Ablation of Protein Kinase CK2β in Skeletal Muscle Fibers Interferes with Their Oxidative Capacity
Abstract
:1. Introduction
2. Results
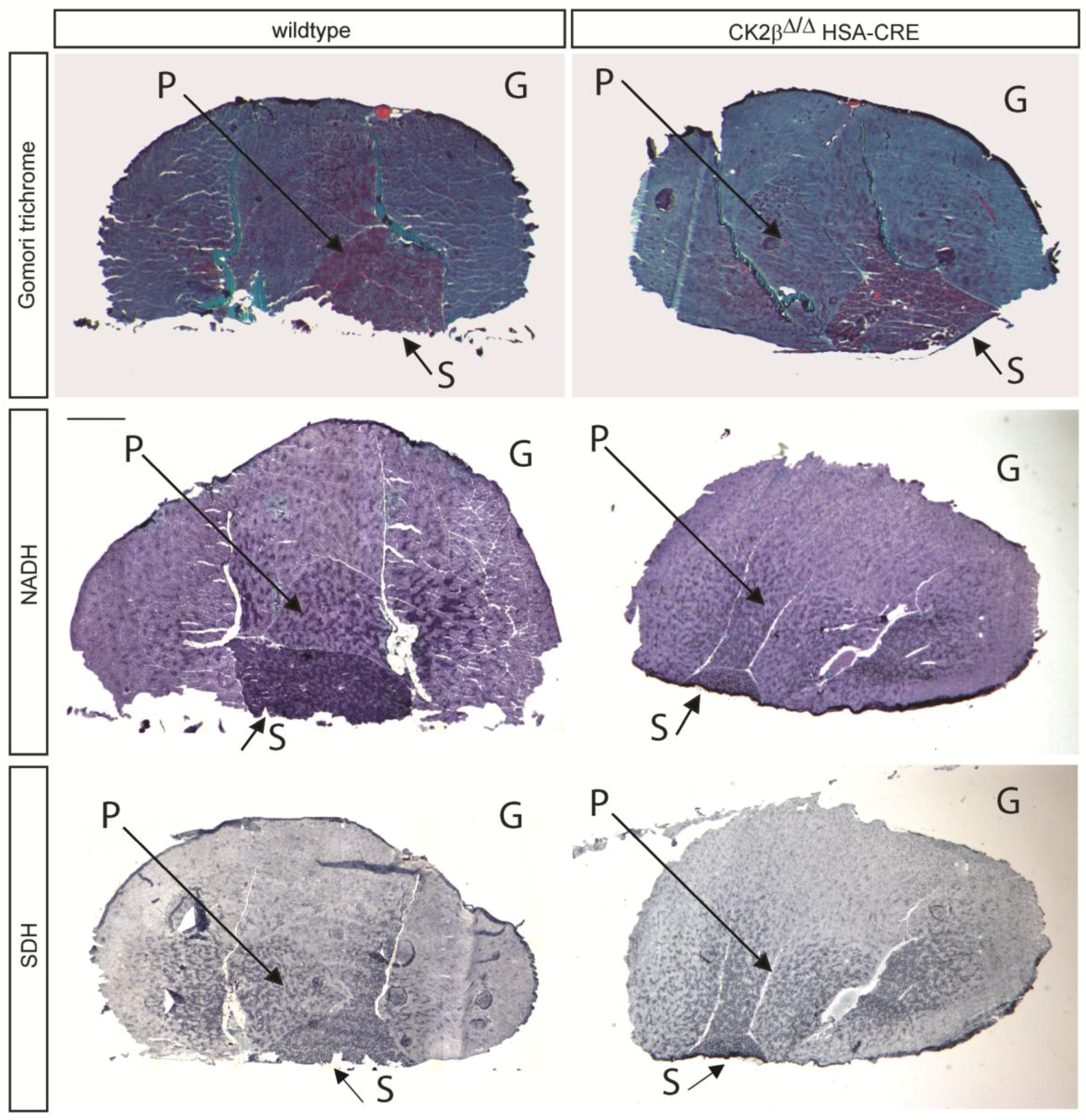
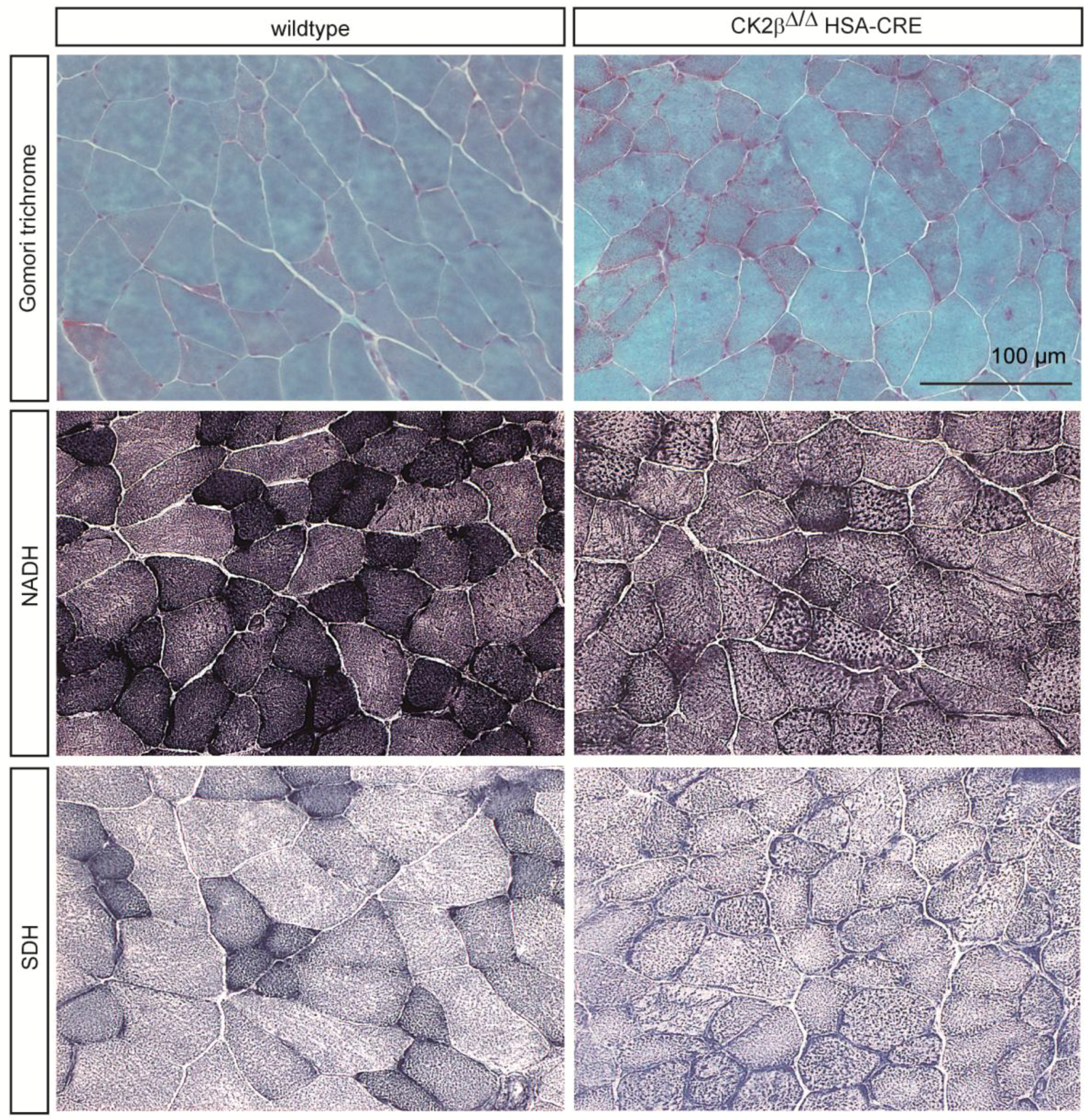
2.1. Gomori Trichrome Staining Revealed Impairments in CK2β-Deficient Muscle Fibers
2.2. Nicotinamide Adenine Dinucleotide (NADH) Dehydrogenase Staining Detected a Diminished Enzymatic Activity in CK2β-Deficient Muscle Fibers
2.3. Succinate Dehydrogenase Activity Is Reduced in CK2β-Deficient Muscle Fibers
3. Discussion
4. Materials and Methods
4.1. Mice Mating and Genotyping
4.2. Dissecting of Skeletal Muscles and Tissue Sections
4.3. Histochemical Stainings, Immunohistochemistry, Imaging, and Data Analysis
4.4. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Olsten, M.E.; Litchfield, D.W. Order or chaos? An evaluation of the regulation of protein kinase ck2. Biochem. Cell Biol. 2004, 82, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Meggio, F.; Pinna, L.A. One-thousand-and-one substrates of protein kinase ck2? FASEB J. 2003, 17, 349–368. [Google Scholar] [CrossRef] [PubMed]
- Escalier, D.; Silvius, D.; Xu, X. Spermatogenesis of mice lacking ck2α′: Failure of germ cell survival and characteristic modifications of the spermatid nucleus. Mol. Reprod. Dev. 2003, 66, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Toselli, P.A.; Russell, L.D.; Seldin, D.C. Globozoospermia in mice lacking the casein kinase II α′ catalytic subunit. Nat. Genet. 1999, 23, 118–121. [Google Scholar] [PubMed]
