Chemical Characterization, Antioxidant, Cytotoxic and Microbiological Activities of the Essential Oil of Leaf of Tithonia Diversifolia (Hemsl) A. Gray (Asteraceae)
Abstract
:1. Introduction
2. Results
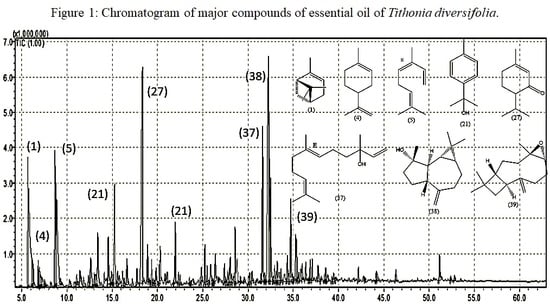
2.1. Chemical Characterization of Essential Oil
2.2. Antioxidant Activity
2.3. Cytotoxicity Test
2.4. Antimicrobial Activity
3. Discussion
3.1. Chemical Characterization of Essential Oil
3.2. Antioxidant Activity
3.3. Cytotoxicity Test
3.4. Antimicrobial Activity
4. Material and Methods
4.1. Collection of Plant Material
4.2. Obtaining Essential Oil
4.3. Identification of the Chemical Composition of the Essential Oil by Gas Chromatography Coupled to Mass Spectrometry (GC-MS) and Magnetic Nuclear Resonance (NMR)
4.4. Analysis of Antioxidant Activity
- (%AA) = 100 − {[(Abssample − Abswhite) × 100] / Abscontrol}
- %AA = percentage of antioxidant activity
- Abssample = Sample Absorbance
- Abswhite = Absorbance of white
- Abscontrol = Control Absorbance
4.5. Cytotoxicity Test
4.6. Microbiological Activity
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fabri, R.L.; Nogueira, M.S.; Dutra, L.B.; Bouzada, M.L.M.; Scio, E. Potencial antioxidante e antimicrobiano de espécies da família Asteraceae. Rev. Bras. Plantas Med. 2011, 13, 183–189. [Google Scholar] [CrossRef] [Green Version]
- Thongsom, M.; Chunglok, W.; Kuanchuea, R. Antioxidant and Hypoglycemic Effects of Tithonia diversifolia Aqueous Leaves Extract in Alloxan-induced Diabetic Mice. Adv. Environ. Biol. 2013, 7, 2116–2125. [Google Scholar]
- Da Gama, R.M.; Guimarães, M.; de Abreu, L.C.; Armando-Junior, J. Phytochemical screening and antioxidant activity of ethanol extract of Tithonia diversifolia (Hemsl) A. Gray dry flowers. Asian Pac. J. Trop. Biomed. 2014, 4, 740–742. [Google Scholar] [CrossRef]
- Di Giacomo, C.; Vanella, L.; Sorrenti, V.; Santangelo, R.; Barbagallo, I.; Calabrese, G.; Genovese, C.; Mastrojeni, S.; Ragusa, S.; Acquaviva, R. Effects of Tithonia diversifolia (Hemsl.) A. Gray Extract on Adipocyte Differentiation of Human Mesenchymal Stem Cells. PLoS ONE 2015, 10, e0122320. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, B.L.; Edrada-Ebel, R.; Da Costa, F.B. Effect of the environment on the secondary metabolic profile of Tithonia diversifolia: A model for environmental metabolomics of plants. Sci. Rep. 2016, 6, 29265. [Google Scholar] [CrossRef] [PubMed]
- Chagas-Paula, D.A.; Oliveira, R.B.; Rocha, B.A.; Da Costa, F.B. Ethnobotany, Chemistry, and Biological Activities of the Genus Tithonia (Asteraceae). Chem. Biodivers. 2012, 9, 210–235. [Google Scholar] [CrossRef] [PubMed]
- Goffin, E.; Ziemons, E.; De Mol, P.; de Madureira, M.D.C.; Martins, A.P.; da Cunha, A.P.; Philippe, G.; Tits, M.; Angenot, L.; Frederich, M. In Vitro Antiplasmodial Activity of Tithonia diversifolia and Identification of its Main Active Constituent: Tagitinin C. Planta Med. 2002, 68, 543–545. [Google Scholar] [CrossRef] [PubMed]
- Agboola, O.O.; Oyedeji, S.; Olowoyo, J.O.; Ajao, A.; Aregbesola, O. Chemical composition and antimicrobial activities of essential oil extracted from Tithonia diversifolia (Asteraceae) flower. Bioresour. Bioprocess. 2016, 1, 169–176. [Google Scholar]
