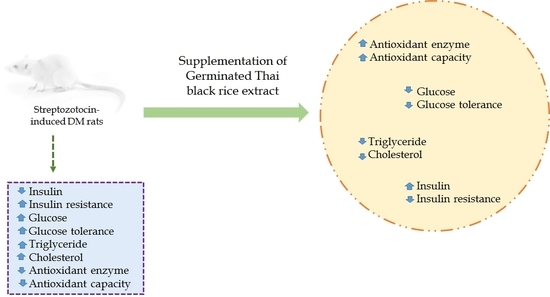
Germinated Thai Black Rice Extract Protects Experimental Diabetic Rats from Oxidative Stress and Other Diabetes-Related Consequences
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling and Extraction
2.2. Analysis of the BR Extract
2.2.1. Determination of γ-Aminobutyric Acid (GABA) Content
2.2.2. Phenolic Acids Determination
2.3. Anthocyanins Determination
2.4. Animal Procedures
2.5. Total Antioxidant Capacity Determination
2.6. Lipid Peroxidation Inhibition Determination
2.7. Antioxidant Enzymes Determination
2.8. Oral Glucose Tolerance Test
2.9. Glucose, Triglyceride, and Cholesterol Determination
2.10. Insulin Determination
2.10.1. Plasma Insulin Determination
2.10.2. Homeostasis Model Assessment
2.11. Statistics
3. Results
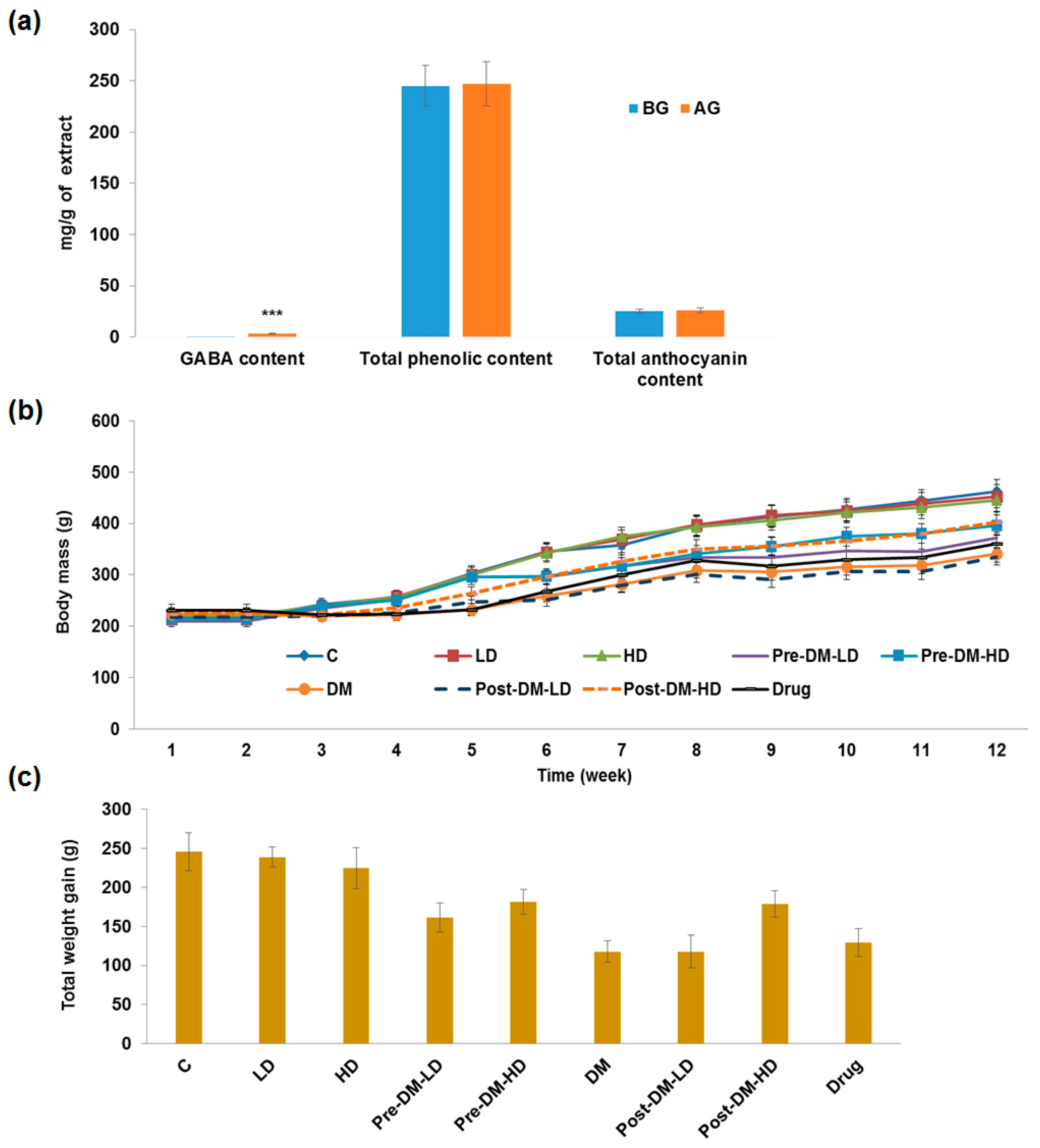
3.1. Germination and Extraction
3.2. In Vivo Study
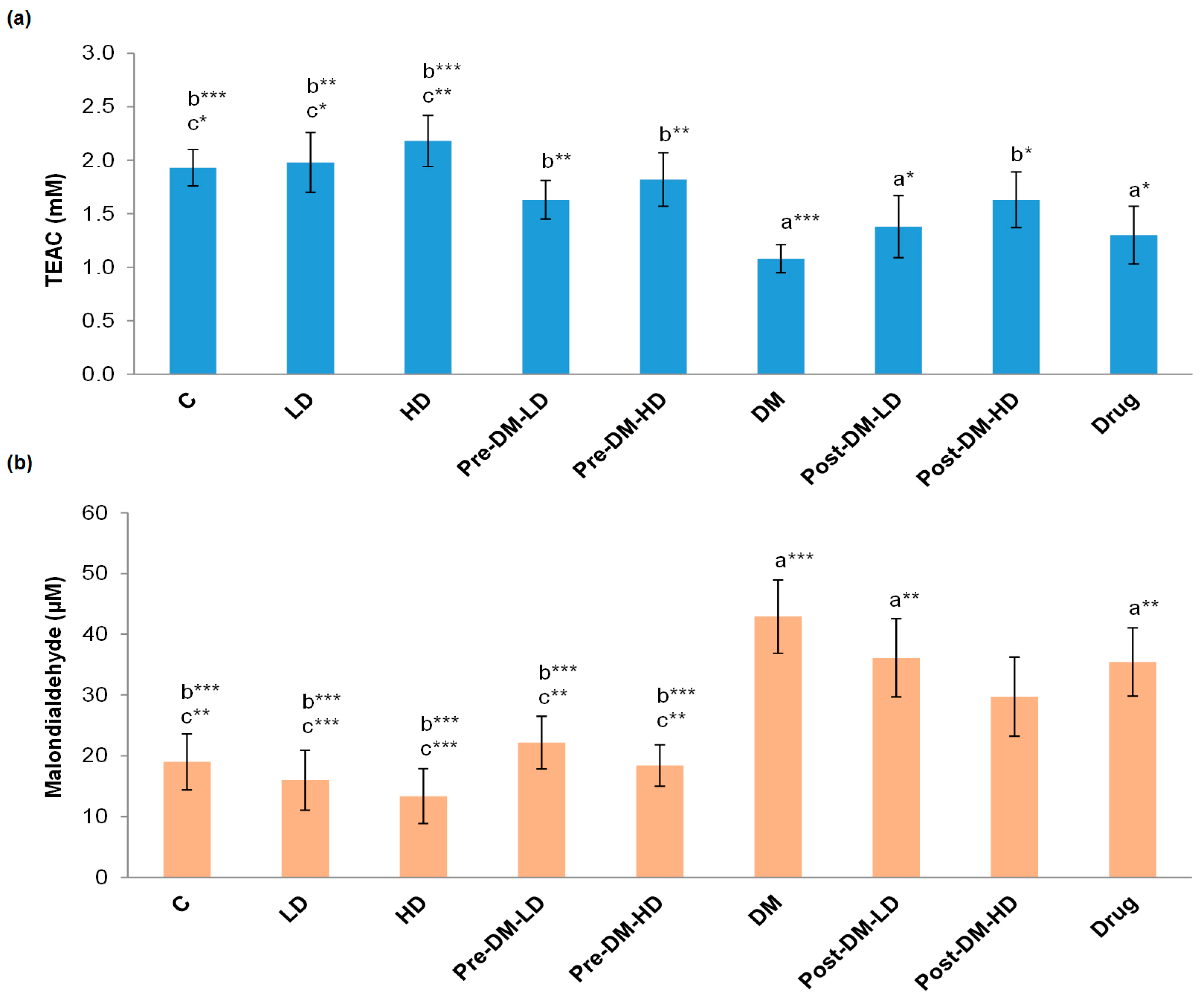
3.2.1. Changes in Antioxidant Capacity
3.2.2. Changes in Antioxidant Enzymes Level
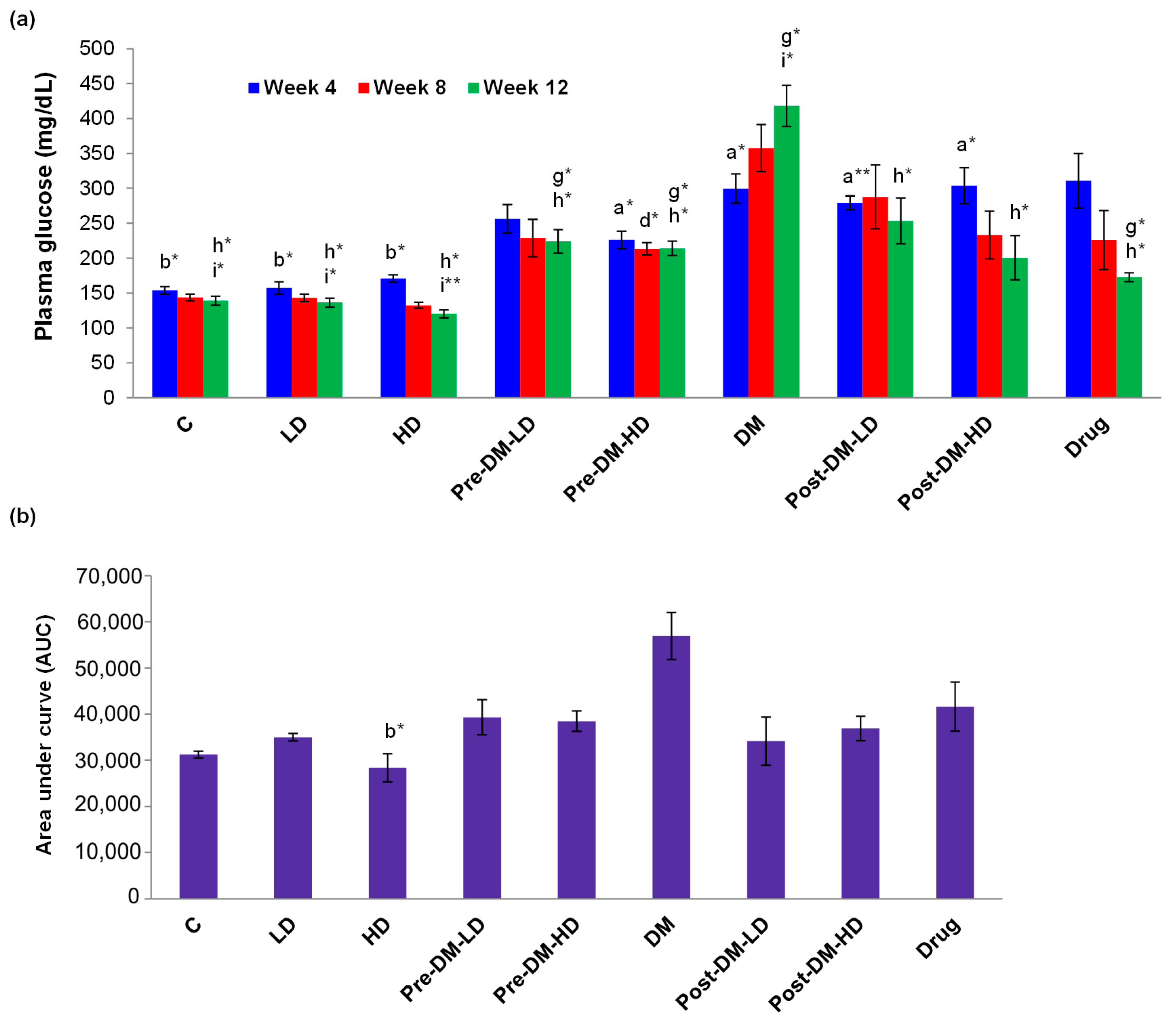
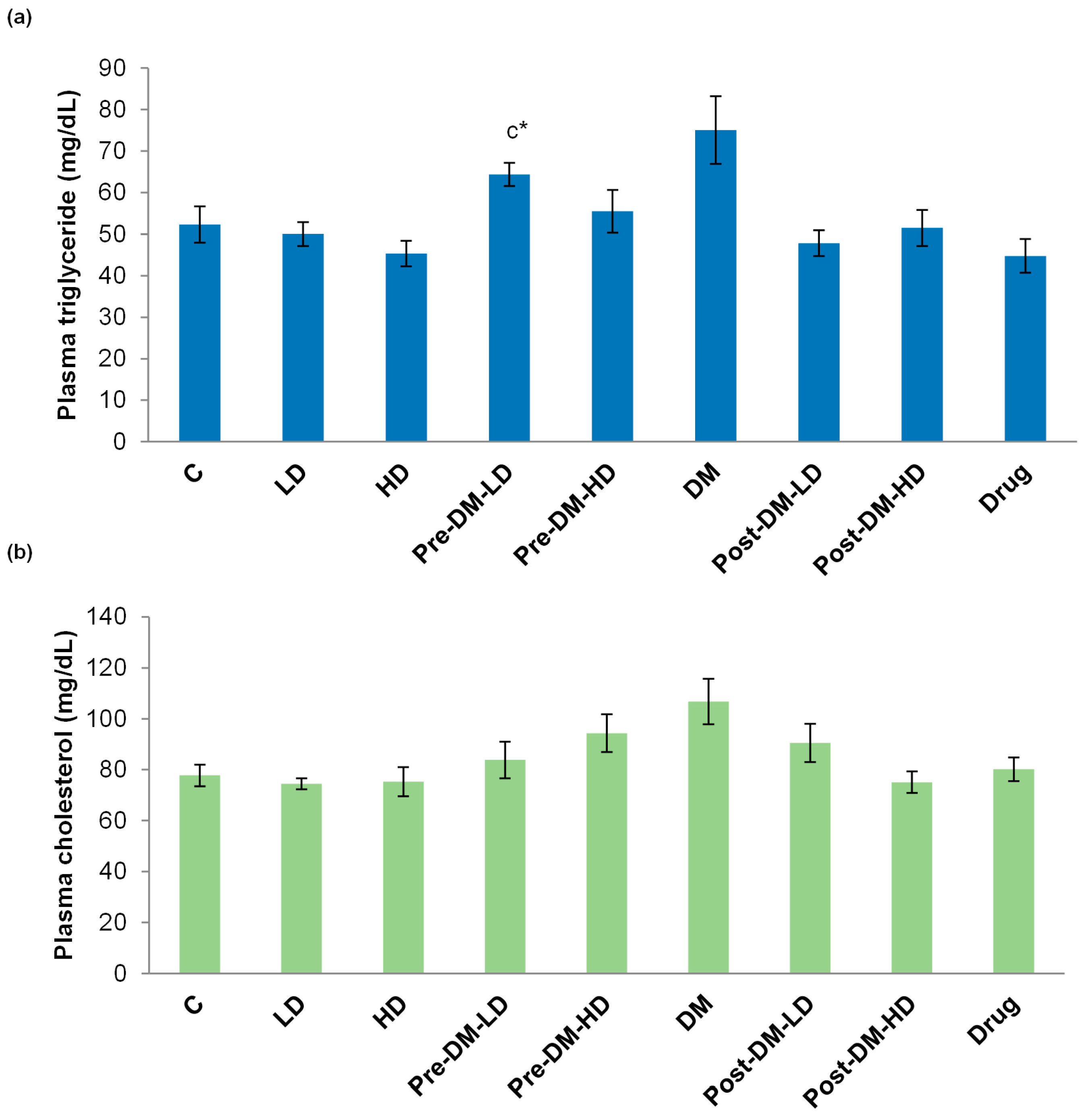
3.2.3. Profiles of Glucose, Glucose Tolerance, Triglyceride, and Cholesterol
3.2.4. Insulin Profile and Insulin Resistance
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Umar, A.; Ahmed, Q.U.; Muhammad, B.Y.; Dogarai, B.B.; Soad, S.Z. Antihyperglycemic activity of the leaves of Tetracera scandens Linn. Merr. (Dilleniaceae) in alloxan induced diabetic rats. J. Ethnopharmacol. 2010, 1, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.E.; Sicree, R.A.; Zimmet, P.Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010, 87, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Whiting, D.R.; Guariguata, L.; Weil, C.; Shaw, J. IDF diabetes atlas: Global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res. Clin. Pract. 2011, 94, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Lachin, J.M.; Walker, E.A.; Nathan, D.M. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar] [PubMed]
