Silencing of Exosomal miR-181a Reverses Pediatric Acute Lymphocytic Leukemia Cell Proliferation
Abstract
:1. Introduction
2. Results
2.1. Characterization of Exosomes
2.2. Leukemia Derived Exosome-Induced Cell Proliferation
- Exosome isolation: Exosomes were isolated from conditioned media (Exo-CM) of two ALL cell lines (JM1 and SUP-B15) and one control B cell line (CL-01). In addition, exosomes were harvested from human serum samples of either patients with childhood acute lymphocytic leukemia (Exo-PALL) or healthy donors (Exo-HD).
- Exosome dose titration and exposure time optimization: In order to establish exosome dose titration and time kinetics of the process, Exo-CM (CL-01 and SUP-B15) was added to JM1 cells (paracrine incubation) in one of three different concentrations (100, 250, and 500 µg/mL) with PBS only (no exosomes) as negative control. Cell counting was carried out at 24 and 48 h under light microscopy. Control Exo-CL-01 did not induce cell proliferation at either time points, while Exo-SUP-B15 significantly induced cell proliferation, compared to PBS negative control, both at 24 and 48 h. Similar results were obtained with autologous incubation (autocrine fashion) of Exo-JM1 on JM1 cells (data not shown). Exosome treatment augmented cell proliferation at a starting exosome concentration (100 µg/mL), with optimal proliferation at 250 µg/mL (Supplementary Figure S1A). Consequently, we chose an exosome concentration of 250 µg/mL (noted saturated cell proliferation induction) as the working dose for future experiments. Similarly, we optimized the dose and time points from HD and PALL-derived serum exosomes. Further, JM1 leukemia cells were treated with Exo-HD and Exo-PALL. The control Exo-HD did not induce cell proliferation compared to PBS at either time points (24 or 48 h), while Exo-PALL significantly induced leukemic cell proliferation compared to Exo-HD and PBS negative control, both at 24 and 48 h (Supplementary Figure S1B). Based on these data, we decided that 250 µg/mL exosome concentration and 24 h incubation are the optimum conditions for the cell proliferation assay and we used these in the subsequent experiments.
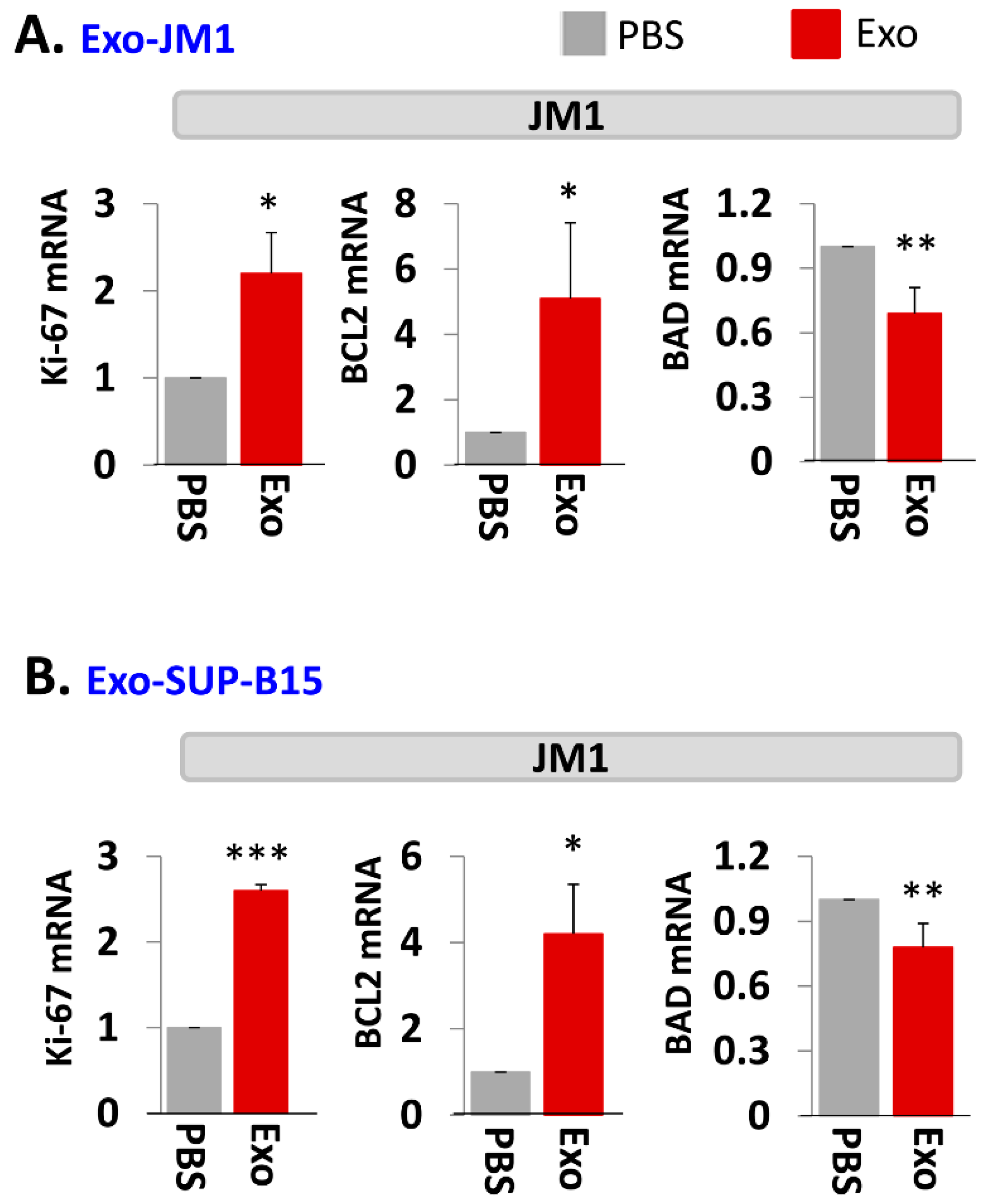
- Exosome-induced leukemia cell proliferation: Consequently, we confirmed that CM-exosomes induce JM1 cell proliferation both in an autocrine and paracrine fashion using Exo-JM1 (Figure 2A) and Exo-SUP-B15 (Figure 2B), respectively. Our data showed that exosomes originated from ALL cell lines (JM1, SUP-B15) promoted cellular proliferation not only in both leukemic B cell lines but also in control human B cells (CL-01) as well (Figure 2A,B).
2.3. Exo-JM1 and Exo-SUP-B15 Treatment Augments Proliferative/Pro-Survival Genes, and Down Regulates Pro-Apoptotic Genes
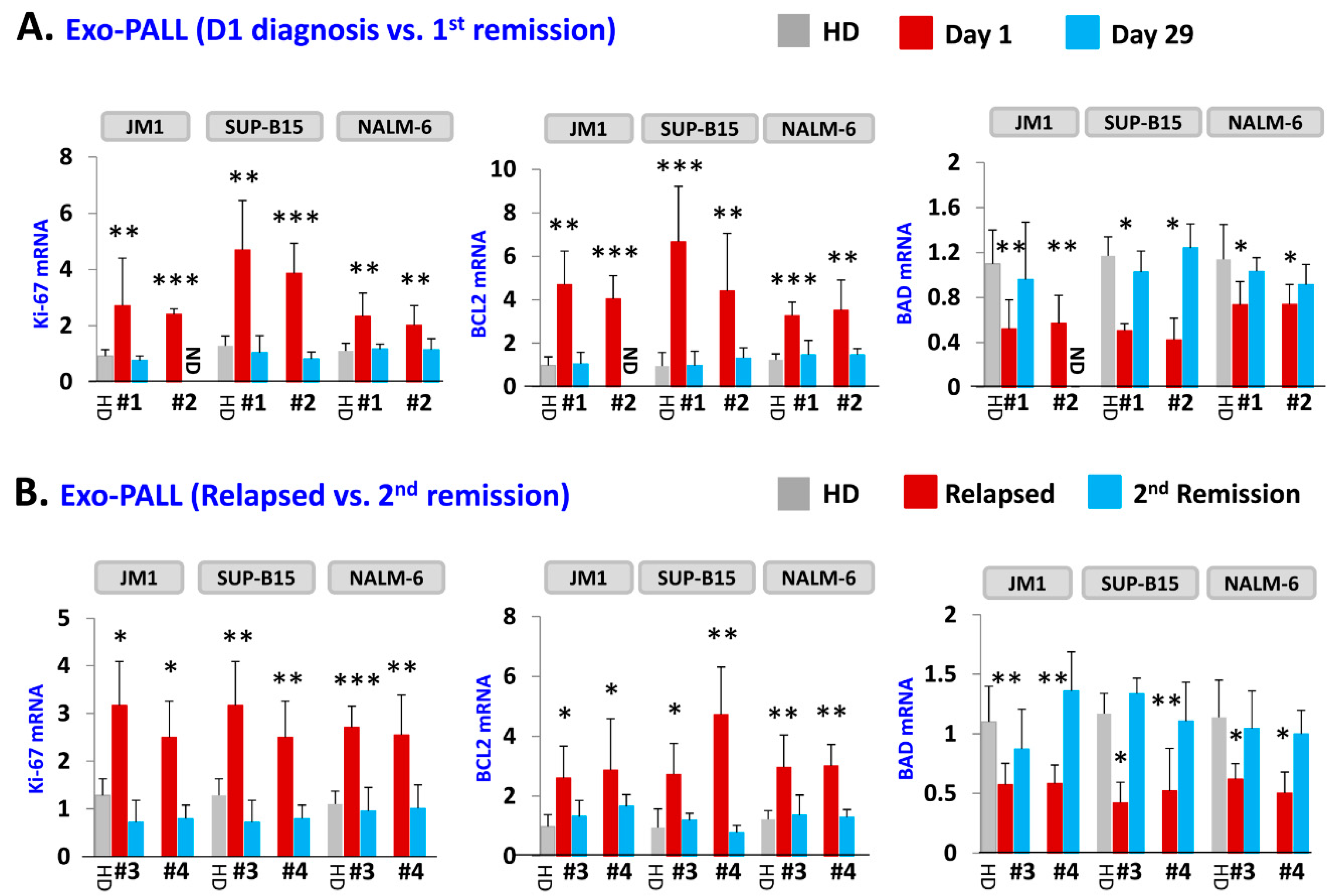
2.4. Exo-PALL Promotes Proliferative/Pro-Survival Genes, and Down-Regulates Pro-Apoptotic Genes