- Wanzala, W.; Osundwa, E.M.; Alwala, O.J.; Gakuubi, M.M. Chemical composition of essential oil of Tithonia diversifolia (Hemsl.) A. Gray from the Southern slopes of Mount Elgon in Western Kenya. Indian J. Med. Res. 2016, 13. [Google Scholar]
- Sousa, I.P.; Chagas-Paula, D.A.; Tiossi, R.F.J.; Silva, E.D.O.; Miranda, M.A.; de Oliveira, R.B.; Spadaro, A.C.C.; Bastos, J.K.; Furtado, N.A.J.C.; Da Costa, F.B. Essential oils from Tithonia diversifolia display potent anti-oedematogenic effects and inhibit acid production by cariogenic bacteria. J. Essent. Oil Res. 2019, 31, 43–52. [Google Scholar] [CrossRef]
- Gu, J.-Q.; Gills, J.J.; Park, E.J.; Mata-Greenwood, E.; Hawthorne, M.E.; Axelrod, F.; Chavez, P.I.; Fong, H.H.S.; Mehta, R.G.; Pezzuto, J.M.; et al. Sesquiterpenoids from Tithonia diversifolia with Potential Cancer Chemopreventive Activity. J. Nat. Prod. 2002, 65, 532–536. [Google Scholar] [CrossRef] [PubMed]
- Tona, L.; Kambu, K.; Ngimbi, N.; Cimanga, K.; Vlietinck, A. Antiamoebic and phytochemical screening of some Congolese medicinal plants. J. Ethnopharmacol. 1998, 61, 57–65. [Google Scholar] [CrossRef]
- Cos, P.; Hermans, N.; De Bruyne, T.; Apers, S.; Sindambiwe, J.B.; Witvrouw, M.; De Clercq, E.; Vanden Berghe, D.; Pieters, L.; Vlietinck, A.J. Antiviral activity of Rwandan medicinal plants against human immunodeficiency virus type-1 (HIV-1). Phytomedicine 2002, 9, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Hilário, R.R.; de Toledo, J.J.; Mustin, K.; Castro, I.J.; Costa-Neto, S.V.; Kauano, É.E.; Eilers, V.; Vasconcelos, I.M.; Mendes-Junior, R.N.; Funi, C.; et al. The Fate of an Amazonian Savanna: Government Land-Use Planning Endangers Sustainable Development in Amapá, the Most Protected Brazilian State. Trop. Conserv. Sci. 2017, 10, 194008291773541. [Google Scholar] [CrossRef]
- Van Den Dool, H.; Kratz, P.D. A Generalization of the Retention Index System Including Linear Temperature Programmed Gas-Liquid Partition Chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectorscopy, 4th ed.; Allured Pub. Corp.: Carol Stream, IL, USA, 2007; ISBN 978-1-932633-21-4. [Google Scholar]
- Lee, S.-G. α-Pinene and myrtenol: Complete1H NMR assignment. Magn. Reson. Chem. 2002, 40, 311–312. [Google Scholar] [CrossRef]
- Aggarwal, K.K.; Khanuja, S.P.S.; Ateeque Ahmad, T.R.; Santha Kuma, V.K.; Kumar, S. Antimicrobial activity profiles of the two enantiomers of limonene and carvone isolated from the oils of Mentha spicata and Anethum sowa. Flavour Fragr. J. 2002, 17, 59–63. [Google Scholar] [CrossRef]
- Castillo, A.M.; Patiny, L.; Wist, J. Fast and accurate algorithm for the simulation of NMR spectra of large spin systems. J. Magn. Reson. Imaging 2011, 209, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Banfi, D.; Patiny, L. Resurrecting and Processing NMR Spectra On-line. Chim. Int. J. Chem. 2008, 62, 280–281. [Google Scholar] [CrossRef]
- Machado, S.M.F.; Ribeiro, V.A.F.A.; Militão, J.S.L.T.; de Morais, S.M.; Machado, M.I.L. Seasonal Variation of (E)-Verolidol in Siparuna guianensis Aublet and 13C-NMR Spectral Assignments of (E)- and (Z)-Nerolidol. J. Essent. Oil Res. 2001, 13, 130–131. [Google Scholar] [CrossRef]
