Synergistic Effect of a HER2 Targeted Thorium-227 Conjugate in Combination with Olaparib in a BRCA2 Deficient Xenograft Model
Abstract
:1. Introduction
2. Results
2.1. Preparation and Characterization of HER2-TTC
2.2. Synergistic Effect of HER2-TTC and PARPi Olaparib on DLD-1 BRCA2 -/- In Vitro
2.3. Specific Tumor Accumulation of HER2-TTC in the HER2 low DLD-1 Xenograft Models
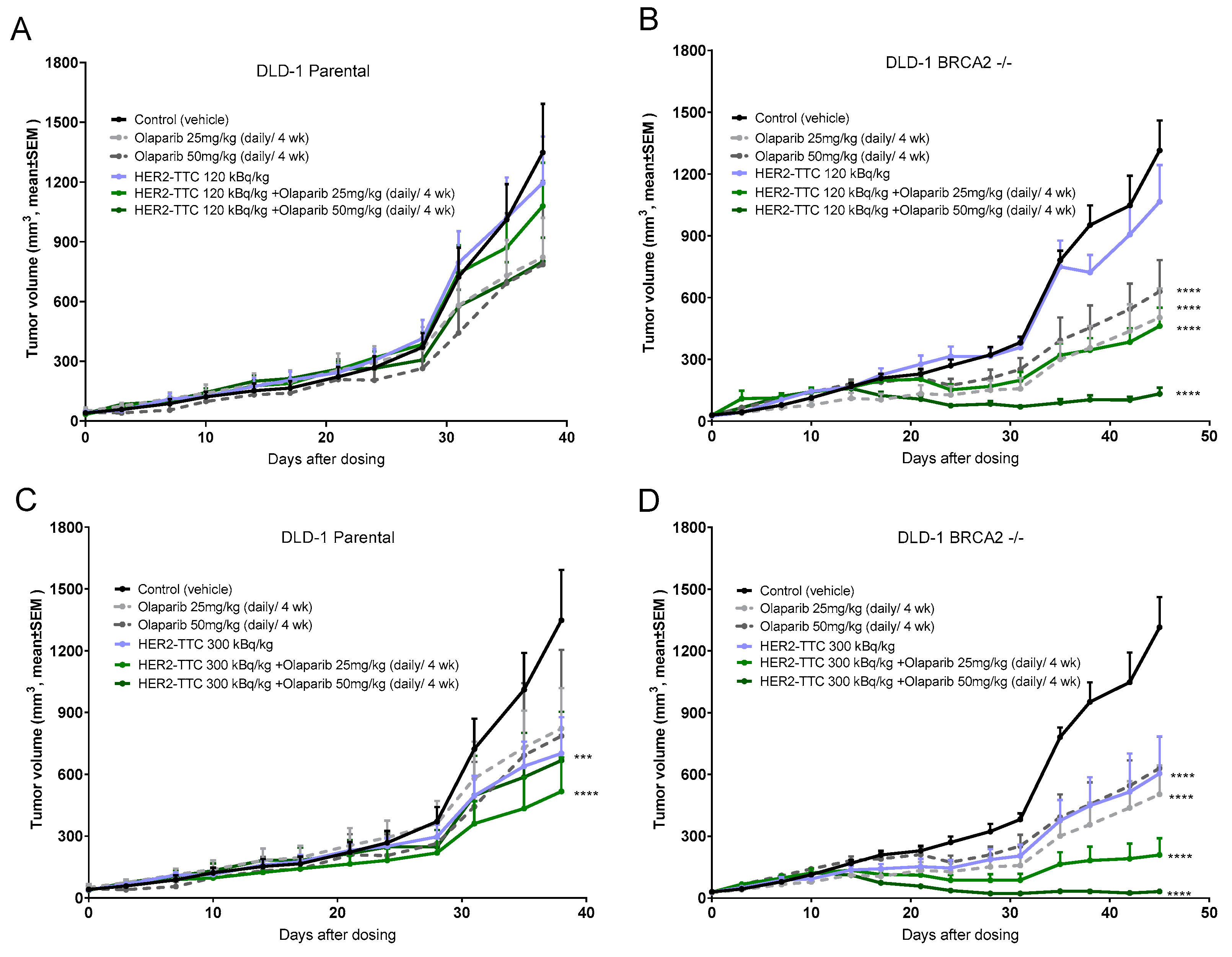
2.4. Significant Dose-Dependent Tumor Growth Inhibition of HER2-TTC and Olaparib in HER2 Low Expressing DLD-1 Xenograft Models after Single Dose Administration
2.5. Synergistic Effect of HER2-TTC in Combination with PARPi Olaparib in the DLD-1 BRCA2 -/- Xenograft Model
2.6. Effect on Hematology of the Combination Compared to Monotherapy
3. Discussion
4. Materials and Methods
5. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Haro, K.J.; Scott, A.C.; Scheinberg, D.A. Mechanisms of resistance to high and low linear energy transfer radiation in myeloid leukemia cells. Blood 2012, 120, 2087–2097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sgouros, G.; Roeske, J.C.; McDevitt, M.R.; Palm, S.; Allen, B.J.; Fisher, D.R.; Brill, A.B.; Song, H.; Howell, R.W.; Akabani, G.; et al. Mird pamphlet No. 22 (abridged): Radiobiology and dosimetry of alpha-particle emitters for targeted radionuclide therapy. J. Nucl. Med. 2010, 51, 311–328. [Google Scholar] [CrossRef]
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fossa, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Suominen, M.I.; Rissanen, J.P.; Kakonen, R.; Fagerlund, K.M.; Alhoniemi, E.; Mumberg, D.; Ziegelbauer, K.; Halleen, J.M.; Kakonen, S.M.; Scholz, A. Survival benefit with radium-223 dichloride in a mouse model of breast cancer bone metastasis. J. Natl. Cancer Inst. 2013, 105, 908–916. [Google Scholar] [CrossRef] [PubMed]
- Ramdahl, T.; Bonge-Hansen, H.T.; Ryan, O.B.; Larsen, S.; Herstad, G.; Sandberg, M.; Bjerke, R.M.; Grant, D.; Brevik, E.M.; Cuthbertson, A.S. An efficient chelator for complexation of thorium-227. Bioorg. Med. Chem. Lett. 2016, 26, 4318–4321. [Google Scholar] [CrossRef] [Green Version]
- Hagemann, U.B.; Sommer, A.; Kristian, A.; Wang, E.; Larsen, Å.; Wirnitzer, U.; Ellinger-Ziegelbauer, H.; Sandmann, S.; Poethko, T.; Karlsson, J.; et al. Preclinical activity of the FGFR2-targeted Thorium-227 conjugate in preclinical models of colorectal, gastric and triple-negative breast cancer. In Proceedings of the AACR Annual Meeting 2017, Washington, DC, USA, 1–5 April 2017. [Google Scholar] [CrossRef]
