Acute Toxicity of the Hydroethanolic Extract of the Flowers of Acmella oleracea L. in Zebrafish (Danio rerio): Behavioral and Histopathological Studies
Abstract
:1. Introduction
2. Results
2.1. Analyses by Ultra-High-Resolution Liquid Chromatography–Electrospray Ionization Mass Spectrometry (UHPLC–ESI-MS)
2.2. Behavioral Analysis
2.3. Determination of LD50 and LC50
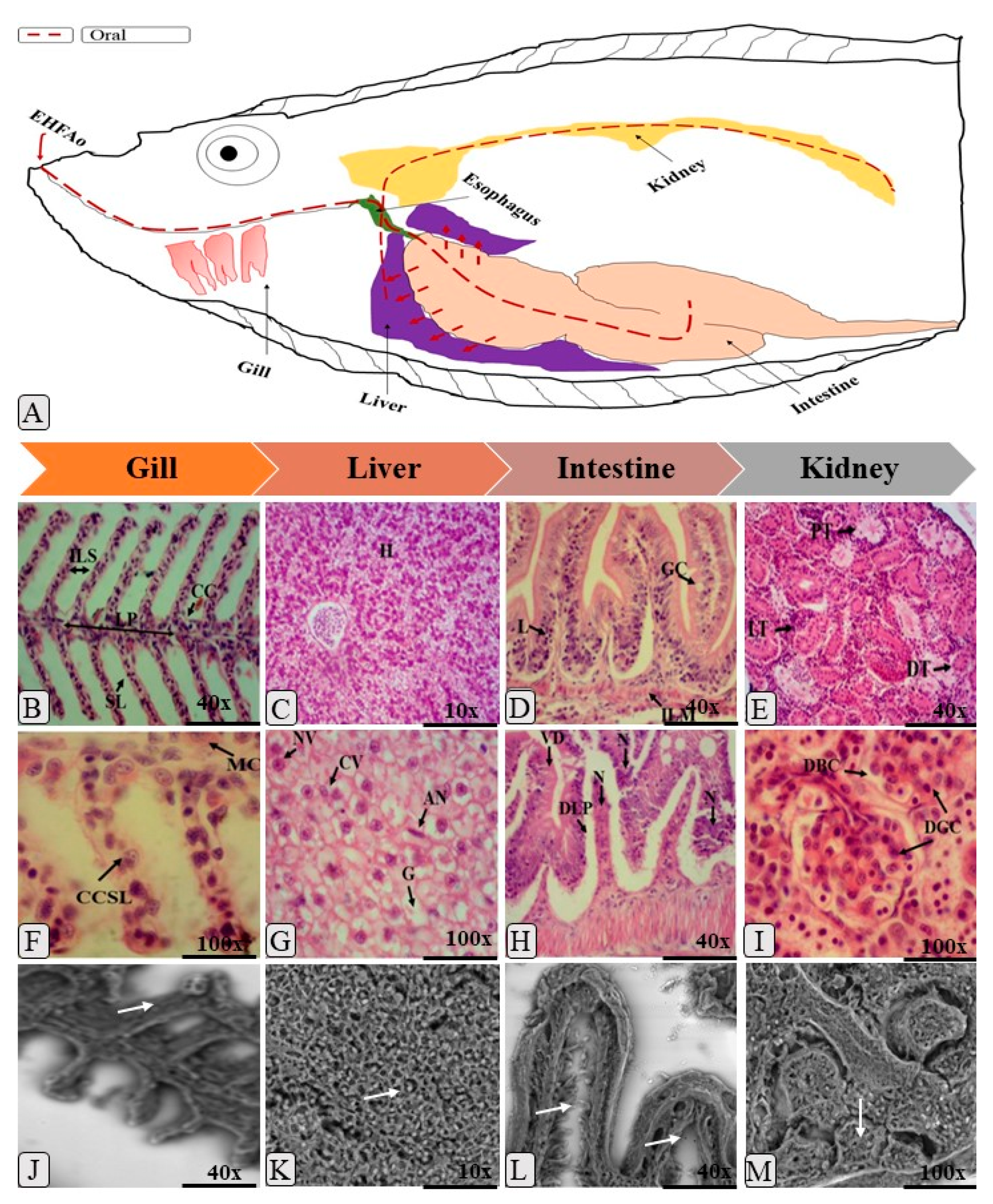
2.4. Histopathology
3. Discussion
4. Material and Methods
4.1. Plant Material
4.2. Obtaining the Hydroethanolic Extract of the Flowers of A. Oleracea (L.) R. K. Jansen (EHFAo)
4.3. Acute Toxicity Study of EHFAo
4.3.1. Animals
4.3.2. Experimental Design
4.3.3. Behavioral Analysis and Mortality
4.3.4. Determination of LD50 and LC50
4.3.5. Histopathological Analysis
4.3.6. Assessment of Histopathological Changes
4.3.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fent, K.; Weston, A.A.; Caminada, D. Ecotoxicology of human pharmaceuticals. Aquat. Toxicol. 2006, 76, 122–159. [Google Scholar] [CrossRef] [PubMed]
- Gaworecki, K.M.; Klaine, S.J. Behavioral and biochemical responses of hybrid striped bass during and after fluoxetine exposure. Aquat. Toxicol. 2008, 88, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Villachica, H.; Carvalho, J.E.U.; Muller, C.H.; Diaz, S.C.; Almanza, M. Frutales y Hortalizas Promissórios de la Amazônia; Lima: TCA, Secretaria Protempore: Brasília, Brazil, 1996; pp. 322–327. [Google Scholar]
- Ratnasooriya, W.D.; Pieris, K.P.P.; Samaratunga, U.; Jayakody, J.R.A.C. Diuretic activity of Spilanthes acmella flowers in rats. J. Ethnopharmacol. 2004, 91, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Lorenzi, H.; Matos, F.J.A. Plantas Medicinais do Brasil: Nativas e Exóticas Cultivadas; Instituto Plantarum: Nova Odessa, Brazil, 2002; p. 396. [Google Scholar]
- Vulpi, T.S.; Morais, C.P.M.; Trindade, A.P.F.; Lima, M.C.H.P.; Velozo, L.S.M.; Kaplan, M.A.C. Análise do óleo e ssencial dos diferentes órgãos de Acmella ci liata Kunth (Asteraceae). Revista Brasileira de Biociências 2007, 5, 1128–1130. [Google Scholar]
- Gerbino, A.; Schena, G.; Milano, S.; Milella, L.; Barbosa, A.F.; Armentano, F.; Procino, G.; Svelto, M.; Carmosino, M. Spilanthol from Acmella oleracea lowers the intracellular levels of cAMP impairing NKCC2 phosphorylation and water channel AQP2 membrane expression in mouse kidney. PLoS ONE 2016, 11, e0156021. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.; Yadav, N.; Kharya, M.D.; Savadi, R. Preliminary studies on diuretic effect of Spilanthes acmella leaves extracts in rats. Int. J. Pharm. Pharm. Sci. 2011, 3, 245–247. [Google Scholar]