- Inagaki, F.; Abe, A. Analysis of lH and 13C Nuclear Magnetic Resonance Spectra of Spathulenol by Two-dimensional Met hods. J. Chem. Soc. Perkin Trans. 1985, 2, 1773–1778. [Google Scholar] [CrossRef]
- Krebs, H.C.; Rakotoarimanga, J.V.; Habermehl, G.G. Isolation of Spatulenol and (−)-Caryophyllene Oxide from Vernonia mollissima Don and ’H and 13C Reassignment by Two-Dimensional NMR Spectroscopy. Magn. Reson. Chem. 1990, 28, 124–128. [Google Scholar] [CrossRef]
- Lawal, O.A.; Kasali, A.A.; Opoku, A.R.; Oyedeji, A.O. Volatile Constituents of the Flowers, Leaves, Stems and Roots of Tithonia diversifolia (Hemsely) A. Gray. J. Essent. Oil Bear. Plant 2012, 15, 816–821. [Google Scholar] [CrossRef]
- Sousa, C.D.; Silva, H.R.; Vieira, G.M., Jr.; Ayres, M.C.; Costa, C.D.; Araújo, D.S.; Cavalcante, L.C.; Barros, E.D.; Araújo, P.D.; Brandão, M.S.; et al. Fenóis totais e atividade antioxidante de cinco plantas medicinais. Quím. Nova 2007, 30, 351–355. [Google Scholar] [CrossRef] [Green Version]
- Bondet, V.; Brand-Williams, W.; Berset, C. Kinetics and Mechanisms of Antioxidant Activity using the DPPH.Free Radical Method. LWT-Food Sci. Technol. 1997, 30, 609–615. [Google Scholar] [CrossRef]
- Zhang, H.-Y.; Gao, Y.; Lai, P.-X. Chemical Composition, Antioxidant, Antimicrobial and Cytotoxic Activities of Essential Oil from Premna microphylla Turczaninow. Molecules 2017, 22, 381. [Google Scholar] [CrossRef] [PubMed]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive Compounds and Antioxidant Activity in Different Types of Berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, G.L.S. Determinação da capacidade antioxidante de produtos naturais in vitro pelo método do DPPH•: Estudo de revisão. Rev. Bras. Plantas Med. 2015, 17, 36–44. [Google Scholar] [CrossRef]
- Ćavar, S.; Maksimović, M.; Šolić, M.E.; Jerković-Mujkić, A.; Bešta, R. Chemical composition and antioxidant and antimicrobial activity of two Satureja essential oils. Food Chem. 2008, 111, 648–653. [Google Scholar] [CrossRef]
- Roberto, D.; Micucci, P.; Sebastian, T.; Graciela, F.; Anesini, C. Antioxidant Activity of Limonene on Normal Murine Lymphocytes: Relation to H2O2 Modulation and Cell Proliferation. Basic Clin. Pharmacol. Toxicol. 2009, 106, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Seol, G.-H.; Kang, P.; Lee, H.S.; Seol, G.H. Antioxidant activity of linalool in patients with carpal tunnel syndrome. BMC Neurol. 2016, 16, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyacienth, D.C.; Almeida, S.S.M.S. Estudo Fitoquímico, Toxicidade em Artemia salina Leach e Atividade Antibacteriana de Pseudoxandra cuspidata Maas. Biota Amaz. 2015, 5, 4–7. [Google Scholar] [CrossRef]
- Candido, L.P. Busca de Extratos e Compostos Ativos com Potencial Herbicida e Inseticida nas Espécies Davilla elliptica St. Hill e Ocotea Pulchella Nees & Mart; Tese, Universidade Federal de São Carlos: São Carlos, SP, USA, 2016. [Google Scholar]
- Li, X.; Huang, G.; Zhao, G.; Chen, W.; Li, J.; Sun, L. Two New Monoterpenes from Tithonia diversifolia and Their Anti-Hyperglycemic Activity. Rec. Nat. Prod. 2013, 7, 351–354. [Google Scholar]
- Silva, E.M.F.; Filho, M. Estudo in vitro do potencial citotóxico da Annona muricata L. Revista de Ciências Farmacêuticas Básica e Aplicada 2015, 36, 277–283. [Google Scholar]
