Nanostructured TiO2 Carbon Paste Based Sensor for Determination of Methyldopa
Abstract
1. Introduction
2. Results
2.1. Graphite Characterization
2.2. Electrochemical Impedance Spectroscopy
2.3. Electrocatalytic Response
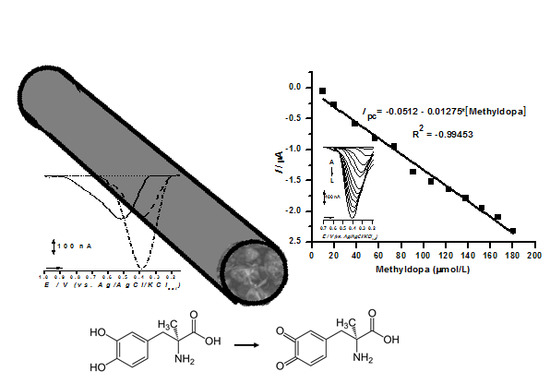
2.4. Sensor Application in Methyldopa Detection
2.5. Sensor Applicability in Commercial Samples
3. Discussion
4. Materials and Methods
4.1. Reagents, Samples, and Solutions
4.2. Synthesis and Characterization of Graphite Powder
4.3. Preparation of Sensors
4.4. Electrochemical Assays
4.5. Real Sample Preparation
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Asadpour-Zeynali, K.; Mollarasouli, F. A novel and facile synthesis of TGA-capped CdSe@Ag2Se core-shell quantum dots as a new substrate for high sensitive and selective methyldopa sensor. Sens. Actuators B 2016, 237, 387–399. [Google Scholar] [CrossRef]
- Fouladgar, M.; Ahmadzadeh, S. Application of a nanostructured sensor based on NiO nanoparticles modified carbon paste electrode for determination of methyldopa in the presence of Folic acid. Appl. Surf. Sci. 2016, 379, 150–155. [Google Scholar] [CrossRef]
- Tajik, S.; Taher, M.A.; Beitollahi, H. First report for simultaneous determination of methyldopa and hydrochlorothiazide using a nanostructured based electrochemical sensor. J. Electroanal. Chem. 2013, 704, 137–144. [Google Scholar] [CrossRef]
- Keyvanfard, M.; Hatami, M.; Gupta, V.K.; Agarwal, S.; Sadeghifar, H.; Khalilzadeh, M.A. Liquid phase analysis of methyldopa in the presence of tyrosine using electrocatalytic effect of a catechol derivative at a surface of NiO nanoparticle modified carbon paste electrode. J. Mol. Liq. 2017, 230, 290–294. [Google Scholar] [CrossRef]
- Moccelini, S.K.; Franzoi, A.C.; Vieira, I.C.; Dupont, J.; Scheeren, C.W. A novel support for laccase immobilization: Cellulose acetate modified with ionic liquid and application in biosensor for methyldopa detection. Biosens. Bioelectron. 2011, 26, 3549–3554. [Google Scholar] [CrossRef] [PubMed]
- Vieira, I.C.; Fatibello-Filho, O. Spectrophotometric determination of methyldopa and dopamine in pharmaceutical formulations using a crude extract of sweet potato root (Ipomoea batatas (L.) Lam.) as enzymatic source. Talanta 1998, 46, 559–564. [Google Scholar] [CrossRef]
- Ribeiro, P.R.S.; Gomes Neto, J.A.; Pezza, L.; Pezza, H.R. Flow-injection spectrophotometric determination of methyldopa in pharmaceutical formulations. Talanta 2005, 67, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Madrakian, T.; Afkhami, A.; Khalafi, L.; Mohammadnejad, M. Spectrophotometric determination of catecholamines based on their oxidation reaction followed by coupling with 4-aminobenzoic acid. J. Braz. Chem. Soc. 2006, 17, 1259–1265. [Google Scholar] [CrossRef]
- Zecevic, M.; Zivanovic, L.J.; Agatonovic-Kustrin, S.; Minic, D. The use of a response surface methodology on HPLC analysis of methyldopa, amiloride and hydrochlorothiazide in tablets. J. Pharm. Biomed. Anal. 2001, 24, 1019–1025. [Google Scholar] [CrossRef]
- Muzzi, C.; Bertocci, E.; Terzuoli, L.; Porcelli, B.; Ciari, I.; Pagani, R.; Guerranti, R. Simultaneous determination of serum concentrations of levodopa, dopamine, 3-O-methyldopa and a-methyldopa by HPLC. Biomed. Pharmacother. 2008, 62, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Li, S.F.; Wu, H.L.; Yu, Y.J.; Li, Y.N.; Nie, J.F.; Fu, H.Y.; Yu, R.Q. Quantitative analysis of levodopa, carbidopa and methyldopa in human plasma samples using HPLC-DAD combined with second-order calibration based on alternating trilinear decomposition algorithm. Talanta 2010, 81, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Manjunatha, J.G.; Deraman, M.; Basri, N.H.; Nor, N.S.M.; Talib, I.A.; Ataollah, N. Sodium dodecyl sulfate modified carbon nanotubes paste electrode as a novel sensor for the simultaneous determination of dopamine, ascorbic acid, and uric acid. C. R. Chim. 2014, 17, 465–476. [Google Scholar] [CrossRef]
