Polyester Sulphonic Acid Interstitial Nanocomposite Platform for Peroxide Biosensor
Abstract
:1. Introduction
2. Experimental
2.1. Reagents
2.2. Instrumentation
2.3. Fabrication of Biosensor
2.4. Biosensor Response
3. Results and Discussion
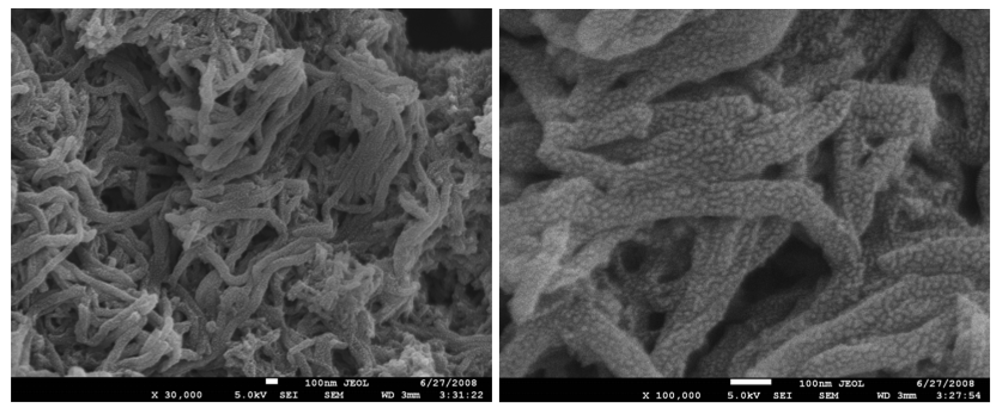
3.1. Characterization of PANI:PESA Composite
3.2. pH Response of Pt/PANI-PESA/HRP
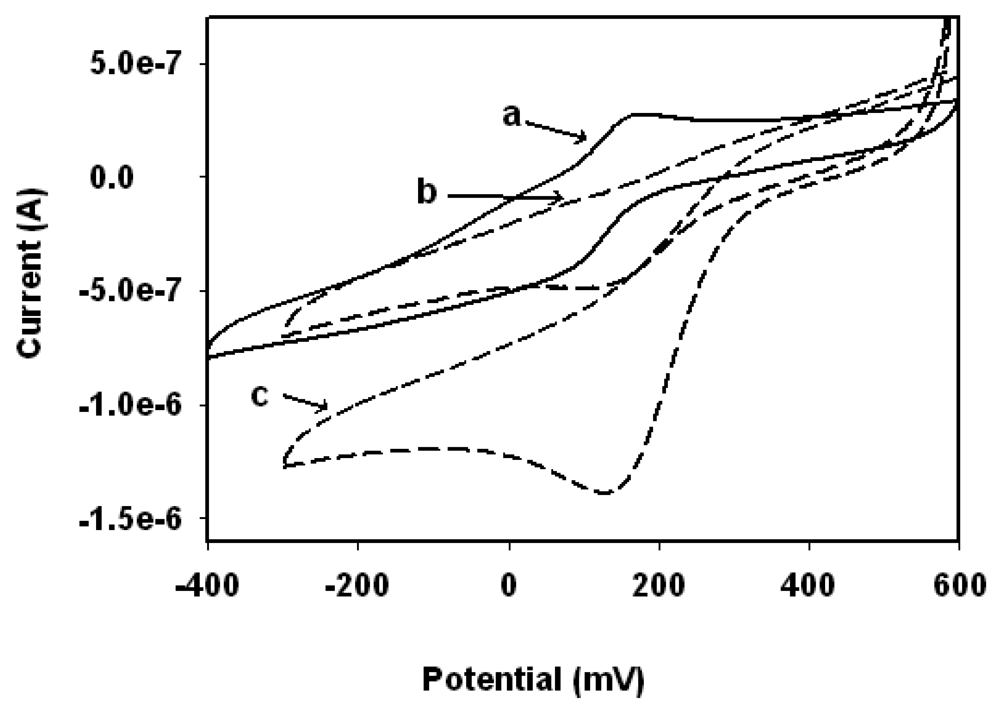
3.3. Biosensor Response to H2O2
3.4. Stability of the Biosensor
4. Conclusions
Reference and Notes
- Heeger, A.J. Semiconducting and metallic polymers: The fourth generation of polymeric materials. Synth. Met. 2002, 125, 23–42. [Google Scholar]
- Cao, Y.; Smith, P.; Heeger, A.J. Counter-ion induced processibility of conducting polyaniline. Synth. Met. 1993, 57, 3514–3519. [Google Scholar]
- Saxena, V.; Mathotra, B.D. Prospects of conducting polymers in molecular electronics. Curr. Appl. Phys. 2003, 3, 293–305. [Google Scholar]
- Pud, A.A. Stability and degradation of conducting polymers in electrochemical systems. Synth. Met. 1994, 66, 1–18. [Google Scholar]
- Min, G. Conducting polymers and their applications in the film industry—polyaniline/polyimide blended films. Synth. Met. 1999, 102, 1163–1166. [Google Scholar]
- Guiseppi-Elie, A.; Wallace, G.G.; Matsue, T. Handbook of Conducting Polymers; Skotheim, T.A., Ed.; Marcel Dekker: New York, NY, USA, 1997; Volume Chapter 34, p. 963. [Google Scholar]
- Atwood, J.; Steed, J. Encyclopedia of Supramolecular Chemistry; Atwood, J.; Steed, J. Taylor and Francis: New York, NY, USA, 2007; Volume 1, p. 1. [Google Scholar]
- Malhotra, B.D.; Turner, A.P.F. Advance in Biosensors: Perspectives in Biosensors; Volume 5, Elsevier Science: Amsterdam, The Netherlands, 2003; p. 186. [Google Scholar]
- Iwuoha, E.I.; Saenzde de Villaerde, D.; Garcia, N.P.; Smyth, M.R.; Pingarron, J.M. Reactivities of organic phase biosensors. 2. The amperometric behaviour of horseradish peroxidase immobilised on a platinum electrode modified with an electrosynthetic polyaniline film. Biosens. Bioelectron. 1997, 12, 749–761. [Google Scholar]
- Adhikari, B.; Majumder, S. Polymer in sensor application. Prog. Polym. Sci. 2004, 29, 699–766. [Google Scholar]