- Eltayeib, A.A.; Ishag, W. Phytochemical screening, antimicrobial, antioxidant and cytotoxicity activities of bark’s crude extracts of Cordia sinensis. Adv. Med. Plant Res. 2015, 2, 39–45. [Google Scholar]
- Riani, L.R.; Macedo, A.L.; Chedier, L.M.; Pimenta, D.S. Chemical Analysis of Essential Oil and Hydrolates of Leaves, Inflorescences and Stems of Piper chimonanthifolium Kunth. Revista Virtual de Química 2017, 9, 1560–1569. [Google Scholar] [CrossRef]
- Estevam, E.B.B.; Silva, E.M.; Miranda, M.L.D.; Alves, J.M.; Pereira, P.S.; Silva, F.G.; Esperandim, V.R.; Martins, C.H.G.; Ambrosio, M.A.L.V.; Tófoli, D.; et al. Avaliação das atividades antibacteriana, tripanocida e citotóxica do extrato hidroalcóolico das raízes de Tradescantia sillamontana Matuda (Veludo Branco) (Commelinaceae). Rev. Bras. Plantas Med. 2016, 18, 415–422. [Google Scholar] [CrossRef]
- Passoni, F.D.; Oliveira, R.B.; Chagas-Paula, D.A.; Gobbo-Neto, L.; Da Costa, F.B. Repeated-dose toxicological studies of Tithonia diversifolia (Hemsl.) A. gray and identification of the toxic compounds. J. Ethnopharmacol. 2013, 147, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Linthoingambi, W.; Mutum, S.S. Antimicrobial activities of different solvent extracts of Tithonia diversifolia (Hemsely) A. Gray. Asian J. Plant. Sci. Res. 2013, 3, 50–54. [Google Scholar]
- Emirdağ-Öztürk, S.; Karayildirim, T.; Anil, H. Synthesis of egonol derivatives and their antimicrobial activities. Bioorgan. Med. Chem. 2011, 19, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Yoshioka, C.; Mano, Y.; Tnabe, W.; Ito, Y.; Okamoto, N.; Shimomura, Y. A nanoparticle formulation of disulfiram prolongs corneal residence time of the drug and reduces intraocular pressure. Exp. Eye Res. 2015, 132, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Ateufack, G.; Nana Yousseu, W.; Dongmo Feudjio, B.; Fonkeng Sama, L.; Kuiate, J.; Kamanyi, A. Antidiarrheal and in vitro antibacterial activities of leaves extracts of Hibiscus asper. Hook. F. (malvaceae). Asian J. Pharm. Clin. Res. 2014, 7, 130–136. [Google Scholar]
- American Type Culture Collection Manuscript in the Following Manner: Escherichia coli (ATCC® 8739TM). 2018. Available online: https://www.atcc.org/ (accessed on 29 January 2019).
- Vahdani, M.; Faridi, P.; Zarshenas, M.M.; Javadpour, S.; Abolhassanzadeh, Z.; Moradi, N.; Bakzadeh, Z.; Karmostaji, A.; Mohagheghzadeh, A.; Ghasemi, Y. Major Compounds and Antimicrobial Activity of Essential Oils from Five Iranian Endemic Medicinal Plants. J. Pharm. 2011, 3, 48–53. [Google Scholar] [CrossRef]
- Odeyemi, A.T.; Agidigbi, T.S.; Adefemi, S.O.; Fasuan, S.O. Antibacterial activities of crude extracts of tithonia diversifolia against common environmental pathogenic bacteria. Experiment 2017, 20, 1421–1426. [Google Scholar]
- Guinoiseau, E.; Luciani, A.; Rossi, P.G.; Quilichini, Y.; Ternengo, S.; Bradesi, P.; Berti, L. Cellular effects induced by Inula graveolens and Santolina corsica essential oils on Staphylococcus Aureus. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 873–879. [Google Scholar] [CrossRef] [PubMed]
- BRASIL. Farmacopéia Brasileira, 5th ed.; Agência Nacional de Vigilância Sanitária (ANVISA): Brasília, Brazil, 2010; p. 546.