- Macedo, I.Y.L.; Garcia, L.F.; Souza, A.R.; Silva, A.M.L.; Fernandez, C.; Santos, M.D.G.; Magalhães, R.S.; Torres, I.M.S.; Gil, E.S. Differential pulse voltammetric determination of albendazole and mebendazole in pharmaceutical formulations based on modified sonogel carbon paste electrodes with perovskite-type LaFeO3 nanoparticles. J. Electrochem. Soc. 2016, 163, B428–B434. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Sanati, A.L.; Gupta, V.K.; Yoosefian, M.; Asif, M.; Bahari, A. A voltammetric biosensor based on ionic liquid/NiO nanoparticle modified carbon paste electrode for the determination of nicotinamide adenine dinucleotide (NADH). Sens. Actuators B Chem. 2014, 204, 647–654. [Google Scholar] [CrossRef]
- El-Ads, E.H.; Galal, A.; Atta, N.F. Electrochemistry of glucose at gold nanoparticles modified graphite/SrPdO3 electrode—Towards a novel non-enzymatic glucose sensor. J. Electroanal. Chem. 2015, 749, 42–52. [Google Scholar] [CrossRef]
- Garcia, L.F.; Souza, A.R.; Lobón, G.S.; Santos, W.T.P.; Alecrim, M.F.; Santiago, M.F.; Sotomayor, R.L.A.; Gil, E.S. Efficient enzyme-free biomimetic sensors for natural phenol detection. Molecules 2016, 21, 1060. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, D.; Fu, J.; Huang, F.; Wei, Q. TiO2-CuCNFs based laccase biosensor for enhanced electrocatalysis in hydroquinone detection. J. Electroanal. Chem. 2016, 766, 16–23. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Saeid, B.; Rezaei, B.; Allafchian, A.R. Differential pulse voltammetric determination of methyldopa using MWCNTs modified glassy carbon decorated with NiFe2O4 nanoparticles. Ionics 2015, 21, 1435–1444. [Google Scholar] [CrossRef]
- Gil, E.S.; Torres, I.M.S.; Moraes, F.C.; Machado, S.A.S.; Matias, A.E.B. Electroanalytical method for the analysis of methyldopa in pharmaceutical tablets using a crude extract of laccase from pycnoporus sanguineus as oxidizing agent. Lat. Am. J. Pharm. 2010, 29, 1277–1282. [Google Scholar]
- Kutluay, A.; Aslanoglu, M. Quantification of methyldopa in pharmaceuticals using a glassy carbon electrode modified with carbon nanotubes. Chin. Chem. Lett. 2016, 27, 91–95. [Google Scholar] [CrossRef]
- Antunes, R.S.; Garcia, L.F.; Somerset, V.S.; Gil, E.S.; Lopes, F.M. The use of a polyphenoloxidase biosensor obtained from the fruit of jurubeba (solanum paniculatum L.) in the determination of paracetamol and other phenolic drugs. Biosensors 2018, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Jana Svítková, J.; Svikruhová, S.S.; Vojs, M.; Marton, M.; Svorc, L. DNA-modified boron-doped diamond electrode as a simple electrochemical platform for detection of damage to DNA by antihypertensive amlodipine. Monatsh. Chem. 2016, 1–9. [Google Scholar] [CrossRef]
- Moraes, J.T.; Salamanca-Neto, C.A.R.; Švorc, L.; Sartori, E.R. Advanced sensing performance towards simultaneous determination of quaternary mixture of antihypertensives using boron-doped diamond electrode. Microchem. J. 2017, 134, 173–180. [Google Scholar] [CrossRef]
- Rezaei, B.; Askarpour, N.; Ensafi, A.A. Adsorptive stripping voltammetry determination of methyldopa on the surface of a carboxylated multiwall carbon nanotubes modified glassy carbon electrode in biological and pharmaceutical samples. Colloids Surf. B Biointerfaces 2013, 109, 253–258. [Google Scholar] [CrossRef] [PubMed]
- FARMACOPÉIA Brasileira, Agência Nacional de Vigilância Sanitária, Fundação Oswaldo Cruz, 5ª edição, 381 Brasília 2010.