- Hagemann, U.B.; Mihaylova, D.; Uran, S.R.; Borrebaek, J.; Grant, D.; Bjerke, R.M.; Karlsson, J.; Cuthbertson, A.S. Targeted alpha therapy using a novel CD70 targeted thorium-227 conjugate in in vitro and in vivo models of renal cell carcinoma. Oncotarget 2017, 8, 56311. [Google Scholar] [CrossRef]
- Karlsson, J.; Hagemann, U.B.; Schatz, C.; Grant, D.; Kristian, A.; Ellingsen, C.; Mihaylova, D.; Geraudie, S.; Indrevoll, B.; Wirnitzer, U.; et al. HER2-targeted thorium-227 conjugate (HER2-TTC): Efficacy in preclinical models of trastuzumab and T-DM1 resistance. In Proceedings of the AACR Annual Meeting 2017, Washington, DC, USA, 1–5 April 2017. [Google Scholar] [CrossRef]
- Hammer, S.; Larssen, A.; Ellingsen, C.; Geraudie, S.; Grant, S.; Indrevoll, B.; Oliver von Ahsen, A.K.; Hagemann, U.B.; Karlsson, J.; Bjerke, R.M.; et al. Preclinical pharmacology of the PSMA-targeted thorium-227 conjugate PSMA-TTC: A novel targeted alpha therapeutic for the treatment of prostate cancer. In Proceedings of the AACR Annual Meeting 2017, Washington, DC, USA, 1–5 April 2017. [Google Scholar] [CrossRef]
- Hagemann, U.B.; Wickstroem, K.; Wang, E.; Shea, A.O.; Sponheim, K.; Karlsson, J.; Bjerke, R.M.; Ryan, O.B.; Cuthbertson, A.S. In vitro and in vivo efficacy of a novel CD33-targeted thorium-227 conjugate for the treatment of acute myeloid leukemia. Mol. Cancer Ther. 2016, 15, 2422–2431. [Google Scholar] [CrossRef]
- Roskoski, R., Jr. The ErbB/HER family of protein-tyrosine kinases and cancer. Pharmacol. Res. 2014, 79, 34–74. [Google Scholar] [CrossRef]
- Frankenberg-Schwager, M.; Frankenberg, D.; Harbich, R. Repair of DNA double-strand breaks as a determinant of RBE of alpha particles. Br. J. Cancer Suppl. 1984, 6, 169–173. [Google Scholar]
- Baidoo, K.E.; Yong, K.; Brechbiel, M.W. Molecular pathways: Targeted alpha-particle radiation therapy. Clin. Cancer Res. 2013, 19, 530–537. [Google Scholar] [CrossRef]
- Choi, J.; Vaidyanathan, G.; Koumarianou, E.; Kang, C.M.; Zalutsky, M.R. Astatine-211 labeled anti-HER2 5F7 single domain antibody fragment conjugates: Radiolabeling and preliminary evaluation. Nucl. Med. Boil. 2018, 56, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Pruszynski, M.; D’Huyvetter, M.; Bruchertseifer, F.; Morgenstern, A.; Lahoutte, T. Evaluation of an anti-HER2 nanobody labeled with 225ac for targeted alpha-particle therapy of cancer. Mol. Pharm. 2018, 15, 1457–1466. [Google Scholar] [CrossRef] [PubMed]