- Ramsewak, R.S.; Erickson, A.J. Bioactive N-isobutylamides from flower buds of Spilantes acmella. Phytochemistry 1999, 26, 729–732. [Google Scholar] [CrossRef]
- Favoreto, R.; Gilbert, B. Acmella oleracea (L.) R. K. Jansen (Asteraceae)—Jambu. Rev. Fitos 2010, 5, 83–90. [Google Scholar]
- Vijendra, N.; Kumar, K.P. Traditional knowledge on ethnomedicinal uses prevailing in tribal pockets of Chhindwara and Betul Districts, Madhya Pradesh, India. Afr. J. Pharm. Pharmacol. 2010, 4, 662–670. [Google Scholar]
- Santesson, C.G. Several drugs of the Cameroon District and their native uses. Archiv Furdie Botanik A 1926, 20, 1–34. [Google Scholar]
- Moreno, S.C.; Carvalho, G.A.; Picanço, M.C.; Morais, E.G.F.; Pereira, R.M. Bioactivity of compounds from Acmella oleracea against Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) and selectivity to two non-target species. Pest. Manag. Sci. 2012, 68, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.M.E.; Chávez, A.G. Alcamidas em plantas: Distribución e importancia. Av. Y. Perspect. 2001, 20, 377–387. [Google Scholar]
- Chakraborty, A.; Devi, R.K.B.; Rita, S.; Sharatchandra, K.H.; Singh, T.I. Preliminary studies on anti-inflammatory and analgesic activities of Spilanthes acmella in experimental animal model. Indian J. Pharmacol. 2004, 36, 148–150. [Google Scholar]
- Andrade, L.C.; Rotta, I.W.; Chaves, S.R.; Uchida, D.T.; Ferreira, F.B.P.; Jacomassi, E.; Boleta-Ceranto, D.C.F.; Gazim, Z.C. Effectiveness of Acmella oleracea for topical anesthesia on buccal mucosa. Revista Odonto Ciência 2013, 28, 61–65. [Google Scholar]
- Freitas-Blanco, V.S.; Franz-Montan, M.; Groppo, F.C.; Carvalho, J.E.; Figueira, G.M.; Serpe, L.; Sousa, I.M.O.; Damasio, V.A.G.; Yamane, L.T.; Paula, E.; et al. Development and evaluation of a novel mucoadhesive film containing Acmella oleracea extract for oral mucosa topical anesthesia. PLoS ONE 2016, 11, e0162850. [Google Scholar] [CrossRef] [PubMed]
- Ratnasooriya, W.D.; Pieris, K.P.P. Attenuation of persistent pain and hyperalgesia by Spilanthus acmella flowers in rats. Pharm. Biol. 2005, 43, 614–619. [Google Scholar] [CrossRef]
- Wu, L.; Huang, S.; Fan, N.; Lin, M.; Hu, C.; Chu, I.; Han, S. Anti-inflammatory effect of Spilanthol from Spilanthes acmella on murine macrophage by down-regulating LPS-induced inflammatory mediators. J. Agric. Food Chem. 2008, 56, 2341–2349. [Google Scholar] [CrossRef]
