Preparation and Bioevaluation of Novel 99mTc-Labeled Complexes with a 2-Nitroimidazole HYNIC Derivative for Imaging Tumor Hypoxia
Abstract
:1. Introduction
2. Results
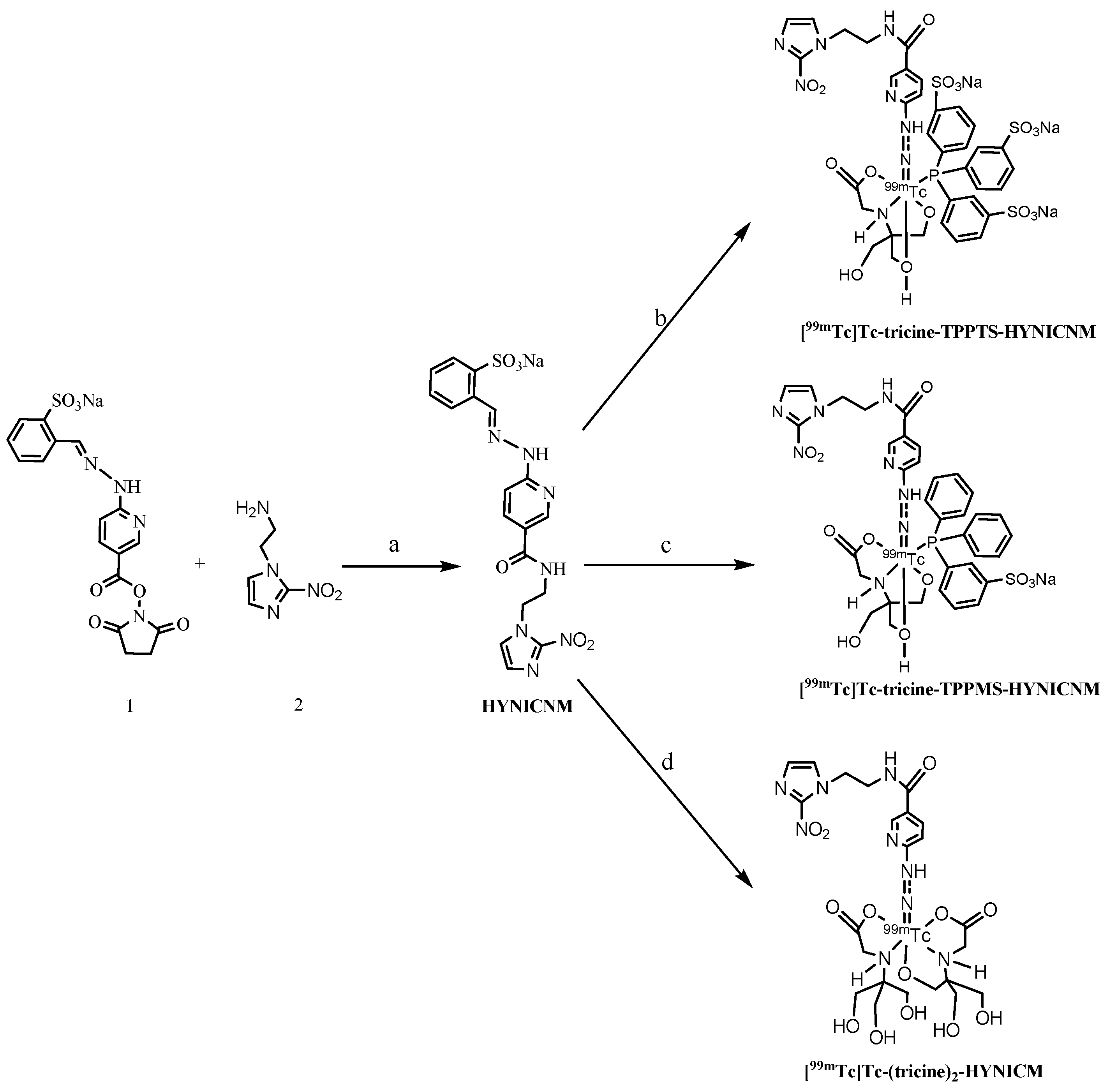