- Jang, N.Y.; Kim, D.H.; Cho, B.J.; Choi, E.J.; Lee, J.S.; Wu, H.G.; Chie, E.K.; Kim, I.A. Radiosensitization with combined use of olaparib and PI-103 in triple-negative breast cancer. BMC Cancer 2015, 15, 89. [Google Scholar] [CrossRef] [PubMed]
- Dillon, M.T.; Good, J.S.; Harrington, K.J. Selective targeting of the g2/m cell cycle checkpoint to improve the therapeutic index of radiotherapy. Clin. Oncol. (R. Coll. Radiol.) 2014, 26, 257–265. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, M.J. Targeting the DNA damage response in cancer. Mol. Cell 2015, 60, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Ame, J.C.; Spenlehauer, C.; de Murcia, G. The parp superfamily. Bioessays 2004, 26, 882–893. [Google Scholar] [CrossRef] [PubMed]
- Tao, G.H.; Yang, L.Q.; Gong, C.M.; Huang, H.Y.; Liu, J.D.; Liu, J.J.; Yuan, J.H.; Chen, W.; Zhuang, Z.X. Effect of PARP-1 deficiency on DNA damage and repair in human bronchial epithelial cells exposed to Benzo(a)pyrene. Mol. Biol. Rep. 2009, 36, 2413–2422. [Google Scholar] [CrossRef]
- Benafif, S.; Hall, M. An update on parp inhibitors for the treatment of cancer. Onco Targets Ther. 2015, 8, 519–528. [Google Scholar]
- Deblonde, G.J.; Lohrey, T.D.; Booth, C.H.; Carter, K.P.; Parker, B.F.; Larsen, A.; Smeets, R.; Ryan, O.B.; Cuthbertson, A.S.; Abergel, R.J. Solution thermodynamics and kinetics of metal complexation with a hydroxypyridinone chelator designed for thorium-227 targeted alpha therapy. Inorg. Chem. 2018, 57, 14337–14346. [Google Scholar] [CrossRef]
- Li, J.Y.; Perry, S.R.; Muniz-Medina, V.; Wang, X.; Wetzel, L.K.; Rebelatto, M.C.; Hinrichs, M.J.; Bezabeh, B.Z.; Fleming, R.L.; Dimasi, N.; et al. A biparatopic HER2-targeting antibody-drug conjugate induces tumor regression in primary models refractory to or ineligible for HER2-targeted therapy. Cancer Cell 2016, 29, 117–129. [Google Scholar] [CrossRef]
- Chou, T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef] [PubMed]