- Belobrajdic, D.P.; Bird, A.R. The potential role of phytochemicals in wholegrain cereals for the prevention of type-2 diabetes. Nutr. J. 2013, 12, 62. [Google Scholar] [CrossRef] [PubMed]
- Venn, B.J.; Mann, J.I. Cereal grains, legumes and diabetes. Eur. J. Clin. Nutr. 2004, 58, 1443–1461. [Google Scholar] [CrossRef] [PubMed]
- De Munter, J.S.; Hu, F.B.; Spiegelman, D.; Franz, M.; van Dam, R.M. Whole grain, bran, and germ intake and risk of type 2 diabetes: A prospective cohort study and systematic review. PLoS Med. 2007, 4, e261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Priebe, M.G.; van Binsbergen, J.J.; de Vos, R.; Vonk, R.J. Whole grain foods for the prevention of type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2008, 23, CD006061. [Google Scholar]
- Dembinska-Kiec, A.; Mykkanen, O.; Kiec-Wilk, B.; Mykkanen, H. Antioxidant phytochemicals against type 2 diabetes. Br. J. Nutr. 2008, 99, ES109–ES117. [Google Scholar] [CrossRef] [PubMed]
- Price, K.D.; Price, C.S.C.; Reynolds, R.D. Hyperglycemiainduced ascorbic acid deficiency promotes endothelial dysfunction and the development of atherosclerosis. Atherosclerosis 2001, 158, 1–12. [Google Scholar] [CrossRef]
- Valabhji, J.; McColl, A.J.; Richmond, W.; Schachter, M.; Rubens, M.B.; Elkeles, R.S. Total antioxidant status and coronary artery calcification in type 1 diabetes. Diabetes Care 2001, 24, 1608–1613. [Google Scholar] [CrossRef] [PubMed]
- Pickup, J.C. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetres. Diabetes Care 2004, 27, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Gariballa, S.; Afandi, B.; Haltem, M.A.; Yassin, J.; Alessa, A. Effect of antioxidants and B-group vitamins on risk of infections in patients with type 2 diabetes mellitus. Nutrients 2013, 5, 711–724. [Google Scholar] [CrossRef] [PubMed]