- Boonen, J.; Baert, B.; Burvenich, C.; Blondeel, P.; Saeger, S.; Spiegeleer, B. LC-MS profiling of N-alkylamides in Spilanthes acmella extract and the transmucosal behaviour of its main bioactive spilanthol. J. Pharm. Biomed. Anal. 2010, 53, 243–249. [Google Scholar] [CrossRef]
- Jacobson, M. The structure of spilanthol. Chem. Ind. 1957, 12, 50–51. [Google Scholar]
- Prachayasittukal, V.; Prachayasittukal, S.; Ruchirawat, S.; Prachayasittikul, V. High therapeutic potential of Spilanthes acmella: A review. EXCLI J. 2013, 12, 291–312. [Google Scholar]
- Santos, I.V.F.; Souza, G.C.; Santana, G.R.; Duarte, J.L.; Fernandes, C.P.; Keita, H.; Velázquez-Moyado, J.A.; Navarrete, A.; Ferreira, I.M.; Carvalho, H.O.; et al. Histopathology in Zebrafish (Danio rerio) to Evaluate the Toxicity of Medicine: An Anti-Inflammatory Phytomedicine with Janaguba Milk (Himatanthus drasticus Plumel), Cap 3; Wiley Blackwell: Hoboken, NJ, USA, 2018. [Google Scholar]
- Souza, G.C.; Duarte, J.L.; Fernandes, C.P.; Velázquez-Moyado, J.A.; Navarrete, A.; Carvalho, J.C.T. Obtainment and study of the toxicity of perillyl alcohol nanoemulsion on zebrafish (Danio rerio). J. Nanomed. Res. 2016, 4, 00093. [Google Scholar]
- Santos, I.V.F.; Duarte, J.L.; Fernandes, C.P.; Keita, H.; Amado, J.R.R.; Velázquez-Moyado, J.A.; Navarrete, A.; Carvalho, J.C.T. Use of zebrafish (Danio rerio) in experimental models for biological assay with natural products. Afr. J. Pharm. Pharmacol. 2016, 10, 883–891. [Google Scholar]
- Borges, S.B.; Keita, H.; Sanchez-Ortiz, B.L.; Sampaio, T.I.S.; Ferreira, I.M.; Lima, E.S.; Silva, M.J.A.; Fernandes, C.P.; Oliveira, A.E.M.F.M.; Conceição, E.C.; et al. Anti-inflammatory activity of nanoemulsions of essential oil from Rosmarinus officinalis L.: In vitro and in zebrafish studies. Inflammopharmacology 2018, 26, 1057–1080. [Google Scholar] [CrossRef] [PubMed]
- Collymore, C.; Rasmussen, S.; Tolwani, R.J. Gavaging Adult Zebrafish. J. Vis. Exp. 2013, 78, e50691. [Google Scholar] [CrossRef] [PubMed]
- Borges, R.B.; Souza, G.C.; Ferreira, A.C.M.; Carvalho, J.C.T. Use of Zebrafish (Danio rerio) in Non-Clinical Toxicological Studies of New Drugs; IntechOpen: London, UK, 2019. [Google Scholar]
- Souza, G.C.; Pereira, A.C.M.; Viana, M.D.; Ferreira, A.M.; Silva, I.D.R.; Oliveira, M.M.R.; Barbosa, W.L.R.; Silva, L.B.; Ferreira, I.M.; Santos, C.B.R.; et al. Acmella oleracea (L) R. K. Jansen Reproductive Toxicity in Zebrafish: An In Vivo and In Silico Assessment. Evid.-Based Complement. Altern. Med. 2019, 2019, 1237301. [Google Scholar] [CrossRef]