- Brahim, S.; Wilson, A.M.; Narinesingh, D.; Iwuoha, E.; Guiseppi-Elie, A. Chemical and biological sensors based on electrochemical detection using conducting electroactive polymers. Microchim. Acta 2003, 143, 123–127. [Google Scholar]
- Ramanavičius, A.; Ramanavičienė, A.; Malinauskas, A. Electrochemical sensors based on conducting polymer-Polypyrrole. Electrochim. Acta 2006, 51, 6025–6037. [Google Scholar]
- Chen, X.; Zhng, J.; Wang, B.; Cheng, G.; Dong, S. Hydrogen peroxide biosensor based on sol-gel-derived glasses doped with Eastman AQ polymer. Anal. Chim. Acta 2001, 434, 255–260. [Google Scholar]
- Michira, I.N.; Klink, M.; Akinyeye, R.O.; Somerset, V.; Sekota, M.; Al-Ahmed, A.; Baker, P.G.L.; Iwuoha, E.I. Recent Advances in Analytical Electrochemistry; Ozoemena, K.I., Ed.; Transworld Research Network: Kerala, India, 2007; p. 137. [Google Scholar]
- Michira, I.; Akinyeye, R.; Somerset, V.; Klink, M.J.; Sekota, M.; Al-Ahmed, A.; Baker, P.G.L.; Iwuoha, E. Synthesis, characterisation of novel polyaniline nanomaterials and application in amperometric biosensors. Macromol. Sympo. 2007, 255, 57–69. [Google Scholar]
- Iwuoha, E.I.; Mavundla, S.E.; Somerset, V.S.; Petrik, L.P.; Klink, M.J.; Sekota, M.; Baker, P. Electrochemical and spectroscopic properties of fly ash-polyaniline matrix nanorod composites. Microchim. Acta 2006, 155, 453–458. [Google Scholar]
- Mathebe, N.G.R.; Morrin, A.; Iwuoha, E.I. Electrochemistry and scanning electron Microscopy of Polyaniline/peroxidase-based biosensor. Talanta 2004, 64, 115–120. [Google Scholar]
- Al-Ahmed, A.; Mohammad, F.; Ab. Rahman, M.Z. Composites of polyaniline and cellulose acetat: Preparation, characterization and thermo-oxidative degardation and stability in terms of Dcelectrical conductivity retention. Synth. Met. 2004, 144, 29–49. [Google Scholar]
- Elkaoutit, M.; Naranjo-Rodriguez, I.; Dominguez, M.; Hernandez-Artiga, M.P.; Bellido-Milla, D.; De Cisneros, J.L.H.H. A third-generation hydrogen peroxide biosensor based on horsreadish peroxidease (HRP) enzyme immobilized in a Nafion-Sonogel-Carbon composite. Electrochim. Acta 2008, 53, 7131–7137. [Google Scholar]