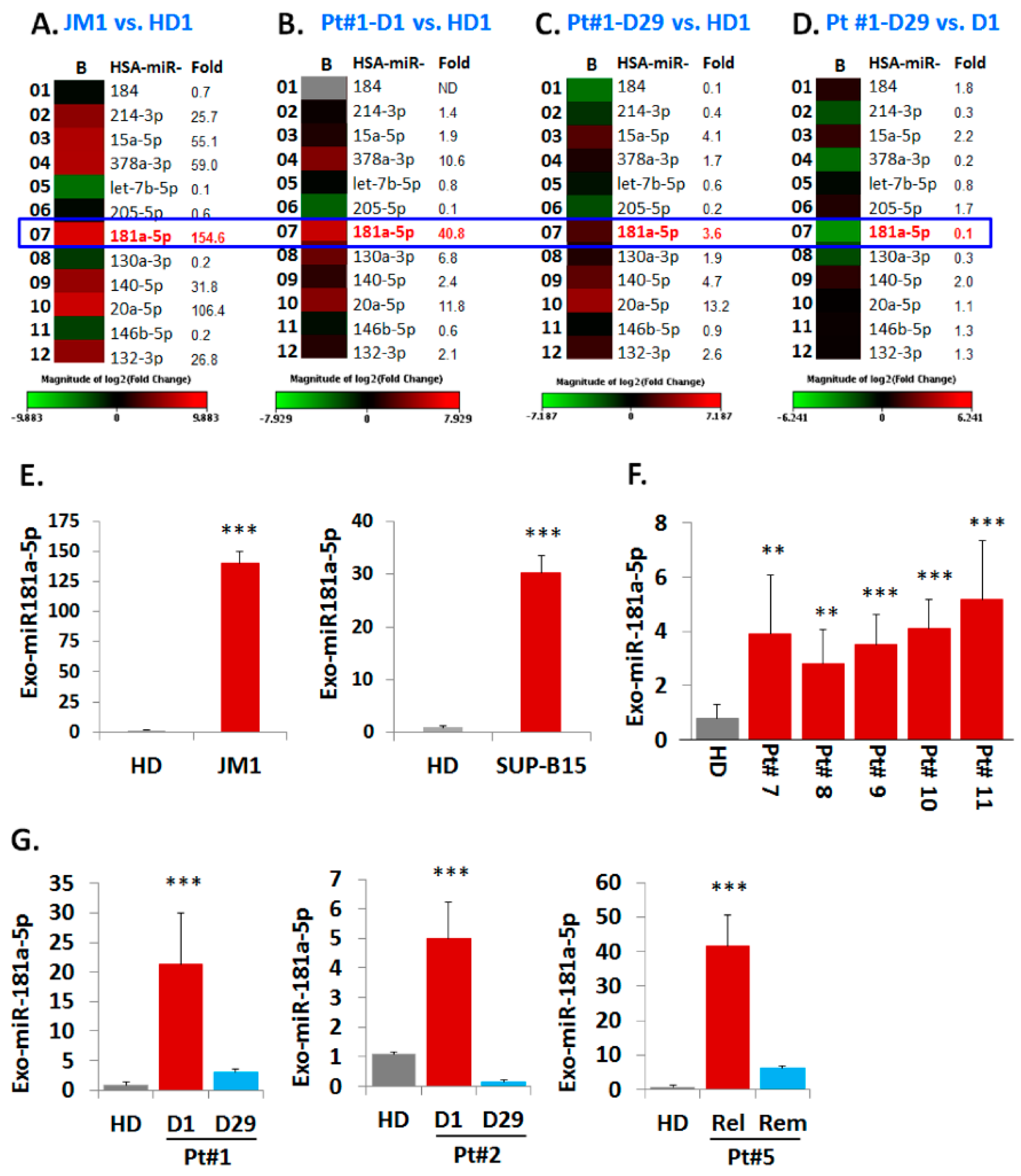
2.5. Analysis of miR Profiles in Exosomes
Exo-miR Expression by Human Cancer Pathway Finder Array and Validation of Elevated Exosomal miR-181a Expression by q-PCR
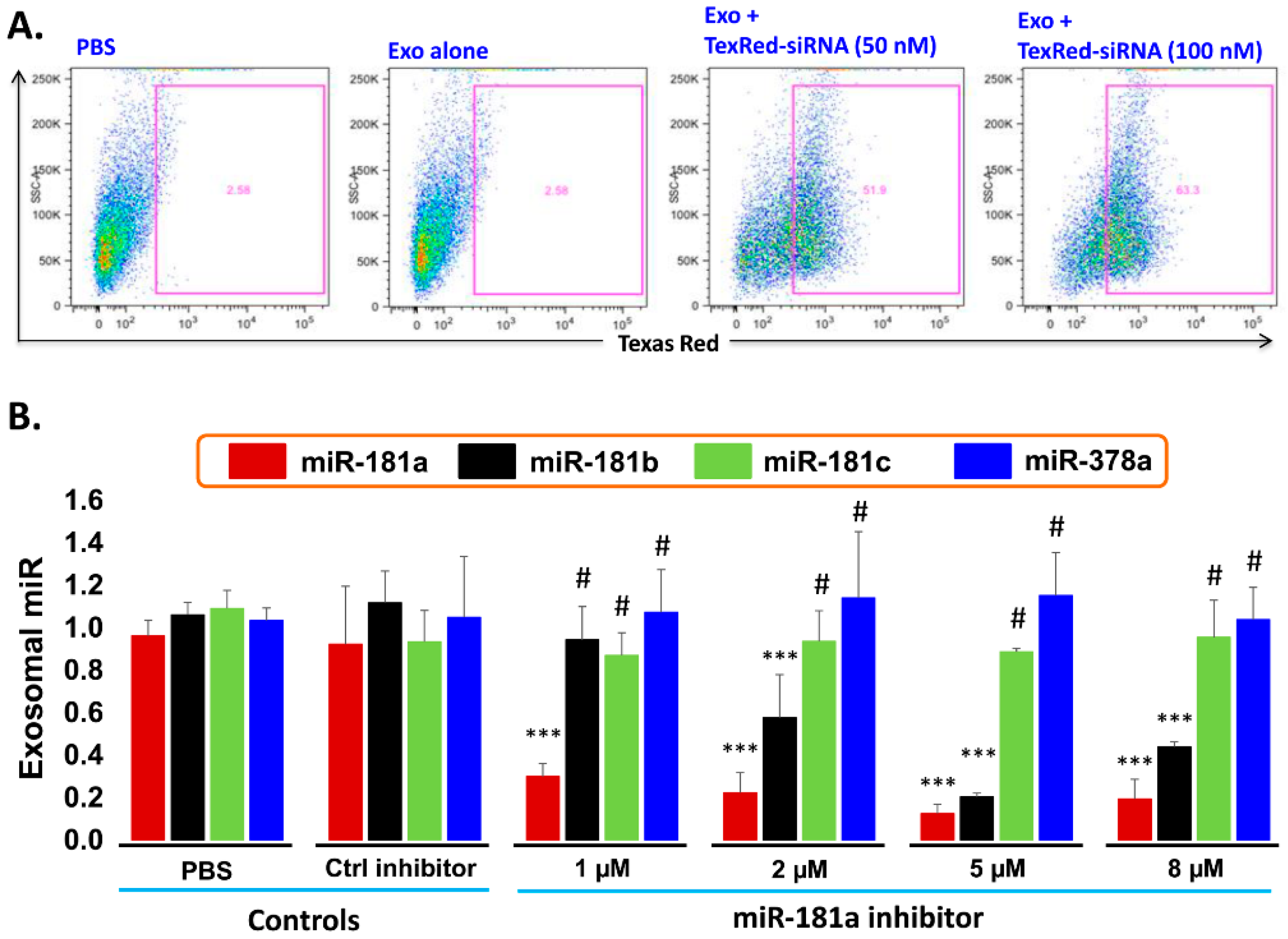
2.6. Transfection Efficiency of TexRed-siRNA into the Target Cell and Silencing of exosomal miR-181a
- Transfection efficiency of siRNA inhibitor in exosomes: To support the idea that miR-181a is a major player in leukemia cell proliferation, we explored miR-181a inhibition by a miScript miRNA inhibitor to reverse the induced cell proliferation. We first established and determined uptake-efficiency of a control siRNA by exosomes, by TexRed as per manufacturer recommendations (Figure 6). We transfected and loaded Exo-JM1 with TexRed-siRNA and co-cultured with JM1 cells. After 24 h, JM1 cells were harvested and analyzed for TexRed-siRNA uptake by the flow cytometer. We observed around 50–60% JM1 cells were TexRed positive (Figure 6A).