- Gothai, S.; Ganesan, P.; Park, S.Y.; Fakurazi, S.; Choi, D.K.; Arulselvan, P. Natural phyto-bioactive compounds for the treatment of type 2 diabetes: Inflammation as a target. Nutrients 2016, 8, 461. [Google Scholar] [CrossRef] [PubMed]
- Pengkumsri, N.; Chaiyasut, C.; Saenjum, C.; Sirilun, S.; Peerajan, S.; Suwannalert, P.; Sirisattha, S.; Sivamaruthi, B.S. Physicochemical and antioxidative properties of black, brown and red rice varieties of northern Thailand. Food Sci. Technol. (Campinas) 2015, 35, 331–338. [Google Scholar] [CrossRef]
- Pengkumsri, N.; Chaiyasut, C.; Sivamaruthi, B.S.; Saenjum, C.; Sirilun, S.; Peerajan, S.; Suwannalert, P.; Sirisattha, S.; Chaiyasut, K.; Kesika, P. The influence of extraction methods on composition and antioxidant properties of rice bran oil. Food Sci. Technol. (Campinas) 2015, 35, 493–501. [Google Scholar] [CrossRef]
- Deng, G.F.; Xu, X.R.; Guo, Y.J.; Xia, E.Q.; Li, S.; Wu, S.; Chen, F.; Ling, W.H.; Li, H.B. Determination of antioxidant property and their lipophilic and hydrophilic phenolic components in cereal grains. J. Funct. Foods 2012, 4, 906–914. [Google Scholar] [CrossRef]
- Zhang, W.M.; Zhang, R.F.; Zhang, F.X.; Liu, R.H. Phenolic profiles and antioxidant activity of black rice bran of different commercially available varieties. J. Agric. Food Chem. 2010, 58, 7580–7587. [Google Scholar] [CrossRef] [PubMed]
- Torimitsu, M.; Nagase, R.; Yanagi, M.; Homma, M.; Sasai, Y.; Ito, Y.; Hayamizu, K.; Nonaka, S.; Hosono, T.; Kise, M.; et al. Replacing White rice with pre-germinated brown rice mildly ameliorates hyperglycemia and imbalance of adipocytokine levels in type 2 diabetes model rats. J. Nutr. Sci. Vitaminol. 