- Tavares, L.A.; Ferreira, A.G. Análises quali-e quantitativa de cafés comerciais via ressonância magnética nuclear. Quím. Nova 2006, 29, 911–915. [Google Scholar] [CrossRef]
- Lopes-Lutz, D.; Alviano, D.S.; Alviano, C.S.; Kolodziejczyk, P.P. Screening of chemical composition, antimicrobial and antioxidant activities of Artemisia essential oils. Phytochemistry 2008, 69, 1732–1738. [Google Scholar] [CrossRef] [PubMed]
- Andrade, M.A.; das Graças Cardoso, M.; Batista, L.R.; Mallet, A.C.; Machado, S.M. Óleos essenciais de Cymbopogon nardus, Cinnamomum zeylanicum e Zingiber officinale: Composição, atividades antioxidante e antibacteriana. Rev. Ciênc. Agron. 2012, 43, 399–408. [Google Scholar] [CrossRef]
- Tepe, B.; Daferera, D.; Sokmen, A.; Sokmen, M.; Polissiou, M. Antimicrobial and antioxidant activities of the essential oil and various extracts of Salvia tomentosa Miller (Lamiaceae). Food Chem. 2005, 90, 333–340. [Google Scholar] [CrossRef]
- Gülle, S.; Erbaş, S.Ç.; Uzel, A. Synthesis and Spectroscopic Studies of Phenanthroimidazole-Imine Derivatives and Evaluation of Their Antioxidant Activity. J. Fluoresc. 2018, 28, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Araujo, M.G.; Cunha, W.R.; Veneziani, R.C. Phytochemical screening and toxicological bioassay with brine shrimp larvae (Artemia salina Leach) of an extract obtained from fruits of Solanum lycocarpum A. St-Hill. (Solanaceae). J. Basic Appl. Pharm. Sci. 2012, 31, 205–209. [Google Scholar]
- Lôbo, K.M.; Athayde, A.C.; Silva, A.M.; Rodrigues, F.F.; Lôbo, I.; Bezerra, D.A.; Costa, J.G. Avaliação da atividade antibacteriana e prospecção fitoquímica de Solanum paniculatum Lam. e Operculina hamiltonii (G. Don) D. F. Austin & Staples, do semi-árido paraibano. Rev. Bras. Plantas Med. 2010, 12, 227–235. [Google Scholar]
- Clinical and Laboratory Standards Institute (Ed.) Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically: M07-A10; Approved Standard, 10th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015; ISBN 978-1-56238-987-1. [Google Scholar]
| N° * | LRI | KI | Compounds | Relative Percentage (%) | Identification # |
|---|---|---|---|---|---|
| 1 | 945 | 939 | α-pinene | 9.9 | MS, LRI, KI |
| 2 | 972 | 975 | Sabinene | 0.85 | MS, IR, KI |
| 3 | 982 | 979 | β-pinene | 1.34 | MS, IR, KI |
| 4 | 1028 | 1029 | Limonene | 5.40 | MS, IR, KI |
| 5 | 1032 | 1037 | (Z)-β-ocimene | 4.02 | MS, IR, KI |
| 6 | 1042 | 1050 | (E)-β-ocimene | 0.09 | MS, IR, KI |
| 7 | 1069 | 1070 | CIS-sabinene hydrate | 0.3 | MS, IR, KI |
| 8 | 1083 | 1088 | Terpinolene | 0.21 | MS, IR, KI |
| 9 | 1090 | 1091 | p-cymenene | 0.31 | MS, IR, KI |
| 10 | 1099 | 1096 | Linalool | 0.32 | MS, IR, KI |
| 11 | 1109 | 1099 | α-pinene oxide | 0.33 | MS, IR, KI |
| 12 | 1121 | 1122 | TRANS-p-mentha-2,8-dien-1-ol | 0.35 | MS, IR, KI |
| 13 | 1127 | 1132 | (4E,6Z)-allo-ocimene | 1.3 | MS, IR, KI |
| 14 | 1135 | 1137 | CIS-p-mentha-2,8-dien-1-ol | 0.39 | MS, IR, KI |
| 15 | 1138 | 1135 | (Z)-myroxide | 0.25 | MS, IR, KI |
| 16 | 1141 | 1141 | CIS-verbenol | 0.43 | MS, IR, KI |