- Kozempel, J.; Mokhodoeva, O.; Vlk, M. Progress in targeted alpha-particle therapy. What we learned about recoils release from in vivo generators. Molecules 2018, 23, 581. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Sachsenmeier, K.; Zhang, L.; Sult, E.; Hollingsworth, R.E.; Yang, H. A new bliss independence model to analyze drug combination data. J. Biomol. Screen. 2014, 19, 817–821. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, J.; Hagemann, U.B.; Schatz, C.; Grant, D.; Ellingsen, C.; Kristian, A.; Mihaylova, D.; Uran, S.R.; Suominen, M.; Bjerke, R.M.; et al. HER2-targeted thorium-227 conjugate (HER2-TTC): Efficacy in a HER2 positive orthotopic bone model. In Proceedings of the AACR Annual Meeting 2017, Washington, DC, USA, 1–5 April 2017. [Google Scholar] [CrossRef]
- Scott, C.L.; Swisher, E.M.; Kaufmann, S.H. Poly (ADP-ribose) polymerase inhibitors: Recent advances and future development. J. Clin. Oncol. 2015, 33, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- Valerie, K.; Povirk, L.F. Regulation and mechanisms of mammalian double-strand break repair. Oncogene 2003, 22, 5792–5812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schultz, N.; Lopez, E.; Saleh-Gohari, N.; Helleday, T. Poly(ADP-ribose) polymerase (PARP-1) has a controlling role in homologous recombination. Nucleic Acids Res. 2003, 31, 4959–4964. [Google Scholar] [CrossRef]
- Maeda, H. Macromolecular therapeutics in cancer treatment: The EPR effect and beyond. J. Control. Release 2012, 164, 138–144. [Google Scholar] [CrossRef]
- Alsop, K.; Fereday, S.; Meldrum, C.; de Fazio, A.; Emmanuel, C.; George, J.; Dobrovic, A.; Birrer, M.J.; Webb, P.M.; Stewart, C.; et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: A report from the Australian ovarian cancer study group. J. Clin. Oncol. 2012, 30, 2654–2663. [Google Scholar] [CrossRef]
- Walsh, C.S. Two decades beyond BRCA1/2: Homologous recombination, hereditary cancer risk and a target for ovarian cancer therapy. Gynecol. Oncol. 2015, 137, 343–350. [Google Scholar] [CrossRef] [Green Version]
- Abbas, N.; Heyerdahl, H.; Bruland, O.S.; Borrebaek, J.; Nesland, J.; Dahle, J. Experimental alpha-particle radioimmunotherapy of breast cancer using 227th-labeled p-benzyl-DOTA-trastuzumab. EJNMMI Res. 2011, 1, 18. [Google Scholar] [CrossRef]
- Reddy, N.; Ong, G.L.; Behr, T.M.; Sharkey, R.M.; Goldenberg, D.M.; Mattes, M.J. Rapid blood clearance of mouse IgG2a and human IgG1 in many nude and nu/+ mouse strains is due to low IgG2a serum concentrations. Cancer Immunol. Immunother. 1998, 46, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Bliss, C.I. The toxicity of poisons applied jointly. Ann. Appl. Biol. 1939, 26, 585–615. [Google Scholar] [CrossRef]