- Nakatani, N.; Nagashima, M. Pungent Alkamides from Spilanthes acmella L. var. oleracea Clarke. Biosci. Biotechnol. Biochem. 1992, 56, 759–762. [Google Scholar] [CrossRef]
- Dubey, S.; Maity, S.; Singh, M.; Saraf, S.A.; Saha, S. Phytochemistry, pharmacology and toxicology of Spilanthes acmella: A review. Adv. Pharmacol. Sci. 2013, 2013, 423750. [Google Scholar]
- Cilia-López, V.G.; Juárez-Flores, B.I.; Aguirre-Rivera, J.R.; Reyes-Aguero, J.A. Analgesic activity of Heliopsis longipes and its effect on the nervous system. Pharm. Biol. 2010, 48, 195–200. [Google Scholar] [CrossRef]
- Tiwari, K.L.; Jadhav, S.K.; Joshi, V. An updated review on medicinal herb Genus Spilanthes. J. Chin. Integr. Med. 2011, 9, 1170–1178. [Google Scholar] [CrossRef]
- Dias, A.M.A.; Santos, P.; Seabraa, I.J.; Junior, R.N.C.; Braga, M.E.M.; de Sousa, H.C. Spilanthol from Spilanthes acmella flowers, leaves and stems obtained by selective supercritical carbon dioxide extraction. J. Supercrit. Fluids. 2012, 61, 62–70. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Rattan, R.S.; Kumar, N.; Singh, B. Insecticidal toxicity of spilanthol from Spilanthes acmella Murr. Against Plutella xylostella L. Am. J. Plant Sci. 2012, 3, 1568–1572. [Google Scholar] [CrossRef]
- Abeysiri, G.R.P.I.; Dharmadasa, R.M.; Abeysinghe, D.C.; Samarasinghe, K. Screening of phytochemical, physico-chemical and bioactivity of different parts of Spilantes acmella Murr. (Asteraceae), a natural remedy for toothache. Ind. Crop. Prod. 2013, 50, 852–856. [Google Scholar] [CrossRef]
- Hernández, I.; Márquez, L.; Martínez, I.; Dieguez, R.; Delporte, C.; Prietoa, S.; Molina Torres, J.; Garrido, G. Anti-inflammatory effects of ethanolic extract and alkamides-derived from Heliopsis longipes roots. J. Ethnopharmacol. 2009, 124, 649–652. [Google Scholar] [CrossRef]
- Demarne, F.; Passaro, G. Use of an Acmella Oleracea Extract for the Botulinum Toxin-Like Effect Thereof in an Anti-Wrinkle Cosmetic Composition. U.S. Patent 7531193, 12 May 2009. [Google Scholar]
- Ribeiro, L.C. Investigação do efeito ictiotóxico do extrato etanolico da raiz de Spilanthes acmella (jambu) em zebrafish através da análise eletrofisiológica e comportamental. 61 f. Master’s Thesis, Programa de Pós-Graduação em Neurociências e Biologia Celular da Universidade Federal do Pará, Instituto de Ciências Biológicas, Belém, Brasil, 2013. [Google Scholar]
- Little, E.E.; Fairchild, J.F.; Delonay, A.J. Behavioral Methods for Assessing Impacts of Contaminants on Early Life Stage Fishes; American Fisheries Society: Bethesda, MD, USA, 1993; pp. 67–76. [Google Scholar]