- Targeted silencing of miR-181a by siRNA inhibitor: Once siRNA uptake was confirmed, Exo-JM1 was transfected and loaded with a specific miR-181a inhibitor and the exosomal miR-181a level was determined by q-PCR. Results showed that transfection of exosomes with an inhibitor resulted in a 70–88% silencing of exosomal miR-181a (Figure 6B). To rule out off-target activity causing non-specific inhibition, we randomly chose to amplify miR-181b, miR-181c, and miR-378 as an off-target hits. The miR-181a inhibitor consistently inhibited specific miR-181a expression at all applied concentrations (1–8 µM). The miR-181a inhibitor did not inhibit non-specific miR-181b at low concentration (1 µM) while miR-181a inhibitor at high concentration (2–8 µM) showed off target activity and miR-181b inhibition was observed. Interestingly, both miR-181c and miR-378a was not inhibited by miR-181a inhibitor at either concentration.
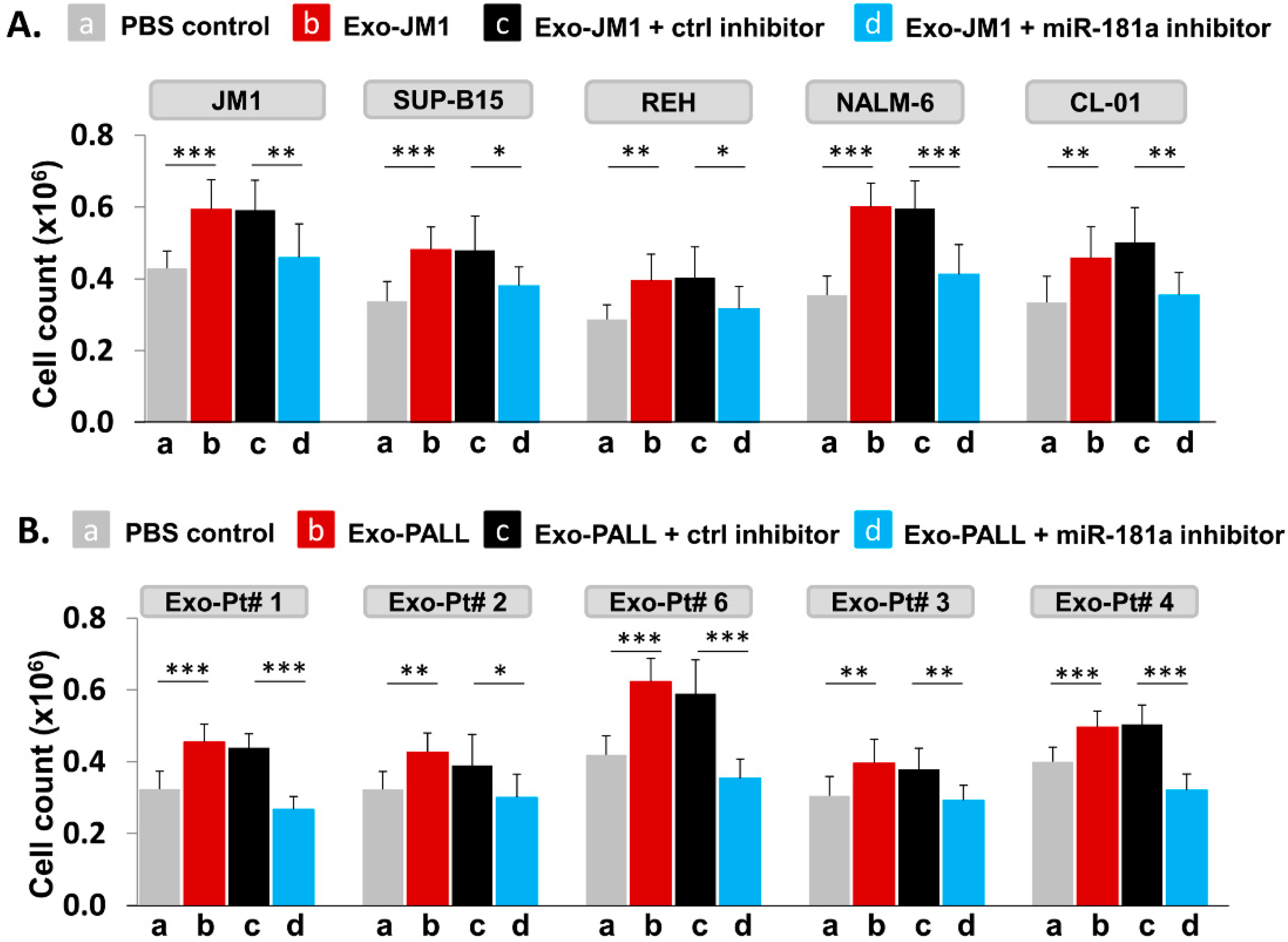
2.7. PALL-Derived Exosomal miR-181a Silencing by miR-181a Inhibitor Reverses Exosome Induced Cell Proliferation
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Cell Culture
4.2. Human Serum Samples
4.3. Depletion of Exosomes from the FBS
4.4. Cell Culture Set up for Exosome Production
4.5. Exosome Isolation
4.6. Characterization of Exosomes
Exosomal CD63 and CD81 Expression by Flow Cytometer
4.7. Exosome Induced-Cell Proliferation Assay
4.8. Cellular RNA Extraction and cDNA Preparation
4.9. Cellular mRNA Expression by q-PCR
4.10. Exosomal RNA Isolation
4.11. Silencing of Exosomal miR-181a with miR-181a Inhibitor Using Exo-Fect™ Exosome Transfection Reagent Kit
4.12. Exosomal miR-181a-5p Expression by PCR
4.13. Exosomal miR-181a Silencing Using a Specific miR-181a Inhibitor
- Confirmation of exosomal uptake of TexRed-siRNA and cellular uptake of exosomes: Exosomes were labeled with TexRed-siRNA (SBI System Bioscience) as per manufacturer’s protocol. TexRed-labeled JM1 exosomes were then co-cultured with JM1 cells for 24 h. Presence of TexRed-siRNA (introduced in target cells by exosomes) into the JM1 cells was assessed by flow cytometer.