- Buchou, T.; Vernet, M.; Blond, O.; Jensen, H.H.; Pointu, H.; Olsen, B.B.; Cochet, C.; Issinger, O.G.; Boldyreff, B. Disruption of the regulatory beta subunit of protein kinase ck2 in mice leads to a cell-autonomous defect and early embryonic lethality. Mol. Cell. Biol. 2003, 23, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Song, D.H.; Dominguez, I.; Mizuno, J.; Kaut, M.; Mohr, S.C.; Seldin, D.C. Ck2 phosphorylation of the armadillo repeat region of beta-catenin potentiates wnt signaling. J. Biol. Chem. 2003, 278, 24018–24025. [Google Scholar] [CrossRef] [PubMed]
- Abicht, A.; Stucka, R.; Schmidt, C.; Briguet, A.; Hopfner, S.; Song, I.H.; Pongratz, D.; Muller-Felber, W.; Ruegg, M.A.; Lochmuller, H. A newly identified chromosomal microdeletion and an n-box mutation of the achr epsilon gene cause a congenital myasthenic syndrome. Brain 2002, 125, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.G.; Wang, Q.; Zhou, J.Z.; Wang, J.; Luo, Z.; Liu, M.; He, X.; Wynshaw-Boris, A.; Xiong, W.C.; Lu, B.; et al. Regulation of achr clustering by dishevelled interacting with musk and pak1. Neuron 2002, 35, 489–505. [Google Scholar] [CrossRef]
- Wang, J.; Jing, Z.; Zhang, L.; Zhou, G.; Braun, J.; Yao, Y.; Wang, Z.Z. Regulation of acetylcholine receptor clustering by the tumor suppressor APC. Nat. Neurosci. 2003, 6, 1017–1018. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y. Wnt signaling in axon guidance. Trends Neurosci. 2004, 27, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Huraskin, D.; Eiber, N.; Reichel, M.; Zidek, L.M.; Kravic, B.; Bernkopf, D.; von Maltzahn, J.; Behrens, J.; Hashemolhosseini, S. Wnt/beta-catenin signaling via axin2 is required for myogenesis and, together with yap/taz and tead1, active in iia/iix muscle fibers. Development 2016, 143, 3128–3142. [Google Scholar] [CrossRef] [PubMed]