| Tissue Type | DLD-1 Parental | DLD-1 BRCA2 -/- | |
|---|---|---|---|
| Colorectal Adenocarcinoma | |||
| Cancer model characteristics | mAb/receptor | 6500 | 3400 |
| Average doubling time in vitro (h)/in vivo (d) | 35/7.3 | 57/8.3 | |
| HER2 IHC score | 1—2+ | 1—2+ | |
| In vitro cytotoxicity | IC50 (kBq/mL HER2-TTC) | 22 | 3 |
| IC50 (pM 227Th) | 86 | 10 | |
| IC50 (µM olaparib) | 5 | 0.03 | |
| Average combination index (CI) | 1 | 0.6 | |
| Biodistribution | Tumor/blood ratio (HER2-TTC, 336 h) | 12.8 ± 2.4 | 17.3 ± 3.5 |
| Tumor/blood ratio (isotype control, 336 h) | 1.2 | 1.5 | |
| Treatment/Control In vivo Efficacy | HER2-TTC, 125 kBq/kg bw | 0.9 (n.s.) | 0.8 (n.s.) |
| HER2-TTC, 300 kBq/kg bw | 0.5 (p = 0.03) | 0.5 (p < 0.0001) | |
| HER2-TTC, 600 kBq/kg bw | 0.3 (p < 0.0001) | 0.1 (p < 0.0001) | |
| Olaparib 25 mg/kg bw | 0.6 (n.s.) | 0.4 (p < 0.0001) | |
| Olaparib 50 mg/kg bw | 0.6 (n.s.) | 0.5 (p < 0.0001) | |
| 125 kBq/kg bw + 25 mg/kg bw olaparib | 0.8 (n.s.) | 0.4 (p < 0.0001) | |
| 125 kBq/kg bw + 50 mg/kg bw olaparib | 0.6 (n.s.) | 0.1 (p < 0.0001) | |
| 300 kBq/kg bw + 25 mg/kg bw olaparib | 0.4 (p < 0.0001) | 0.2 (p < 0.0001) | |
| 300 kBq/kg bw + 50 mg/kg bw olaparib | 0.5 (p = 0.009) | 0.03 (p < 0.0001) | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wickstroem, K.; Karlsson, J.; Ellingsen, C.; Cruciani, V.; Kristian, A.; Hagemann, U.B.; Bjerke, R.M.; Ryan, O.B.; Linden, L.; Mumberg, D.; et al. Synergistic Effect of a HER2 Targeted Thorium-227 Conjugate in Combination with Olaparib in a BRCA2 Deficient Xenograft Model. Pharmaceuticals 2019, 12, 155. https://doi.org/10.3390/ph12040155
Wickstroem K, Karlsson J, Ellingsen C, Cruciani V, Kristian A, Hagemann UB, Bjerke RM, Ryan OB, Linden L, Mumberg D, et al. Synergistic Effect of a HER2 Targeted Thorium-227 Conjugate in Combination with Olaparib in a BRCA2 Deficient Xenograft Model. Pharmaceuticals. 2019; 12(4):155. https://doi.org/10.3390/ph12040155
Chicago/Turabian StyleWickstroem, Katrine, Jenny Karlsson, Christine Ellingsen, Véronique Cruciani, Alexander Kristian, Urs B. Hagemann, Roger M. Bjerke, Olav B. Ryan, Lars Linden, Dominik Mumberg, and et al. 2019. "Synergistic Effect of a HER2 Targeted Thorium-227 Conjugate in Combination with Olaparib in a BRCA2 Deficient Xenograft Model" Pharmaceuticals 12, no. 4: 155. https://doi.org/10.3390/ph12040155