| 17 | 1145 | 1144 | TRANS-verbenol | 1.68 | MS, IR, KI |
| 18 | 1161 | 1164 | CIS-Chrysanthenol | 0.28 | MS, IR, KI |
| 19 | 1171 | 1169 | Borneol | 1.44 | MS, IR, KI |
| 20 | 1180 | 1177 | Terpinen-4-ol | 0.31 | MS, IR, KI |
| 21 | 1187 | 1182 | p-cymen-8-ol | 3.0 | MS, IR, KI |
| 22 | 1195 | 1193 | Dihydro carveol | 0.37 | MS, IR, KI |
| 23 | 1207 | 1205 | Verbenone | 0.49 | MS, IR, KI |
| 24 | 1218 | 1216 | TRANS-carveol | 1.0 | MS, IR, KI |
| 25 | 1231 | 1229 | CIS-carveol | 0.2 | MS, IR, KI |
| 26 | 1243 | 1243 | Carvone | 0.42 | MS, IR, KI |
| 27 | 1257 | 1252 | Piperitone | 11.72 | MS, IR, KI |
| 28 | 1290 | 1290 | Thymol | 0.5 | MS, IR, KI |
| 29 | 1298 | 1299 | Carvacrol | 0.57 | MS, IR, KI |
| 30 | 1337 | 1343 | Piperitenone | 1.47 | MS, IR, KI |
| 31 | 1349 | 1389 | 2-Dodecanone | 0.3 | MS, IR, KI |
| 32 | 1411 | 1426 | 2,5-dimethoxy-p-cymene | 0.83 | MS, IR, KI |
| 33 | 1417 | 1466 | (E)-caryophyllene | 0.43 | MS, IR, KI |
| 34 | 1477 | 1488 | (E)-β-ionone | 0.64 | MS, IR, KI |
| 35 | 1489 | 1493 | α-zingiberene | 1.21 | MS, IR, KI |
| 36 | 1503 | 1505 | (E,E)-a-farnesene | 0.13 | MS, IR, KI |
| 37 | 1563 | 1563 | (E)-nerolidol | 3.78 | MS, IR, KI |
| 38 | 1578 | 1578 | Spathulenol | 10.8 | MS, IR, KI |
| 39 | 1581 | 1583 | Caryophyllene oxide | 3.43 | MS, IR, KI |
| 40 | 1584 | 1590 | Globulol | 2.64 | MS, IR, KI |
| 41 | 1624 | 1631 | β-muurola-4,10(14)-dienol | 1.8 | MS, IR, KI |
| 42 | 1641 | 1640 | epi-a-cadinol | 2.04 | MS, IR, KI |
| 43 | 1654 | 1654 | α-cadinol | 1.35 | MS, IR, KI |
| 44 | 1657 | 1659 | Selin-11-en-4-a-ol | 0.91 | MS, IR, KI |
| 45 | 1663 | 1671 | 14-hydroxy-(Z)-caryophyllene | 0.22 | MS, IR, KI |
| 46 | 1669 | 1662 | Allohimachalol | 0.82 | MS, IR, KI |
| 47 | 1683 | 1671 | Bulnesol | 0.38 | MS, IR, KI |
| 48 | 1696 | 1698 | (2Z,6Z)-farnesol | 0.49 | MS, IR, KI |
| 49 | 2106 | 1943 | Phytol | 0.78 | MS, IR, KI |
| Total | 80.26 |
| Position | 1H NMR | 1H NMR [17] | 13C NMR | 13C NMR [17] |
|---|---|---|---|---|
| 1 | 1.931 | 1.931 | 47.05 | 46.99 |
| 2 | - | - | 145.06 | 144.54 |
| 3 | 5.203 | 5.186 | 116.01 | 116.10 |
| 4 | (2.231; 2.210) | 2.232 | 31.26 | 31.25 |
| 5 | 2.065 | 2.067 | 40.74 | 40.69 |
| 6 | - | - | 37.96 | 37.97 |
| 7 | (1.616; 1.559) | (1.15; 2.334) | 31.46 | 31.45 |
| 8 | 1.282 | 1.264 | 26.35 | 26.35 |
| 9 | 0.853 | 0.834 | 20.80 | 20.80 |
| 10 | 1.673 | 1.659 | 22.97 | 23.01 |
| Position | 1H NMR | 1H NMR [18] | 13C NMR | 13C NMR [18] |
|---|---|---|---|---|
| 1 | - | - | 133.62 | 133.30 |
| 2 | 2.081 | 2.082 | 27.91 | 27.90 |
| 3 | (1.673; 1.495) | (1.675; 1.495) | 30.59 | 30.60 |
| 4 | 1.673 | 1.675 | 41.08 | 41.10 |
| 5 | (2.288; 2.081) | (2.289; 2.082) | 30.80 | 30.80 |
| 6 | 5.455 | 5.209 | 120.64 | 120.70 |
| 7 | 1.791 | 1.516 | 23.46 | 23.30 |
| 8 | - | - | 159.27 | 149.70 |
| 9 | 1.712 | 1.558 | 20.67 | 20.60 |
| 10 | 4.770 | (4.952; 4.949) | 108.35 | 108.40 |
| Position | 1H NMR | 1H NMR [19] | 13C NMR | 13C NMR [19] |
|---|---|---|---|---|
| 1 | (4.995; 5.234) | (4.998; 5.233) | 112.57 | 112.1 |
