High Concentrations of Sodium Chloride Improve Microbicidal Activity of Ibuprofen against Common Cystic Fibrosis Pathogens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Reagents
2.1.2. Microorganisms
2.2. Methods
2.2.1. Microdilution Antibacterial Test for Minimum Inhibitory Concentration (MIC)
2.2.2. Effect of IBU on Bacterial Growth
2.2.3. Langmuir Film Balance Experiments
2.2.4. Preclinical Toxicological Study
3. Results
3.1. Determination of Minimum Inhibitory Concentration (MIC) of Ibuprofen
3.2. Bactericidal Effect of IBU-Na as a Function of Time
3.3. IBU-Na Activity as a Function of Initial Inoculum Title
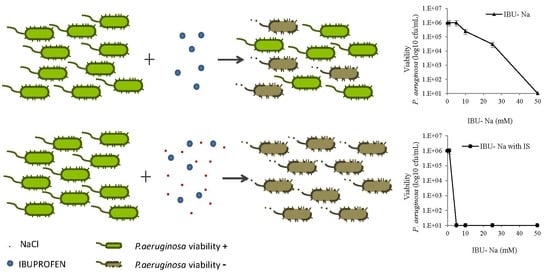
3.4. Effect of Ionic Strength on the Bactericidal Effect of IBU-Na
3.5. Effect of Ionic Strength on the Effect of Other Microbicides
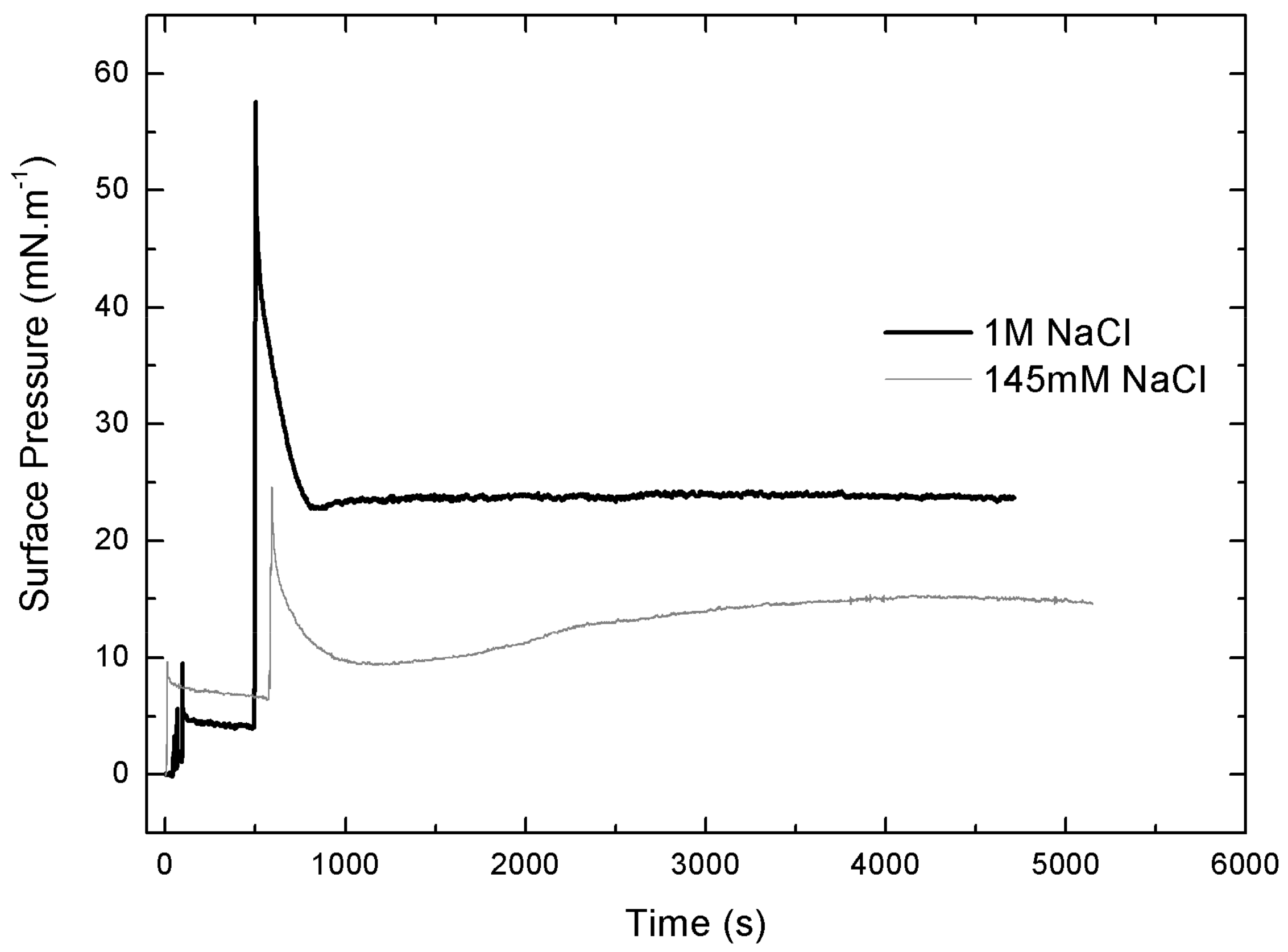
3.6. Langmuir Film Balance
3.6.1. Langmuir’s Monolayers
3.6.2. Gibbs Monolayers
3.6.3. Penetration Studies
3.7. Preclinical Toxicity Study
4. Discussion
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Cantas, L.; Shah, Q.A.; Cavaco, L.M.; Manaia, C.M.; Walsh, F.; Popowska, M.; Garelick, H.; Bürgmann, H.; Sørum, H. A brief multi-disciplinary review on antimicrobial resistance in medicine and its linkage to the global environmental microbiota. Front. Microbiol. 2014, 4, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, J.; Wright, G.D. Bacterial resistance to aminoglycoside antibiotics. Trends Microbiol. 1997, 5, 234–240. [Google Scholar] [CrossRef]
- Higgins, C.F. Multiple molecular mechanisms for multidrug resistance transporters. Nature 2007, 446, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Hooper, D.C. Mechanisms of action and resistance of older and newer fluoroquinolones. Clin. Infect. Dis. 2000, 31, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Nikaido, H. Multidrug Resistance in Bacteria. Annu. Rev. Biochem. 2009, 78, 119–146. [Google Scholar] [CrossRef] [PubMed]
- Poole, K. Aminoglycoside Resistance in Pseudomona aeruginosa. Antimicrob. Agents Chemother. 2005, 49, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Livermore, D.M. The need for new antibiotics. Clin. Microbiol. Infect. 2004, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Barrett, C.T.; Barrett, J.F. Antibacterials: Are the new entries enough to deal with the emerging resistance problems? Curr. Opin. Biotechnol. 2003, 14, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Cole, S.T. Who will develop new antibacterial agents? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369. [Google Scholar] [CrossRef] [PubMed]
- Conly, J.M.; Johnston, B.L. Where are all the new antibiotics? The new antibiotic paradox. Can. J. Infect. Dis. Med. Microbiol. 2005, 16, 159–160. [Google Scholar] [CrossRef] [PubMed]
- Franco, B.E.; Martínez, M.A.; Sánchez Rodríguez, M.A.; Wertheimer, A.I. The determinants of the antibiotic resistance process. Infect. Drug Resist. 2009, 2, 1–11. [Google Scholar] [PubMed]