- Dose titration of miR-181a inhibitor: Exo-JM1 were transfected with different concentrations of the miR181a inhibitor (1, 2, 5, 8 µM) to determine optimal dosing. Then, RNA was isolated from transfected exosomes for cDNA synthesis and miR-181a expression by q-PCR.
- Exosomal miR-181a silencing: 300 µg of exosomes (either CM- or serum-derived) was transfected under following conditions: (1) transfection reagents only; (2) transfection reagent and control inhibitor; (3) transfection reagent with miR-181a inhibitor (1.0 µM). Each transfection mixture was co-cultured with JM1 cells for 24 h, allowing for exosomal uptake by the target cells. Cell count was carried out by light microscopy.
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yáñez-Mó, M.; Siljander, P.R.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [Green Version]
- Thakur, B.K.; Zhang, H.; Becker, A.; Matei, I.; Huang, Y.; Costa-Silva, B.; Zheng, Y.; Hoshino, A.; Brazier, H.; Xiang, J.; et al. Double-stranded DNA in exosomes: A novel biomarker in cancer detection. Cell Res. 2014, 24, 766–769. [Google Scholar] [CrossRef] [Green Version]
- Théry, C.; Zitvogel, L.; Amigorena, S. Exosomes: Composition, biogenesis and function. Nat. Rev. Immunol. 2002, 2, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Momen-Heravi, F.; Bala, S.; Bukong, T.; Szabo, G. Exosome-mediated delivery of functionally active miRNA-155 inhibitor to macrophages. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1517–1527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, C.P.; Mardini, O.; Ericsson, M.; Prabhakar, S.; Maguire, C.; Chen, J.W.; Tannous, B.A.; Breakefield, X.O. Dynamic biodistribution of extracellular vesicles in vivo using a multimodal imaging reporter. ACS Nano 2014, 8, 483–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umezu, T.; Ohyashiki, K.; Kuroda, M.; Ohyashiki, J.H. Leukemia cell to endothelial cell communication via exosomal miRNAs. Oncogene 2013, 32, 2747–2755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugita, F.; Maki, K.; Nakamura, Y.; Sasaki, K.; Mitani, K. Overexpression of MIR9 indicates poor prognosis in acute lymphoblastic leukemia. Leuk. Lymphoma 2014, 55, 78–86. [Google Scholar] [CrossRef]
- Yeh, C.H.; Moles, R.; Nicot, C. Clinical significance of microRNAs in chronic and acute human leukemia. Mol. Cancer 2016, 15, 37. [Google Scholar] [CrossRef] [Green Version]
- Manier, S.; Liu, C.J.; Avet-Loiseau, H.; Park, J.; Shi, J.; Campigotto, F.; Salem, K.Z.; Huynh, D.; Glavey, S.V.; Rivotto, B.; et al. Prognostic role of circulating exosomal miRNAs in multiple myeloma. Blood 2017, 129, 2429–2436. [Google Scholar] [CrossRef]
- Kahlert, C.; Kalluri, R. Exosomes in tumor microenvironment influence cancer progression and metastasis. J. Mol. Med. (Berl. Ger.) 2013, 91, 431–437. [Google Scholar] [CrossRef] [Green Version]
- Tuschl, T.; Zamore, P.D.; Lehmann, R.; Bartel, D.P.; Sharp, P.A. Targeted mRNA degradation by double-stranded RNA in vitro. Genes Dev. 1999, 13, 3191–3197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [Green Version]
- Morozova, N.; Zinovyev, A.; Nonne, N.; Pritchard, L.L.; Gorban, A.N.; Harel-Bellan, A. Kinetic signatures of microRNA modes of action. RNA 2012, 18, 1635–1655. [Google Scholar] [CrossRef] [Green Version]
- Garofalo, M.; Condorelli, G.L.; Croce, C.M.; Condorelli, G. MicroRNAs as regulators of death receptors signaling. Cell Death Differ. 2010, 17, 200–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schotte, D.; Pieters, R.; Den Boer, M.L. MicroRNAs in acute leukemia: From biological players to clinical contributors. Leukemia 2012, 26, 1–12. [Google Scholar] [CrossRef]
- Yang, Z.; Wan, X.; Gu, Z.; Zhang, H.; Yang, X.; He, L.; Miao, R.; Zhong, Y.; Zhao, H. Evolution of the mir-181 microRNA family. Comput. Biol. Med. 2014, 52, 82–87. [Google Scholar] [CrossRef]