- Picton, C.; Woodgett, J.; Hemmings, B.; Cohen, P. Multisite phosphorylation of glycogen synthase from rabbit skeletal muscle. Phosphorylation of site 5 by glycogen synthase kinase-5 (casein kinase-ii) is a prerequisite for phosphorylation of sites 3 by glycogen synthase kinase-3. FEBS Lett. 1982, 150, 191–196. [Google Scholar] [CrossRef]
- Cheusova, T.; Khan, M.A.; Schubert, S.W.; Gavin, A.C.; Buchou, T.; Jacob, G.; Sticht, H.; Allende, J.; Boldyreff, B.; Brenner, H.R.; et al. Casein kinase 2-dependent serine phosphorylation of musk regulates acetylcholine receptor aggregation at the neuromuscular junction. Genes Dev. 2006, 20, 1800–1816. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, D.; Straubinger, M.; Hashemolhosseini, S. Protein kinase ck2 interacts at the neuromuscular synapse with rapsyn, rac1, 14-3-3gamma, and dok-7 proteins and phosphorylates the latter two. J. Biol. Chem. 2015, 290, 22370–22384. [Google Scholar] [CrossRef] [PubMed]
- Heuss, D.; Klascinski, J.; Schubert, S.W.; Moriabadi, T.; Lochmuller, H.; Hashemolhosseini, S. Examination of transcript amounts and activity of protein kinase ck2 in muscle lysates of different types of human muscle pathologies. Mol. Cell Biochem. 2008, 316, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.W. Selected Histochemical and Histopathological Methods; C.C. Thomas: Springfield, IL, USA, 1966. [Google Scholar]
- Sheehan, D.C.; Hrapchak, B.B. Theory and Practice of Histotechnology, 2nd ed.; Battelle Press: Columbus, OH, USA, 1987. [Google Scholar]
- Kravic, B.; Huraskin, D.; Frick, A.D.; Jung, J.; Redai, V.; Palmisano, R.; Marchetto, S.; Borg, J.P.; Mei, L.; Hashemolhosseini, S. LAP proteins are localized at the post-synaptic membrane of neuromuscular junctions and appear to modulate synaptic morphology and transmission. J. Neurochem. 2016, 139, 381–395. [Google Scholar] [CrossRef] [PubMed]