| 2 | 6.393 | 6.395 | 141.16 | 137.5 |
| 3 | - | - | 133.74 | 133.8 |
| 4 | 5.368 | 5.367 | 133.25 | 133.1 |
| 5 | 2.471 | 2.528 | 27.21 | 27.2 |
| 6 | 5.191 | 5.189 | 122.3 | 122.1 |
| 7 | - | - | 132.02 | 132.0 |
| 8 | 1.582 | 1.58 | 21.81 | 21.8 |
| 9 | 1.749 | 1.754 | 14.92 | 15.1 |
| 10 | 1.569 | 1.58 | 21.81 | 21.8 |
| Position | 1H NMR | 1H NMR [19] | 13C NMR | 13C NMR [19] |
|---|---|---|---|---|
| 1 | - | - | 141.16 | 139.8 |
| 2 | 7.160 | 7.104 | 128.87 | 128.7 |
| 3 | 7.243 | 7.243 | 124.30 | 124.3 |
| 4 | - | - | 150.27 | 146.3 |
| 5 | 7.243 | 7.243 | 124.30 | 124.3 |
| 6 | 7.160 | 7.104 | 128.87 | 128.7 |
| 7 | 2.252 | 2.252 | 21.41 | 21.3 |
| 8 | - | - | 70.49 | 71.4 |
| 9 | 1.382 | 1.381 | 31.74 | 31.8 |
| 10 | 1.382 | 1.381 | 31.74 | 31.8 |
| Position | 1H NMR | 1H NMR [20] | 13C NMR | 13C NMR [20] |
|---|---|---|---|---|
| 1 | - | - | 201.32 | 200.0 |
| 2 | 5.963 | 5.951 | 126.83 | 126.8 |
| 3 | - | 161.08 | 161.6 | |
| 4 | (2.296; 2.312) | (2.298; 2.309) | 30.59 | 30.5 |
| 5 | (1.911; 1.846) | (1.910; 1.843) | 22.97 | 23.2 |
| 6 | 2.679 | 2.679 | 51.58 | 51.6 |
| 7 | 2.186 | 2.179 | 24.06 | 24.1 |
| 8 | 1.911 | 1.910 | 25.83 | 25.9 |
| 9 | 0.952 | 0.956 | 20.26 | 20.1 |
| 10 | 0.952 | 0.956 | 20.18 | 20.1 |
| Position | 1H NMR | 1H NMR [21] | 13C NMR | 13C NMR [21] |
|---|---|---|---|---|
| 1 | (5.087; 5.017) | (5.085; 5.016) | 111.65 | 111.54 |
| 2 | 5.860 | 5.878 | 144.52 | 144.86 |
| 3 | - | - | 70.49 | 73.01 |
| 4 | (1.382; 1.623) | (1.388; 1.602) | 41.74 | 41.91 |
| 5 | 2.034 | 2.033 | 22.61 | 22.61 |
| 6 | 5.277 | 5.283 | 124.25 | 124.09 |
| 7 | - | - | 133.74 | 134.63 |
| 8 | 1.894 | 1.894 | 39.65 | 39.46 |
| 9 | 2.229 | 2.200 | 26.45 | 26.41 |
| 10 | 5.287 | 5.288 | 124.25 | 124.13 |
| 11 | - | - | 130.66 | 130.79 |
| 12 | 1.530 | 1.538 | 25.66 | 25.55 |
| 13 | 1.530 | 1.538 | 16.98 | 17.33 |
| 14 | 1.604 | 1.602 | 15.88 | 15.66 |
| 15 | 1.382 | 1.388 | 27.37 | 27.31 |
| Position | 1H NMR | 1H NMR [22] | 13C NMR | 13C NMR [22] |
|---|---|---|---|---|
| 1 | 2.384 | 2.387 | 53.40 | 53.37 |
| 2 | (1.996; 1.681) | (1.996; 1.686) | 26.67 | 26.68 |
| 3 | (1.530; 1.771) | (1.538; 1.778) | 41.74 | 41.71 |
| 4 | - | - | 80.99 | 80.90 |
| 5 | 1.530 | 1.538 | 54.33 | 54.27 |
| 6 | 0.853 | 0.837 | 29.92 | 29.90 |
| 7 | 0.912 | 0.912 | 27.46 | 27.46 |
| 8 | (1.530; 1.521) | (1.538; 1.522) | 24.78 | 24.74 |
| 9 | (2.380; 2.384) | (2.381; 2.387) | 38.86 | 38.83 |
| 10 | - | - | 153.43 | 153.38 |
| 11 | - | - | 20.18 | 20.21 |
| 12 | 1.141 | 1.143 | 28.65 | 28.62 |
| 13 | 1.141 | 1.143 | 16.32 | 16.29 |
| 14 | (4.875; 5.065) | (4.861; 5.061) | 28.7 | 28.65 |
| 15 | 1.327 | 1.329 | 106.25 | 106.22 |
| Position | 1H NMR | 1H NMR [23] | 13C NMR | 13C NMR [23] |
|---|---|---|---|---|
| 1 | 1.73 | 1.76 | 50.7 | 50.9 |
| 2 | (1.57; 1.63) | (1.45; 1.63) | 27.9 | 27.2 |
| 3 | (0.94; 2.03) | (0.95; 2,06) | 39.1 | 39.2 |
| 4 | - | - | 54.3 | 59.6 |
| 5 | 2.85 | 2.86 | 6.37 | 63.6 |
| 6 | (1.28; 2.29) | (1.28; 2.23) | 30.1 | 30.1 |
| 7 | (2.16; 2.85) | (2.11; 2.37) | 29.8 | 29.8 |