- Kruszewska, H.; Zar Ba, T.; Tyski, S. Examination of antimicrobial activity of selected Non-antibiotic medicinal preparations. Acta Pol. Pharm. Drug Res. 2012, 69, 1368–1371. [Google Scholar]
- Obad, J.; Suskovic, J.; Kos, B. Antimicrobial activity of ibuprofen: New perspectives on an ‘‘Old’’ non-antibiotic drug. Eur. J. Pharm. Sci. 2015, 71, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Elvers, K.T.; Wright, S.J. Antibacterial activity of the anti-inflammatory compound ibuprofen. Lett. Appl. Microbiol. 1995, 20, 82–84. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, A.; Gomaa, A.; Mohamed, F.; Ragab, R.; Eid, M.; Ahmed, A.; Khalaf, A.; Kamal, M.; Mokhtar, S.; Mohamed, H.; et al. Antibacterial, Anti-biofilm Activity of Some Non-steroidal Anti-Inflammatory Drugs and N-acetyl Cysteine against Some Biofilm Producing Uropathogens. Am. J. Epidemiol. Infect. Dis. 2015, 3, 1–9. [Google Scholar] [CrossRef]
- Hersh, E.V.; Hammond, B.F.; Fleury, A.A. Antimicrobial activity of flurbiprofen and ibuprofen in vitro against six common periodontal pathogens. J. Clin. Dent. 1991, 3, 1–5. [Google Scholar] [PubMed]
- Sanyal, A.K.; Roy, D.; Chowdhury, B.; Banerjee, A.B. Ibuprofen, a unique anti-inflammatory compound with antifungal activity against dermatophytes. Lett. Appl. Microbiol. 1993, 17, 109–111. [Google Scholar] [CrossRef]
- Donaldson, S.H.; Bennett, W.D.; Zeman, K.L.; Knowles, M.R.; Tarran, R.; Boucher, R.C. Mucus clearance and lung function in cystic fibrosis with hypertonic saline. N. Engl. J. Med. 2006, 354, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.; Regnis, J.A.; Bailey, D.L.; King, M.; Bautovich, G.J.; Bye, P.T. Effect of hypertonic saline, amiloride, and cough on mucociliary clearance in patients with cystic fibrosis. Am. J. Respir. Crit. Care Med. 1996, 153, 1503–1509. [Google Scholar] [CrossRef] [PubMed]
- Wark, P.; McDonald, V.M. Nebulised hypertonic saline for cystic fibrosis. Cochrane Database Syst. Rev. 2009, 2, CD001506. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 9th ed.; Approved Standard; CLSI document M07-A9; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- AWWA/WEF. Standard Methods for the Examination of Water and Wastewater, 21st ed.; AWWA/WEF: Denver, CO, USA, 2005; Part 9215. [Google Scholar]
- Halder, S.; Yadav, K.K.; Sarkar, R.; Mukherjee, S.; Saha, P.; Haldar, S.; Karmakar, S.; Sen, T. Alteration of Zeta potential and membrane permeability in bacteria: A study with cationic agents. SpringerPlus 2015, 4, 672. [Google Scholar] [CrossRef] [PubMed]
- Pillet, F.; Formosa-Dague, C.; Baaziz, H.; Dague, E.; Rols, M. Cell wall as a target for bacteria inactivation by pulsed electric fields. Sci. Rep. 2016, 6, 19778. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Grigg, J. New insights into pulmonary inflammation in cystic fibrosis. Arch. Dis. Child. 2006, 91, 786–788. [Google Scholar] [CrossRef] [PubMed]
