Vapors
Abstract
:1. Introduction
2. Results and Discussion
2.1. Instrumental Aspects
2.2. Humidity Sensor
2.3. Tetrachloroethylene Sensor
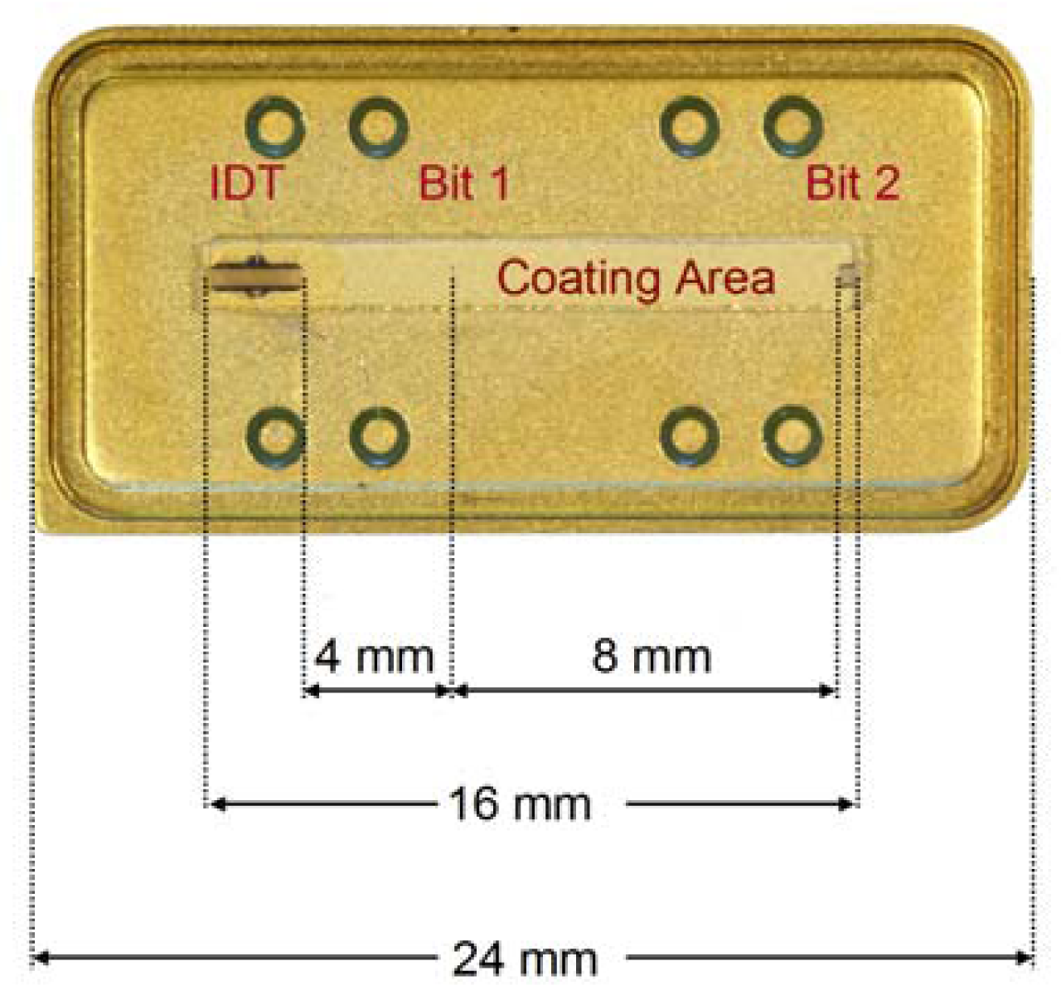
3. Experimental
3.1. Devices and Measurements
3.2. Sensor Materials
4. Conclusions
Acknowledgments
References
- Schiopu, P.; Cristea, I.; Grosu, N.; Craciun, A. Recent developments in surface acoustic wave sensors. Proc. SPIE 7297 2009. art. No. 72972G.. [Google Scholar]
- Wolff, U.; Dickert, F.L.; Fischerauer, G.K.; Greibl, W.; Ruppel, C.C.W. SAW Sensors for harsh environments. IEEE Sens. J. 2000, 1, 4–13. [Google Scholar]
- Lu, C.J.; Zellers, E.T. A dual-adsorbent preconcentrator for a portable indoor-VOC microsensor system. Anal Chem. 2001, 73, 3449–3457. [Google Scholar]
- Penza, M.; Aversa, P.; Cassano, G.; Wlodarski, W.; Kalantar-Zadeh, K. Layered SAW sensor with single-walled carbon nanotube-based nanocomposite coating. Sens. Actuat. B 2007, 127, 168–178. [Google Scholar]
- Dickert, F.L.; Forth, P.; Bulst, W.-E.; Fischerauer, G.; Knauer, U. SAW devices—sensitivity enhancement in going from 80 MHz to 1 GHz. Sens. Actuat. B 1998, 46, 120–125. [Google Scholar]
- Caliendo, C. Acoustic method of investigating the material properties and humidity sensing behavior of polymer coated piezoelectric substrates. J. Appl. Phys. 2006, 100. art. No. 054081. [Google Scholar]
- Penza, M.; Tagliente, M.A.; Aversa, P.; Cassano, G.; Capodieci, L. Single-walled carbon nanotubes nanocomposite microacoustic organic vapor sensors. Mat. Sci. Eng. C 2006, 26, 1165–1170. [Google Scholar]
- Springer, A.; Weigel, R.; Pohl, A.; Seifert, F. Wireless identification and sensing using surface acoustic wave devices. Mechatronics 1999, 9, 745–756. [Google Scholar]
- Janata, J. Principles of Chemical Sensors; Plenum Press: New York NY, USA,, 1989; pp. 69–75. [Google Scholar]
- Seifert, F.; Bulst, W.-E.; Ruppel, C.C.W. Mechanical sensors based on surface acoustic waves. Sens. Actuat. A 1994, 44, 231–239. [Google Scholar]
- Dickert, F.L.; Sikorski, R. Supramolecular strategies in chemical sensing. Mat. Sci. Eng. C 1999, 10, 39–46. [Google Scholar]
- Penza, M.; Cassano, G. Relative humidity sensing by PVA-coated dual resonator SAW oscillator. Sens. Actuat. B 2000, 68, 300–306. [Google Scholar]
- Dickert, F.L.; Hayden, O. Molecular imprinting in chemical sensing. TrAC 1999, 18, 192–199. [Google Scholar]
- Dickert, F.L.; Schuster, O. Mass sensitive detection of solvent vapors with calix[n]arenes. Conformational adaptation to the analyte. Adv Mater. 1993, 5, 826–829. [Google Scholar]







© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lieberzeit, P.A.; Palfinger, C.; Dickert, F.L.; Fischerauer, G. SAW RFID-Tags for Mass-Sensitive Detection of Humidity and Vapors. Sensors 2009, 9, 9805-9815. https://doi.org/10.3390/s91209805
Lieberzeit PA, Palfinger C, Dickert FL, Fischerauer G. SAW RFID-Tags for Mass-Sensitive Detection of Humidity and Vapors. Sensors. 2009; 9(12):9805-9815. https://doi.org/10.3390/s91209805
Chicago/Turabian StyleLieberzeit, Peter A., Christian Palfinger, Franz L. Dickert, and Gerhard Fischerauer. 2009. "SAW RFID-Tags for Mass-Sensitive Detection of Humidity and Vapors" Sensors 9, no. 12: 9805-9815. https://doi.org/10.3390/s91209805