| 8 | - | - | 150.2 | 151.7 |
| 9 | (2.85) | 2.60 | 48.7 | 48.7 |
| 10 | (1.43; 1.48) | (1.43; 1.47) | 39.7 | 39.8 |
| 11 | - | - | 33.5 | 33.9 |
| 12 | (4.80; 4.97) | (4.81; 4.99) | 16.9 | 16.9 |
| 13 | 1.20 | 1.19 | 112.7 | 112.7 |
| 14 | (0.98; 1.00) | (0.98; 1.01) | 29.8 | 29.8 |
| 15 | (0.98; 1.00) | (0.98; 1.01) | 20.8 | 21.6 |
| Concentration (mg·mL−1) | |||||||
|---|---|---|---|---|---|---|---|
| Plant species | 5 | 2.5 | 1 | 0.75 | 0.5 | 0.25 | IC50 |
| T. diversifolia | 54.6 ± 0.06 a | 37.4 ± 0.27 b | 27.4 ± 0.41 c | 23.9 ± 0.55 d | 21.8 ± 0.13 eg | 20.2 ± 0.79 fg | 4.30 |
| Concentration (µg·mL−1) | |||||||
|---|---|---|---|---|---|---|---|
| compound | 250 | 125 | 62.5 | 31.25 | 15.62 | 7.81 | IC50 |
| Ascorbic acid | 99.99 ± 0.0 | 99.99 ± 0.0 | 99.99 ± 0.0 | 99.93 ± 0.02 | 30 ± 0.10 | 18.57 ± 0.52 | 16.71 |
| Concentration (µg·mL−1) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Plant species | 1250 | 1000 | 500 | 250 | 100 | 50 | 10 | LC50 |
| T. diversifolia | 100 a | 100 a | 100 a | 100 a | 100 a | 98.1 b | 83.5 c | 3.11 |
| Plant Species T. diversifolia | ||||
|---|---|---|---|---|
| Bacterium | MIC (mg·mL−1) | |||
| 100 | 50 | 25 | 12.5 | |
| Staphylococcus aureus | + | + | NA | NA |
| Escherichia coli | + | + | + | + |
| Pseudomonas aeruginosa | + | + | NA | NA |
| Amoxicillin (Positive Control) | + | + | + | + |
| DMSO (Negative control) | NA | NA | NA | NA |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira Farias, A.L.; Lobato Rodrigues, A.B.; Lopes Martins, R.; de Menezes Rabelo, É.; Ferreira Farias, C.W.; Moreira da Silva de Almeida, S.S. Chemical Characterization, Antioxidant, Cytotoxic and Microbiological Activities of the Essential Oil of Leaf of Tithonia Diversifolia (Hemsl) A. Gray (Asteraceae). Pharmaceuticals 2019, 12, 34. https://doi.org/10.3390/ph12010034
Ferreira Farias AL, Lobato Rodrigues AB, Lopes Martins R, de Menezes Rabelo É, Ferreira Farias CW, Moreira da Silva de Almeida SS. Chemical Characterization, Antioxidant, Cytotoxic and Microbiological Activities of the Essential Oil of Leaf of Tithonia Diversifolia (Hemsl) A. Gray (Asteraceae). Pharmaceuticals. 2019; 12(1):34. https://doi.org/10.3390/ph12010034
Chicago/Turabian StyleFerreira Farias, Ana Luzia, Alex Bruno Lobato Rodrigues, Rosany Lopes Martins, Érica de Menezes Rabelo, Carlos Wagner Ferreira Farias, and Sheylla Susan Moreira da Silva de Almeida. 2019. "Chemical Characterization, Antioxidant, Cytotoxic and Microbiological Activities of the Essential Oil of Leaf of Tithonia Diversifolia (Hemsl) A. Gray (Asteraceae)" Pharmaceuticals 12, no. 1: 34. https://doi.org/10.3390/ph12010034
APA StyleFerreira Farias, A. L., Lobato Rodrigues, A. B., Lopes Martins, R., de Menezes Rabelo, É., Ferreira Farias, C. W., & Moreira da Silva de Almeida, S. S. (2019). Chemical Characterization, Antioxidant, Cytotoxic and Microbiological Activities of the Essential Oil of Leaf of Tithonia Diversifolia (Hemsl) A. Gray (Asteraceae). Pharmaceuticals, 12(1), 34. https://doi.org/10.3390/ph12010034