2010, 56, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Usuki, S.; Ito, Y.; Morikawa, K.; Kise, M.; Ariga, T.; Rivner, M.; Robert, K.Y. Effect of pre-germinated brown rice intake on diabetic neuropathy in streptozotocin-induced diabetic rats. Nutr. Metab. 2007, 4, 25. [Google Scholar] [CrossRef] [PubMed]
- Imam, M.U.; Musa, S.N.A.; Azmi, N.H.; Ismail, M. Effects of White Rice, Brown Rice and Germinated Brown Rice on Antioxidant Status of Type 2 Diabetic Rats. Int. J. Mol. Sci. 2012, 13, 12952–12969. [Google Scholar] [CrossRef] [PubMed]
- Woraharn, S.; Lailerd, N.; Sivamaruthi, B.S.; Wangcharoen, W.; Sirisattha, S.; Peerajan, S.; Chaiyasut, C. Evaluation of factors that influence the l-glutamic and γ-aminobutyric acid production during Hericium erinaceus fermentation by lactic acid bacteria. CyTA J. Food 2016, 14, 47–54. [Google Scholar] [CrossRef]
- Blondel, O.; Bailbe, D.; Portha, B. In vivo insulin resistance in streptozotocin-diabetic rats. Evidence for reversal following oral vanadate treatment. Diabetologia 1989, 32, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Suwannalert, P.; Rattanachitthawat, S.; Chaiyasut, C.; Riengrojpitak, S. High levels of 25-hydroxyvitamin D3 [25(OH)D3] and α-tocopherol prevent oxidative stress in rats that consume Thai brown rice. J. Med. Plant Res. 2010, 4, 120–124. [Google Scholar]
- Yang, H.; Fan, S.; Song, D.; Wang, Z.; Ma, S.; Li, S.; Li, X.; Xu, M.; Xu, M.; Wang, X. Long-term streptozotocin-induced diabetes in rats leads to severe damage of brain blood vessels and neurons via enhanced oxidative stress. Mol. Med. Rep. 2013, 7, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Matthews, J.N.; Altman, D.G.; Campbell, M.J.; Royston, P. Analysis of serial measurements in medical research. BMJ 1990, 300, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.H. Stimulation of gamma-aminobutyric acid synthesis activity in brown rice by a chitosan/glutamic acid germination solution and calcium/calmodulin. J. Biochem. Mol. Biol. 2003, 36, 319–325. [Google Scholar] [PubMed]