- Lin, Y.; Zhao, J.; Wang, H.; Cao, J.; Nie, Y. miR-181a modulates proliferation, migration and autophagy in AGS gastric cancer cells and downregulates MTMR3. Mol. Med. Rep. 2017, 15, 2451–2456. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, J.; Li, S.; Ma, R.; Cao, H.; Ji, M.; Jing, C.; Tang, J. The function role of miR-181a in chemosensitivity to adriamycin by targeting Bcl-2 in low-invasive breast cancer cells. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2013, 32, 1225–1237. [Google Scholar] [CrossRef]
- Yang, C.; Tabatabaei, S.N.; Ruan, X.; Hardy, P. The Dual Regulatory Role of MiR-181a in Breast Cancer. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2017, 44, 843–856. [Google Scholar] [CrossRef]
- Nishimura, J.; Handa, R.; Yamamoto, H.; Tanaka, F.; Shibata, K.; Mimori, K.; Takemasa, I.; Mizushima, T.; Ikeda, M.; Sekimoto, M.; et al. microRNA-181a is associated with poor prognosis of colorectal cancer. Oncol. Rep. 2012, 28, 2221–2226. [Google Scholar] [CrossRef] [Green Version]
- Parikh, A.; Lee, C.; Joseph, P.; Marchini, S.; Baccarini, A.; Kolev, V.; Romualdi, C.; Fruscio, R.; Shah, H.; Wang, F.; et al. microRNA-181a has a critical role in ovarian cancer progression through the regulation of the epithelial-mesenchymal transition. Nat. Commun. 2014, 5, 2977. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Z.; He, C.; Wang, D.; Yuan, X.; Chen, J.; Jin, J. MicroRNAs expression signatures are associated with lineage and survival in acute leukemias. Blood Cells Mol. Dis. 2010, 44, 191–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Kuscu, C.; Banach, A.; Zhang, Q.; Pulkoski-Gross, A.; Kim, D.; Liu, J.; Roth, E.; Li, E.; Shroyer, K.R.; et al. miR-181a-5p Inhibits Cancer Cell Migration and Angiogenesis via Downregulation of Matrix Metalloproteinase-14. Cancer Res. 2015, 75, 2674–2685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korhan, P.; Erdal, E.; Atabey, N. MiR-181a-5p is downregulated in hepatocellular carcinoma and suppresses motility, invasion and branching-morphogenesis by directly targeting c-Met. Biochem. Biophys. Res. Commun. 2014, 450, 1304–1312. [Google Scholar] [CrossRef] [PubMed]
- Pui, C.H. Recent research advances in childhood acute lymphoblastic leukemia. J. Formos. Med. Assoc. Taiwan Yi Zhi 2010, 109, 777–787. [Google Scholar] [CrossRef] [Green Version]
- Redaelli, A.; Laskin, B.L.; Stephens, J.M.; Botteman, M.F.; Pashos, C.L. A systematic literature review of the clinical and epidemiological burden of acute lymphoblastic leukaemia (ALL). Eur. J. Cancer Care 2005, 14, 53–62. [Google Scholar] [CrossRef]
- Payne, K.J.; Dovat, S. Ikaros and tumor suppression in acute lymphoblastic leukemia. Crit. Rev. Oncog. 2011, 16, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Burke, M.J.; Bhatla, T. Epigenetic modifications in pediatric acute lymphoblastic leukemia. Front. Pediatr. 2014, 2, 42. [Google Scholar] [CrossRef]
- Chatterton, Z.; Morenos, L.; Mechinaud, F.; Ashley, D.M.; Craig, J.M.; Sexton-Oates, A.; Halemba, M.S.; Parkinson-Bates, M.; Ng, J.; Morrison, D.; et al. Epigenetic deregulation in pediatric acute lymphoblastic leukemia. Epigenetics 2014, 9, 459–467. [Google Scholar] [CrossRef] [Green Version]
- Endo, R.; Nakamura, T.; Kawakami, K.; Sato, Y.; Harashima, H. The silencing of indoleamine 2,3-dioxygenase 1 (IDO1) in dendritic cells by siRNA-loaded lipid nanoparticles enhances cell-based cancer immunotherapy. Sci. Rep. 2019, 9, 11335. [Google Scholar] [CrossRef] [Green Version]