- Lobón, G.S.; Yepez, A.; Garcia, L.F.; Morais, R.L.; Vaz, B.G.; Carvalho, V.V.; Oliveira, G.A.R.; Luque, R.; Gil, E.S. Efficient electrochemical remediation of microcystin-LR in tap water using designer TiO2@carbon electrodes. Sci. Rep. 2017, 7, 41326. [Google Scholar] [CrossRef] [PubMed]
- Pifferi, V.; Rimoldi, L.; Meroni, D.; Segrado, F.; Soliveri, G.; Ardizzone, S.; Falciola, L. Electrochemical characterization of insulating silica-modified electrodes: Transport properties and physicochemical features. Electrochem. Commun. 2017, 81, 102. [Google Scholar] [CrossRef]
- Maccarrone, F.; Paffuti, G. Capacitance and forces for thick circular electrodes. J. Electrostat. 2018, 94, 30. [Google Scholar] [CrossRef]
- Uddin, M.S.; Das, H.T.; Maiyalagan, T.; Elumalai, P. Influence of designed electrode surfaces on double layer capacitance in aqueous electrolyte: Insights from standard models. App. Sur. Sci. 2018, 449. [Google Scholar] [CrossRef]




| Material | Analyses EDX | |||||
|---|---|---|---|---|---|---|
| % Ti | % Pd | % C | % O | % Si | % Al | |
| Carbon graphite (C) | - | - | 76.10 | 15.19 | 6.16 | 2.54 |
| TiO2@C | 4.00 2.26 | - | 70.33 73.26 | 18.14 18.61 | 5.32 4.10 | 2.21 1.77 |
| PdO@C | - | 10.63 13.70 | 58.46 52.42 | 14.36 14.38 | 12.16 14.10 | 4.39 5.40 |
| Electrodes | |||
|---|---|---|---|
| Circuit Elements | CPE | PdO@CPE | TiO2@CPE |
| Rs | 610 Ω | 424 Ω | 176 Ω |
| Rp | 4.57 kΩ | 1480 kΩ | 2.87 kΩ |
| C | 0.064 µF | 0.514 µF | 3.65 µF |
| Y | 5.69 µMho.s1/2 | 3.66 µMho.s1/2 | 162 µMho.s1/2 |
| Electrode | Methods | LOD (µmol/L) | Linear Range (µmol/L) | Reference |
|---|---|---|---|---|
| TiO2@CPE | DPVreduction | 1.00 | 10–180 | This work |
| GCE-TGA-capped-CdSe@Ag2Se | DPVoxidation | 0.04 | 0.09–60 | [1] |
| 5ADB-CTNs-CPE | SWV | 0.048 | 0.1–210 | [3] |
| NiO-IL-CPE | SWV | 0.06 | 0.1–700 | [2] |
| GCE-NiFe2O4-MWCNTs | DPVoxidation | 0.08 | 0.5-900 | [18] |
| GCE/Lacc | DPVreduction | 4.5 | 25-100 | [19] |
| GCE-MWCNTs | SWV | 0.001 | 0.005-0.388 | [20] |
| Method | Added Methyldopa (mg) | Recovered Methyldopa (mg) | Mean Recovery (%) ± RSD a |
|---|---|---|---|
| Pure standard | 250 | 250.72 | 100.29% (± 3.94) |
| Standard plus placebo b | 250 | 251.15 | 100.46% (± 4.33) |
| Method | Sample | Labeled Concentration (mg/tablet) | Experimental Concentration (mg) | Mean Recovery (%) ± RSD a |
|---|---|---|---|---|
| Official Method | tablet | 250 | 250.62 | 100.25% (± 4.14) |
| DPV at TiO2@CP | tablet | 250 | 251.02 | 100.41% (± 4.64) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia, L.F.; Cunha, C.E.P.d.; Moreno, E.K.G.; Thomaz, D.V.; Sanz Lobón, G.; Luque, R.; Somerset, V.; De Souza Gil, E. Nanostructured TiO2 Carbon Paste Based Sensor for Determination of Methyldopa. Pharmaceuticals 2018, 11, 99. https://doi.org/10.3390/ph11040099
Garcia LF, Cunha CEPd, Moreno EKG, Thomaz DV, Sanz Lobón G, Luque R, Somerset V, De Souza Gil E. Nanostructured TiO2 Carbon Paste Based Sensor for Determination of Methyldopa. Pharmaceuticals. 2018; 11(4):99. https://doi.org/10.3390/ph11040099
Chicago/Turabian StyleGarcia, Luane Ferreira, Carlos Eduardo Peixoto da Cunha, Emily Kussmaul Gonçalves Moreno, Douglas Vieira Thomaz, Germán Sanz Lobón, Rafael Luque, Vernon Somerset, and Eric De Souza Gil. 2018. "Nanostructured TiO2 Carbon Paste Based Sensor for Determination of Methyldopa" Pharmaceuticals 11, no. 4: 99. https://doi.org/10.3390/ph11040099
APA StyleGarcia, L. F., Cunha, C. E. P. d., Moreno, E. K. G., Thomaz, D. V., Sanz Lobón, G., Luque, R., Somerset, V., & De Souza Gil, E. (2018). Nanostructured TiO2 Carbon Paste Based Sensor for Determination of Methyldopa. Pharmaceuticals, 11(4), 99. https://doi.org/10.3390/ph11040099