- Charoenthaikij, P.; Jangchud, K.; Jangchud, A.; Prinyawiwatkul, W.; Tungtrakul, P. Germination conditions affect selected quality of composite wheat-germinated brown rice flour and bread formulations. J. Food Sci. 2010, 75, S312–S318. [Google Scholar] [CrossRef] [PubMed]
- Roohinejad, S.; Omidizadeh, A.; Mirhosseini, H.; Saari, N.; Mustafa, S.; Hussin, A.S.M.; Hamid, A.; Abd Manap, M.Y. Effect of pre-germination time on amino acid profile and gamma amino butyric acid (GABA) contents in different varieties of Malaysian brown rice. Int. J. Food Prop. 2011, 14, 1386–1399. [Google Scholar] [CrossRef]
- Nakagawa, T.; Yokozawa, T.; Kim, H.J.; Shibahara, N. Protective effects of gamma-aminobutyric acid in rats with streptozotocin-induced diabetes. J. Nutr. Sci. Vitaminol. 2005, 51, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Roohinejad, S.; Omidizadeh, A.; Mirhosseini, H.; Saari, N.; Mustafa, S.; Yusof, R.M.; Hussin, A.S.M.; Hamid, A.; Abd Manap, M.Y. Effect of pre-germination time of brown rice on serum cholesterol levels of hypercholesterolaemic rats. J. Sci. Food Agric. 2010, 90, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Middleton, M.; Kandaswami, C.; Theoharides, C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease and cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar] [PubMed]
- Testa, R.; Bonfigli, A.R.; Genovese, S.; de Nigris, V.; Ceriello, A. The possible role of flavonoids in the prevention of diabetic complications. Nutrients 2016, 8, 310. [Google Scholar] [CrossRef] [PubMed]