- Simeone, L.; Straubinger, M.; Khan, M.A.; Nalleweg, N.; Cheusova, T.; Hashemolhosseini, S. Identification of erbin interlinking musk and erbb2 and its impact on acetylcholine receptor aggregation at the neuromuscular junction. J. Neurosci. 2010, 30, 6620–6634. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Rueden, C.T.; Hiner, M.C.; Eliceiri, K.W. The imagej ecosystem: An open platform for biomedical image analysis. Mol. Reprod. Dev. 2015, 82, 518–529. [Google Scholar] [CrossRef] [PubMed]

| Densitometry | Wildtype | CK2β∆/∆ HSA-Cre |
|---|---|---|
| Plantaris | ||
| NADH dehydrogenase | 221.6 ± 1.37, N = 43 | 165.9 ± 2.97, N = 46 |
| SDH | 186.4 ± 2.24, N = 50 | 109.8 ± 1.88, N = 46 |
| Soleus | ||
| NADH dehydrogenase | 221.4 ± 0.87, N = 37 | 169.5 ± 2.46, N = 47 |
| SDH | 193.8 ± 1.30, N = 35 | 141.0 ± 2.90, N = 42 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eiber, N.; Simeone, L.; Hashemolhosseini, S. Ablation of Protein Kinase CK2β in Skeletal Muscle Fibers Interferes with Their Oxidative Capacity. Pharmaceuticals 2017, 10, 13. https://doi.org/10.3390/ph10010013
Eiber N, Simeone L, Hashemolhosseini S. Ablation of Protein Kinase CK2β in Skeletal Muscle Fibers Interferes with Their Oxidative Capacity. Pharmaceuticals. 2017; 10(1):13. https://doi.org/10.3390/ph10010013
Chicago/Turabian StyleEiber, Nane, Luca Simeone, and Said Hashemolhosseini. 2017. "Ablation of Protein Kinase CK2β in Skeletal Muscle Fibers Interferes with Their Oxidative Capacity" Pharmaceuticals 10, no. 1: 13. https://doi.org/10.3390/ph10010013
APA StyleEiber, N., Simeone, L., & Hashemolhosseini, S. (2017). Ablation of Protein Kinase CK2β in Skeletal Muscle Fibers Interferes with Their Oxidative Capacity. Pharmaceuticals, 10(1), 13. https://doi.org/10.3390/ph10010013