- Everds, N.E.; Snyder, P.W.; Bailey, K.L.; Bolon, B.; Creasy, D.M.; Foley, G.L.; Rosol, T.J.; Sellers, T. Interpreting Stress Responses during Routine Toxicity Studies: A Review of the Biology, Impact, and Assessment. Toxicol. Pathol. 2013, 41, 560–614. [Google Scholar] [CrossRef]
- Nomura, E.C.O.; Rodrigues, M.R.A.; Silva, C.F.S.; Hamm, L.A.; Nascimento, A.M.; de Souza, L.M.; Cipriani, T.R.; Baggio, C.H.; de Paula Werner, M.F. Antinociceptive effects of ethanolic extract from the flowers of Acmella oleracea (L.) R.K. Jansen in mice. J. Ethnopharmacol. 2013, 150, 583–589. [Google Scholar] [CrossRef]
- Takashima, F.; Hibiya, T. (Eds.) An atlas of fish histology. In Normal and Pathological Features, 2nd ed.; Kodansha Ltd.: Tokyo, Japan, 1995. [Google Scholar]
- Barron, M.G. Bioaccumulation and bioconcentration in aquatic organisms. In Handbook of Ecotoxicology; Hoffman, D.J., Rattner, B.A., Burton, G.A., Jr., Cairns, J., Jr., Eds.; Lewis Publishers: Boca Raton, FL, USA, 1995; pp. 652–666. [Google Scholar]
- Barreto, T.R. Alterações morfofuncionais e metabólicas no teleósteo de água doce matrinxã, Brycon cephalus (GÜNTHER, 1869) exposto ao organofosforado metil paration (Folisuper 600 BR®). 105f. Master’s Thesis, Programa de Pós-Graduação em Ciências Fisiológicas da Universidade Federal de São Carlos, São Paulo, Brasil, 2007. [Google Scholar]
- Ringolin-Sá, O. Toxicidade do Herbicida Roundup (glifosato) e do Acaricida Omite (propargito) nas Fases Iniciais da Ontogenia do Bagre Rhamdia Hilarii (Valenciennes, 1840) (Pimelodidae, Siluriformes); Universidade Federal de São Carlos: São Carlos, Brasil, 1999. [Google Scholar]
- Heath, A.G. Water Pollution and Fish Physiology; CRC Press: Boca Raton, FL, USA, 1987; p. 245. [Google Scholar]
- Muller, R.; Lloyd, R. Sublethal and Chronic Effects of Pollutants on Freshwater Fish; Fishing News Books: Oxford, UK, 1994; p. 378. [Google Scholar]
- Carvalho, J.C.T.; Keita, H.; Santana, G.R.; Souza, G.C.; Santos, I.V.F.; Amado, J.R.R.; Kourouma, A.; Prada, A.L.; Carvalho, H.O.; Silva, M.L. Effects of Bothrops alternatus venom in zebrafish: A histopathological study. Inflammopharmacology 2017, 25, 273–284. [Google Scholar] [CrossRef]
- Rezende, K.F.O.; Santos, R.M.; Borges, J.C.S.; Salvo, L.M.; Da Silva, J.R.M.H.C. Histopathological and genotoxic effects of pollution on Nile Tilapia (Oreochromis niloticus, Linnaeus, 1758) in the Billings Reservoir (Brazil). Toxicol. Mech. Methods 2014, 24, 404–411. [Google Scholar] [CrossRef]