- Chiang, A.N.; Wu, H.L.; Yeh, H.I.; Chu, C.S.; Lin, H.C.; Lee, W.C. Antioxidant effects of black rice extract through the induction of superoxide dismutase and catalase activities. Lipids 2006, 41, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.H.; Park, M.Y.; Kim, H.W.; Lee, Y.M.; Hwang, K.A.; Park, J.H.; Park, D.S.; Kwon, O. Black rice (Oryza sativa L.) extract attenuates hepatic steatosis in C57BL/6 J mice fed a high-fat diet via fatty acid oxidation. Nutr. Metab. 2012, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Posuwan, J.; Prangthip, P.; Leardkamolkarn, V.; Yamborisut, U.; Surasiang, R.; Charoensiri, R.; Kongkachuichai, R. Long-term supplementation of high pigmented rice bran oil (Oryza sativa L.) on amelioration of oxidative stress and histological changes in streptozotocin-induced diabetic rats fed a high fat diet; Riceberry bran oil. Food Chem. 2013, 138, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, H.; Seki, T.; Ariga, T. The effect of pre-germinated brown rice intake on blood glucose and PAI-1 levels in streptozotocin-induced diabetic rats. Biosci. Biotechnol. Biochem. 2004, 68, 444–447. [Google Scholar] [CrossRef] [PubMed]
- Senadheera, S.P.A.S.; Ekanayake, S.; Wanigatunge, C. Anti-Diabetic Properties of Rice-Based Herbal Porridges in Diabetic Wistar Rats. Phytother. Res. 2014, 28, 1567–1572. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Kim, E.K.; Lee, M.S.; Lee, H.K.; Hwang, W.S.; Choe, S.J.; Kim, T.Y.; Han, S.J.; Kim, H.J.; Kim, D.J.; et al. Intake of brown rice lees reduces waist circumference and improves metabolic parameters in type 2 diabetes. Nutr. Res. 2011, 31, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T.; Ueno, Y.; Kojo, H.; Yoshikawa, T.; Osawa, T. Gene expression profile of isolated rat adipocytes treated with anthocyanins. Biochim. Biophys. Acta 2005, 15, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakasam, B.; Vareed, S.K.; Olson, L.K.; Nair, M.G. Insulin secretion by bioactive anthocyanins and anthocyanidins present in fruits. J. Agric. Food Chem. 2005, 53, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Sivamaruthi, B.S.; Pengkumsri, N.; Saelee, M.; Kesika, P.; Sirilun, S.; Peerajan, S.; Chaiyasut, C. Impact of physical treatments on stability and radical scavenging capacity of anthocyanidins. Int. J. Pharm. Pharm. Sci. 2015, 8, 162–167. [Google Scholar]
- Woraharn, S.; Lailerd, N.; Sivamaruthi, B.S.; Wangcharoen, W.; Sirisattha, S.; Peerajan, S.; Chaiyasut, C. Screening and kinetics of glutaminase and glutamate decarboxylase producing lactic acid bacteria from fermented Thai foods. Food Sci. Technol. (Campinas) 2014, 34, 793–799. [Google Scholar] [CrossRef]
- Woraharn, S.; Lailerd, N.; Sivamaruthi, B.S.; Wangcharoen, W.; Peerajan, S.; Sirisattha, S.; Chaiyasut, C. Development of fermented Hericium erinaceus juice with high content of l-glutamine and l-glutamic acid. Int. J. Food Sci. Technol. 2015, 50, 2104–2112. [Google Scholar] [CrossRef]
- Yoo, J.Y.; Kim, S.S. Probiotics and prebiotics: Present status and future perspectives on metabolic disorders. Nutrients 2016, 8, 173. [Google Scholar] [CrossRef] [PubMed]
- Henderson, A.J.; Ollila, C.A.; Kumar, A.; Borresen, E.C.; Raina, K.; Agarwal, R.; Ryan, E.P. Chemopreventive properties of dietary rice bran: Current status and future prospects. Adv. Nutr. Int. Rev. J. 2012, 3, 643–653. [Google Scholar] [CrossRef] [PubMed]


| No | Group (G) | Week 1 | Week 2, 3 | Week 4–12 |
|---|---|---|---|---|
| 1 | C (G1) | ND & NDW | ND & NDW | ND & NDW |
| 2 | LD (G2) | 500 mg/kg BW. of GBRE, ND & NDW | Buffer injection (i.p.) *, ND & NDW | 500 mg/kg BW. of GBRE, ND & NDW |
| 3 | HD (G3) | 1000 mg/kg BW. of GBRE, ND & NDW | Buffer injection (i.p.) *, ND & NDW | 1000 mg/kg BW. of GBRE, ND & NDW |