- Corvol, H.; Fitting, C.; Shadelat, K.; Jacquo, T.; Tabari, O.; Boul, M. Distinct cytokine production by lung and blood neutrophils from children with cystic fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003, 284, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Konstan, M.W. Ibuprofen therapy for cystic fibrosis lung disease: Revisited. Curr. Opin. Pulm. Med. 2008, 14, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Konstan, M.W.; Byard, P.J.; Hoppel, C.L.; Davis, P.B. Effect of high-dose ibuprofen in patients with cystic fibrosis. N. Engl. J. Med. 1995, 332, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Konstan, M.W.; Krenicky, J.E.; Finney, M.R.; Kirchner, H.L.; Hilliard, K.A.; Hilliard, J.B.; Davis, P.B.; Hoppel, C.L. Effect of ibuprofen on neutrophil migration in vivo in cystic fibrosis. J. Pharmacol. Exp. Ther. 2003, 306, 1086–1091. [Google Scholar] [CrossRef] [PubMed]
- Konstan, M.W.; Vargo, K.M.; Davis, P.B. Ibuprofen Attenuates the Inflammatory Response to Pseudomonas aeruginosa in a Rat Model of Chronic Pulmonary Infection. Am. Rev. Respir. Dis. 1990, 141, 186–192. [Google Scholar] [CrossRef] [PubMed]









| TRT | Dose IBU [mM] | Acute Damage Parameters | Sub-Acute Damage Parameters | |||||
|---|---|---|---|---|---|---|---|---|
| Alveolar Infiltrate | Interstitial Infiltrate | Hyaline Membranes | Protein Material | Septal Thickening | Masson Bodies | Granulomas and Giant Cells | ||
| A | 25 | 0 a | 1 | 1 | 1 | 1 | NO | NO |
| 50 | 0 b | 0 Capillary congestion | 0 | 0 | 1 Low and limited | NO | NO | |
| B | 25 | 0 | 1 | 0 | 0 | 1 | NO | NO |
| 50 | 0 b | 1 | 0 | 0 | 1 | NO | NO | |
| C | 25 | 0 c | 1 | 0 | 0 | 1 | NO | NO |
| 50 | 0 d | 1 | 0 | 0 | 1 | NO | NO | |
| D | 25 | 0 c | 1 | 0 | 0 | 1 | NO | NO |
| 50 | 0 d | 1 | 0 | 0 | 1 | NO | NO | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz, A.J.; Alasino, R.V.; Garro, A.G.; Heredia, V.; García, N.H.; Cremonezzi, D.C.; Beltramo, D.M. High Concentrations of Sodium Chloride Improve Microbicidal Activity of Ibuprofen against Common Cystic Fibrosis Pathogens. Pharmaceuticals 2018, 11, 47. https://doi.org/10.3390/ph11020047
Muñoz AJ, Alasino RV, Garro AG, Heredia V, García NH, Cremonezzi DC, Beltramo DM. High Concentrations of Sodium Chloride Improve Microbicidal Activity of Ibuprofen against Common Cystic Fibrosis Pathogens. Pharmaceuticals. 2018; 11(2):47. https://doi.org/10.3390/ph11020047
Chicago/Turabian StyleMuñoz, Adrián J., Roxana V. Alasino, Ariel G. Garro, Valeria Heredia, Néstor H. García, David C. Cremonezzi, and Dante M. Beltramo. 2018. "High Concentrations of Sodium Chloride Improve Microbicidal Activity of Ibuprofen against Common Cystic Fibrosis Pathogens" Pharmaceuticals 11, no. 2: 47. https://doi.org/10.3390/ph11020047