- Campagna, A.F. Effects of the copper in the survival, growth and gill morphology of Danio rerio (Cypriniformes, Cyprinidae). Acta Limnol. Bras. 2008, 20, 253–259. [Google Scholar]
- Smart, G. The effect of ammonia exposure on gill structure of the rainbow trout (Salmo gairdneri). J. Fish Biol. 1976, 8, 471–475. [Google Scholar] [CrossRef]
- Rand, G.M.; Petrocelli, S.R. Fundamentals of Aquatic Toxicology: Methods and Applications; Hemisphere: Washington, DC, USA, 1985. [Google Scholar]
- Nogueira, D.J. Utilização das brânquias de Pimelodus maculatus (Lacèpéde, 1803) (Siluriformes; Pimelodidae) como biomarcador de poluição no reservatório da UHE Marechal Mascarenhas de Moraes, Minas Gerais, Brasil. Biotemas 2011, 24, 51–58. [Google Scholar] [CrossRef]
- Holden, J.A.; Layfield, L.L.; Matthews, J.L. The Zebrafish: Atlas of Macroscopic and Microscopic Anatomy; Cambridge University Press: Cambridge, UK, 2012; pp. 58–100. [Google Scholar]
- Takashima, F.; Hibiya, T. An atlas of fish histology-normal and pathological features. Kodansha Ltd. Tóquio 1984, 69, 406. [Google Scholar]
- Abel, P.D. Toxic action of several lethal concentrations of an anionic detergent on the gills of the brown trout (Salmo trutta L.). J. Fish Biol. 1976, 9, 441–446. [Google Scholar] [CrossRef]
- Goksøyr, A. Use of cytochrome P450 lA (CYP1A) in fish as a biomarker of aquatic pollution. Arch. Toxicol. Suppl. 1995, 17, 80–95. [Google Scholar] [PubMed]
- Vliegenthart, A.D.; Tucker, C.S.; Del Pozo, J.; Dear, J.W. Zebrafish as model organisms for studying drug-induced liver injury. Br. J. Clin. Pharmacol. 2014, 78, 1217–1227. [Google Scholar] [CrossRef]
- Alvarez-Pellitero, P.; Sitja-Bobadilla, A. Pathology of Myxosporea in marine fish culture. Dis. Aquat. Org. 2011, 17, 229–238. [Google Scholar] [CrossRef]
- Roberts, R.J.; Ellis, A.E. The anatomy and physiology of teleosts. In Fish Pathology, 3rd eds.; Roberts, R.J., Ed.; W. B. Saunders: Philadelphia, PA, USA, 2012; pp. 12–54. [Google Scholar]
- Leary, S.; Anthony, R.; Cartner, S.; Corey, D.; Grandin, T.; Greenacre, C.; Gwaltney-Brant, S.; McCrackin, M.A.; Meyer, R.; Miller, D.; et al. AVMA Guidelines for the Euthanasia of Animals; American Veterinary Medical Association: Schaumburg, IL, USA, 2013. [Google Scholar]
- Sampaio, T.I.S.; Melo, N.C.; Paiva, B.T.F.; Aleluia, G.A.S.; Silva Neto, F.L.P.; Silva, H.R.; Keita, H.; Cruz, R.A.S.; Sánchez-Ortiz, B.L.; Pineda-Peña, E.A.; et al. Leaves of Spondias mombin L. a traditional anxiolytic and antidepressant: Pharmacological evaluation on zebrafish (Danio rerio). J. Ethnopharmacol. 2018, 224, 563–578. [Google Scholar] [CrossRef]