| 4 | PreLD (G4) | 500 mg/kg BW. of GBRE, ND & NDW | STZ injection (i.p.) *, ND & NDW | 500 mg/kg BW. of GBRE, ND & NDW |
| 5 | PreHD (G5) | 1000 mg/kg BW. of GBRE, ND & NDW | STZ injection (i.p.) *, ND & NDW | 1000 mg/kg BW. of GBRE, ND & NDW |
| 6 | DM (G6) | ND & NDW | STZ injection (i.p.) *, ND & NDW | ND & NDW |
| 7 | PostLD (G7) | ND & NDW | STZ injection (i.p.) *, ND & NDW | 500 mg/kg BW. of GBRE, ND & NDW |
| 8 | PostHD (G8) | ND & NDW | STZ injection (i.p.) *, ND & NDW | 1000 mg/kg BW. of GBRE, ND & NDW |
| 9 | Drug (G9) | ND & NDW | STZ injection (i.p.) *, ND & NDW | 50 mg/kg BW. of MFN, ND & NDW |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaiyasut, C.; Sivamaruthi, B.S.; Pengkumsri, N.; Keapai, W.; Kesika, P.; Saelee, M.; Tojing, P.; Sirilun, S.; Chaiyasut, K.; Peerajan, S.; et al. Germinated Thai Black Rice Extract Protects Experimental Diabetic Rats from Oxidative Stress and Other Diabetes-Related Consequences. Pharmaceuticals 2017, 10, 3. https://doi.org/10.3390/ph10010003
Chaiyasut C, Sivamaruthi BS, Pengkumsri N, Keapai W, Kesika P, Saelee M, Tojing P, Sirilun S, Chaiyasut K, Peerajan S, et al. Germinated Thai Black Rice Extract Protects Experimental Diabetic Rats from Oxidative Stress and Other Diabetes-Related Consequences. Pharmaceuticals. 2017; 10(1):3. https://doi.org/10.3390/ph10010003
Chicago/Turabian StyleChaiyasut, Chaiyavat, Bhagavathi Sundaram Sivamaruthi, Noppawat Pengkumsri, Waranya Keapai, Periyanaina Kesika, Manee Saelee, Parichart Tojing, Sasithorn Sirilun, Khontaros Chaiyasut, Sartjin Peerajan, and et al. 2017. "Germinated Thai Black Rice Extract Protects Experimental Diabetic Rats from Oxidative Stress and Other Diabetes-Related Consequences" Pharmaceuticals 10, no. 1: 3. https://doi.org/10.3390/ph10010003