- Rezaei-Zarchi, S.; Saboury, A.A.; Javed, A. Electrochemical study of horseradish peroxidase using the Nanosilver-modified graphite electrode and its application to hydrogen peroxide biosensor. J. New Mat. Elect. Syst. 2008, 11, 199–203. [Google Scholar]
- Chen, C.C.; Gu, Y. Enhancing the sensitivity and stability of HRP/PANI/Pt electrode by implanted bovine serum albumin. Biosens. Bioelectron. 2008, 23, 765–770. [Google Scholar]
- Luo, X.; Killars, A.J.; Morrin, A.; Smyth, M.R. In situ electropolymerized silica- polyaniline core-shell structures: Electrode modification and enzyme biosensor enhancement. Electrochim. Acta. 2007, 52, 1865–1870. [Google Scholar]
- Xu, Q.; Zhu, J.J.; Hu, X.Y. Ordered mesoporous polyaniline film as a new matrix for enzyme immobilization and biosensor construction. Anal. Chim. Acta 2007, 597, 151–156. [Google Scholar]
- Luo, X.; Killars, A.J.; Morrin, A.; Smyth, M.R. Enhancement of a conducting polymer -based biosensor using carbon nanotubes -doped polyaniline. Anal. Chim. Acta 2006, 575, 39–44. [Google Scholar]
- Chen, X.; Li, C.; Liu, Y.; Du, Z.; Xu, S.; Li, L.; Zhang, M.; Wang, T. Electrocatalytic activity of horseradish peroxidase/chitosan/carbon microsphere microbiocomposites to hydrogen peroxide. Talanta 2008, 77, 37–41. [Google Scholar]








© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Al-Ahmed, A.; Ndangili, P.M.; Jahed, N.; Baker, P.G.L.; Iwuoha, E.I. Polyester Sulphonic Acid Interstitial Nanocomposite Platform for Peroxide Biosensor. Sensors 2009, 9, 9965-9976. https://doi.org/10.3390/s91209965
Al-Ahmed A, Ndangili PM, Jahed N, Baker PGL, Iwuoha EI. Polyester Sulphonic Acid Interstitial Nanocomposite Platform for Peroxide Biosensor. Sensors. 2009; 9(12):9965-9976. https://doi.org/10.3390/s91209965
Chicago/Turabian StyleAl-Ahmed, Amir, Peter M. Ndangili, Nazeem Jahed, Priscilla G. L. Baker, and Emmanuel I. Iwuoha. 2009. "Polyester Sulphonic Acid Interstitial Nanocomposite Platform for Peroxide Biosensor" Sensors 9, no. 12: 9965-9976. https://doi.org/10.3390/s91209965
APA StyleAl-Ahmed, A., Ndangili, P. M., Jahed, N., Baker, P. G. L., & Iwuoha, E. I. (2009). Polyester Sulphonic Acid Interstitial Nanocomposite Platform for Peroxide Biosensor. Sensors, 9(12), 9965-9976. https://doi.org/10.3390/s91209965