- Melo, N.C.; Sánchez-Ortiz, B.L.; Sampaio, T.I.S.; Pereira, A.C.M.; Silva Neto, F.L.P.; Silva, H.R.; Cruz, R.A.S.; Keita, H.; Pereira, A.M.S.; Carvalho, J.C.T. Anxiolytic and Antidepressant Effects of the Hydroethanolic Extract from the Leaves of Aloysia polystachya (Griseb.) Moldenke: A Study on Zebrafish (Danio rerio). Pharmaceuticals 2019, 12, 106. [Google Scholar] [CrossRef] [Green Version]
- Poleksic, V.; Mitrovic-Tutundzic, V. Fish gills as a monitor of sublethal and chronic effects of pollution. In Sublethal and Chronic Effects of Pollutants on Freshwater Fish.; Müller, R., Lloyd, R., Eds.; Fishing New Books ltd. Farnham: Oxford, UK, 1994; pp. 339–352. [Google Scholar]





| Group | Stage I | Stage II | Stage III | Total | % | Médian |
|---|---|---|---|---|---|---|
| Control | 0/3 | 0/2 | 0/4 | 0/9 | 0 | 0.0 ± 0.0 |
| 44.457 mg/kg | 1/3 | 2/2 | 3/4 | 6/9 | 66.6 | 58.6 ± 1.97 |
| 88. 914 mg/kg | 1/3 | 2/2 | 3/4 | 6/9 | 66.6 | 59 ± 2.58 |
| 199.53 mg/kg | 2/3 | 2/2 | 3/4 | 7/9 | 77.7 | 76.9 ± 2.22 |
| 218.83 mg/kg | 2/3 | 2/2 | 4/4 | 8/9 | 88.8 | 87.6 ± 1.99 |
| 448.81 mg/kg | 3/3 | 2/2 | 4/4 | 9/9 | 100 | 100 ± 0.0 |
| Group | Stage I | Stage II | Stage III | Total | % | Median |
|---|---|---|---|---|---|---|
| Control | 0/3 | 0/2 | 0/4 | 0/9 | 0 | 0.0 ± 0.0 |
| 250 µg/L | 2/3 | 2/2 | 3/4 | 7/9 | 77.7 | 75.2 ± 1.97 |
| 300 µg/L | 3/3 | 2/2 | 3/4 | 8/9 | 88.8 | 86.9 ± 2.01 |
| 350 µg/L | 3/3 | 2/2 | 3/4 | 9/9 | 100 | 100 ± 0.0 |
| 400 µg/L | 3/3 | 2/2 | 4/4 | 9/9 | 100 | 100 ± 0.0 |
| 450 µg/L | 3/3 | 2/2 | 4/4 | 9/9 | 100 | 100 ± 0.0 |
| Group/Tissue | Control | 44.457 mg/kg | 88.914 mg/kg | 199.53 mg/kg | 281.83 mg/kg | 448.81 mg/kg | |
|---|---|---|---|---|---|---|---|
| Gill | Total changes | 0/22 | 3/22 | 4/22 | 4/22 | 4/22 | 3/22 |
| % | 0 | 13.6 | 18.1 | 18.1 | 18.1 | 13.6 | |
| Median | 0 ± 0.0 | 0.66 ± 0.14 | 0.81 ± 0.14 | 0.75 ± 0.25 | 0.91 ± 0.14 | 0.66 ± 0.14 | |
| Liver | Total changes | 0/20 | 10/20 | 12/20 | 15/20 | 17/20 | 15/20 |
| % | 0 | 50 | 60 | 75 | 85 | 75 | |
| Median | 0 ± 0.0 | 29.9 ± 1.60 | 33.5 ± 1.37 | 39.3 ± 2.37 | 40.5 ± 3.10 | 12.7 ± 3.78 | |
| Intestine | Total changes | 0/21 | 11/21 | 13/21 | 11/21 | 13/21 | 19/21 |
| % | 0 | 52.3 | 61.9 | 52.3 | 61.9 | 90.4 | |
| Median | 0 ± 0.0 | 26.9 ± 0.62 | 29.2 ± 1.32 | 32.4 ± 1.37 | 32.7 ± 1.32 | 39.5 ± 1.56 | |