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Théry, C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA 2016, 113, E968–E977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haque, S.; Vaiselbuh, S.R. Exosomes molecular diagnostics: Direct conversion of exosomes into the cDNA for gene amplification by two-step polymerase chain reaction. J. Biol. Methods 2018, 5, e96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.K.; Park, S.R.; Jung, B.K.; Jeon, Y.K.; Lee, Y.S.; Kim, M.K.; Kim, Y.G.; Jang, J.Y.; Kim, C.W. Exosomes derived from mesenchymal stem cells suppress angiogenesis by down-regulating VEGF expression in breast cancer cells. PLoS ONE 2013, 8, e84256. [Google Scholar] [CrossRef] [Green Version]
- Raimondo, S.; Saieva, L.; Corrado, C.; Fontana, S.; Flugy, A.; Rizzo, A.; De Leo, G.; Alessandro, R. Chronic myeloid leukemia-derived exosomes promote tumor growth through an autocrine mechanism. Cell Commun. Signal. CCS 2015, 13, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyu, X.; Li, J.; Yun, X.; Huang, R.; Deng, X.; Wang, Y.; Chen, Y.; Xiao, G. miR-181a-5p, an inducer of Wnt-signaling, facilitates cell proliferation in acute lymphoblastic leukemia. Oncol. Rep. 2017, 37, 1469–1476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verduci, L.; Azzalin, G.; Gioiosa, S.; Carissimi, C.; Laudadio, I.; Fulci, V.; Macino, G. microRNA-181a enhances cell proliferation in acute lymphoblastic leukemia by targeting EGR1. Leuk. Res. 2015, 39, 479–485. [Google Scholar] [CrossRef] [Green Version]
- Tong, S.J.; Liu, J.; Wang, X.; Qu, L.X. microRNA-181 promotes prostate cancer cell proliferation by regulating DAX-1 expression. Exp. Ther. Med. 2014, 8, 1296–1300. [Google Scholar] [CrossRef]
- Jianwei, Z.; Fan, L.; Xiancheng, L.; Enzhong, B.; Shuai, L.; Can, L. MicroRNA 181a improves proliferation and invasion, suppresses apoptosis of osteosarcoma cell. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 2013, 34, 3331–3337. [Google Scholar] [CrossRef]
- Mi, Y.; Zhang, D.; Jiang, W.; Weng, J.; Zhou, C.; Huang, K.; Tang, H.; Yu, Y.; Liu, X.; Cui, W.; et al. miR-181a-5p promotes the progression of gastric cancer via RASSF6-mediated MAPK signalling activation. Cancer Lett. 2017, 389, 11–22. [Google Scholar] [CrossRef]
- Torri, A.; Carpi, D.; Bulgheroni, E.; Crosti, M.C.; Moro, M.; Gruarin, P.; Rossi, R.L.; Rossetti, G.; Di Vizio, D.; Hoxha, M.; et al. Extracellular MicroRNA Signature of Human Helper T Cell Subsets in Health and Autoimmunity. J. Biol. Chem. 2017, 292, 2903–2915. [Google Scholar] [CrossRef] [Green Version]
- Wahlgren, J.; De, L.K.T.; Brisslert, M.; Vaziri Sani, F.; Telemo, E.; Sunnerhagen, P.; Valadi, H. Plasma exosomes can deliver exogenous short interfering RNA to monocytes and lymphocytes. Nucleic Acids Res. 2012, 40, e130. [Google Scholar] [CrossRef] [Green Version]
- McKelvey, K.J.; Powell, K.L.; Ashton, A.W.; Morris, J.M.; McCracken, S.A. Exosomes: Mechanisms of Uptake. J. Circ. Biomark. 2015, 4, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greco, K.A.; Franzen, C.A.; Foreman, K.E.; Flanigan, R.C.; Kuo, P.C.; Gupta, G.N. PLK-1 Silencing in Bladder Cancer by siRNA Delivered with Exosomes. Urology 2016, 91, 241.e1–241.e7. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. 2006. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haque, S.; Vaiselbuh, S.R. Silencing of Exosomal miR-181a Reverses Pediatric Acute Lymphocytic Leukemia Cell Proliferation. Pharmaceuticals 2020, 13, 241. https://doi.org/10.3390/ph13090241
Haque S, Vaiselbuh SR. Silencing of Exosomal miR-181a Reverses Pediatric Acute Lymphocytic Leukemia Cell Proliferation. Pharmaceuticals. 2020; 13(9):241. https://doi.org/10.3390/ph13090241
Chicago/Turabian StyleHaque, Shabirul, and Sarah R. Vaiselbuh. 2020. "Silencing of Exosomal miR-181a Reverses Pediatric Acute Lymphocytic Leukemia Cell Proliferation" Pharmaceuticals 13, no. 9: 241. https://doi.org/10.3390/ph13090241