| Kidney | Total changes | 0/22 | 11/22 | 13/22 | 14/22 | 17/22 | 13/22 |
| % | 0 | 50 | 59 | 63.6 | 77.2 | 59 | |
| Median | 0 ± 0.0 | 12.9 ± 1.46 | 40.9 ± 2.87 | 38.4 ± 1.46 | 41.0 ± 3.78 | 13.3 ± 1.30 | |
| Group/Tissue | Control | 250 µg/L | 300 µg/L | 350 µg/L | 400 µg/L | 450 µg/L | |
|---|---|---|---|---|---|---|---|
| Gill | Total changes | 0/22 | 17/22 | 21/22 | 19/22 | 11/22 | 9/22 |
| % | 0 | 77.2 | 95.4 | 86.3 | 50 | 40.9 | |
| Median | 0 ± 0.0 | 6.6 ± 1.79 | 37.3 ± 1.66 | 37.4 ± 1.46 | 28.2 ± 1.80 | 5.8 ± 0.38 | |
| Liver | Total changes | 0/20 | 8/20 | 16/20 | 15/20 | 14/20 | 18/20 |
| % | 0 | 40 | 80 | 75 | 70 | 90 | |
| Median | 0 ± 0.0 | 10.8 ± 1.77 | 40.1 ± 2.96 | 40.3 ± 1.44 | 35.9 ± 1.46 | 19.3 ± 1.37 | |
| Intestine | Total changes | 0/21 | 7/21 | 9/21 | 12/21 | 12/21 | 11/21 |
| % | 0 | 33.3 | 42.8 | 57.1 | 57.1 | 52.3 | |
| Median | 0 ± 0.0 | 3.83 ± 1.44 | 4.08 ± 3.81 | 4.08 ± 3.81 | 3.91 ± 1.44 | 2.83 ± 0.76 | |
| Kidney | Total changes | 0/22 | 22/22 | 17/22 | 19/22 | 17/22 | 13/22 |
| % | 0 | 100 | 77.2 | 86.3 | 77.2 | 59 | |
| Median | 0 ± 0.0 | 38.0 ± 2.50 | 43.4 ± 1.25 | 43.8 ± 3.82 | 43.1 ± 1.77 | 21.1 ± 1.15 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Custodio de Souza, G.; Dias Ribeiro da Silva, I.; Duarte Viana, M.; Costa de Melo, N.; Sánchez-Ortiz, B.L.; Maia Rebelo de Oliveira, M.; Ramos Barbosa, W.L.; Maciel Ferreira, I.; Tavares Carvalho, J.C. Acute Toxicity of the Hydroethanolic Extract of the Flowers of Acmella oleracea L. in Zebrafish (Danio rerio): Behavioral and Histopathological Studies. Pharmaceuticals 2019, 12, 173. https://doi.org/10.3390/ph12040173
Custodio de Souza G, Dias Ribeiro da Silva I, Duarte Viana M, Costa de Melo N, Sánchez-Ortiz BL, Maia Rebelo de Oliveira M, Ramos Barbosa WL, Maciel Ferreira I, Tavares Carvalho JC. Acute Toxicity of the Hydroethanolic Extract of the Flowers of Acmella oleracea L. in Zebrafish (Danio rerio): Behavioral and Histopathological Studies. Pharmaceuticals. 2019; 12(4):173. https://doi.org/10.3390/ph12040173
Chicago/Turabian StyleCustodio de Souza, Gisele, Ianna Dias Ribeiro da Silva, Muller Duarte Viana, Nayara Costa de Melo, Brenda Lorena Sánchez-Ortiz, Monaliza Maia Rebelo de Oliveira, Wagner Luiz Ramos Barbosa, Irlon Maciel Ferreira, and José Carlos Tavares Carvalho. 2019. "Acute Toxicity of the Hydroethanolic Extract of the Flowers of Acmella oleracea L. in Zebrafish (Danio rerio): Behavioral and Histopathological Studies" Pharmaceuticals 12, no. 4: 173. https://doi.org/10.3390/ph12040173