2.1. Synthesis of HYNICNM
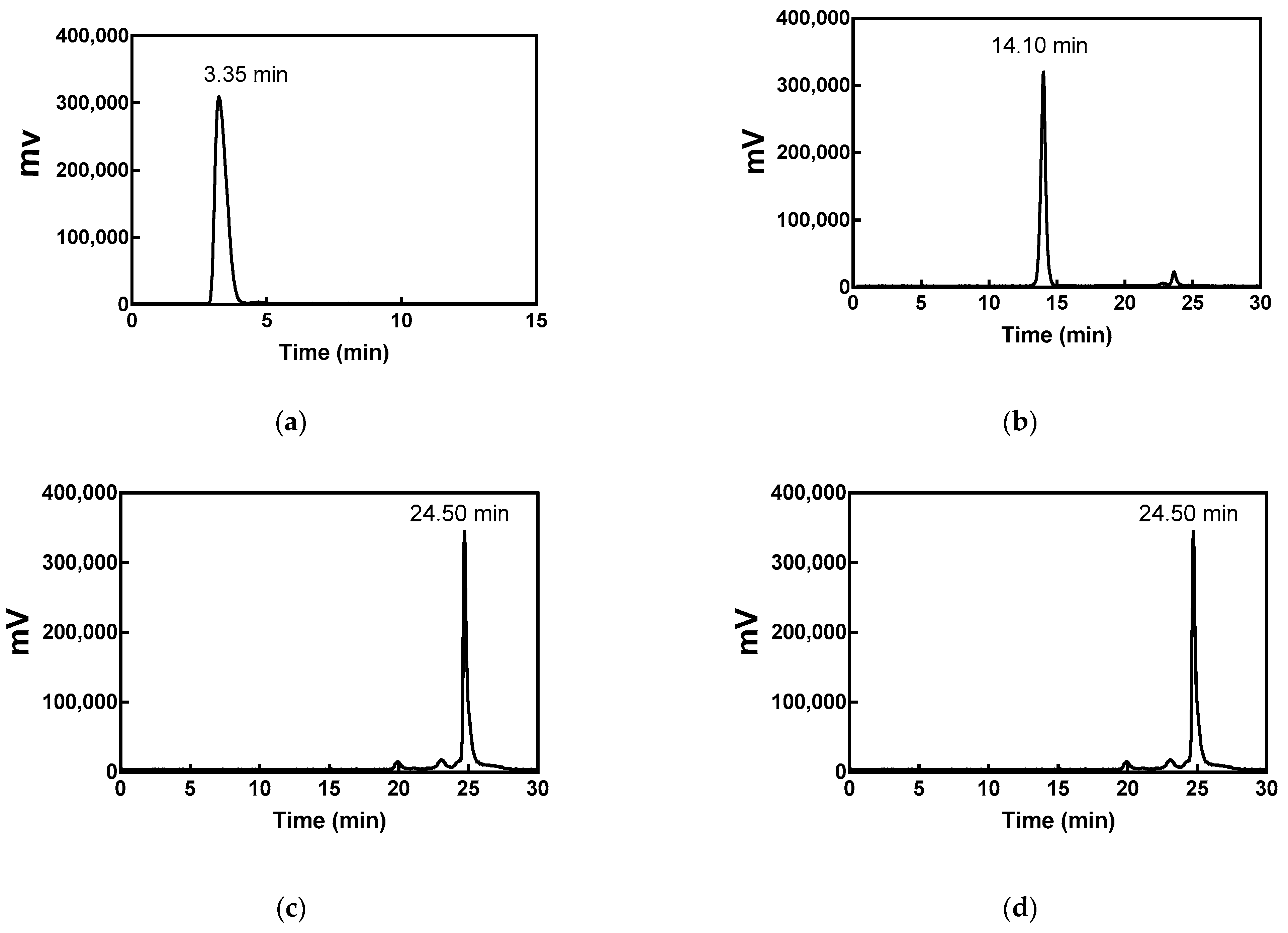
2.2. Radiolabeling and Quality Control
2.3. In Vitro Stability Study and Partition Coefficient
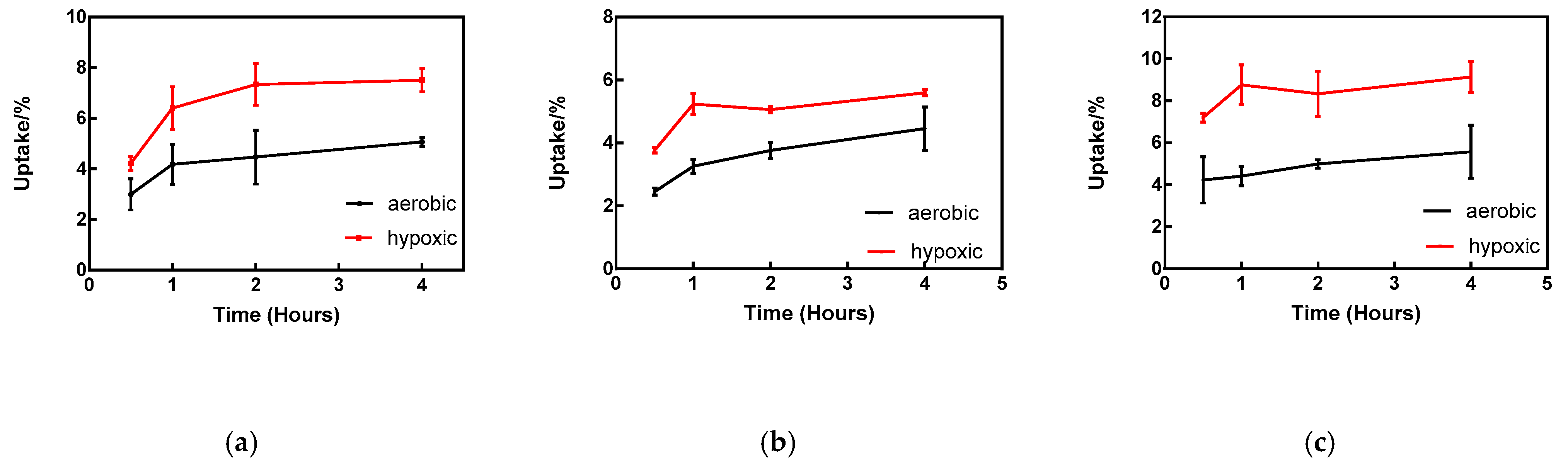
2.4. In Vitro Cellular Uptake
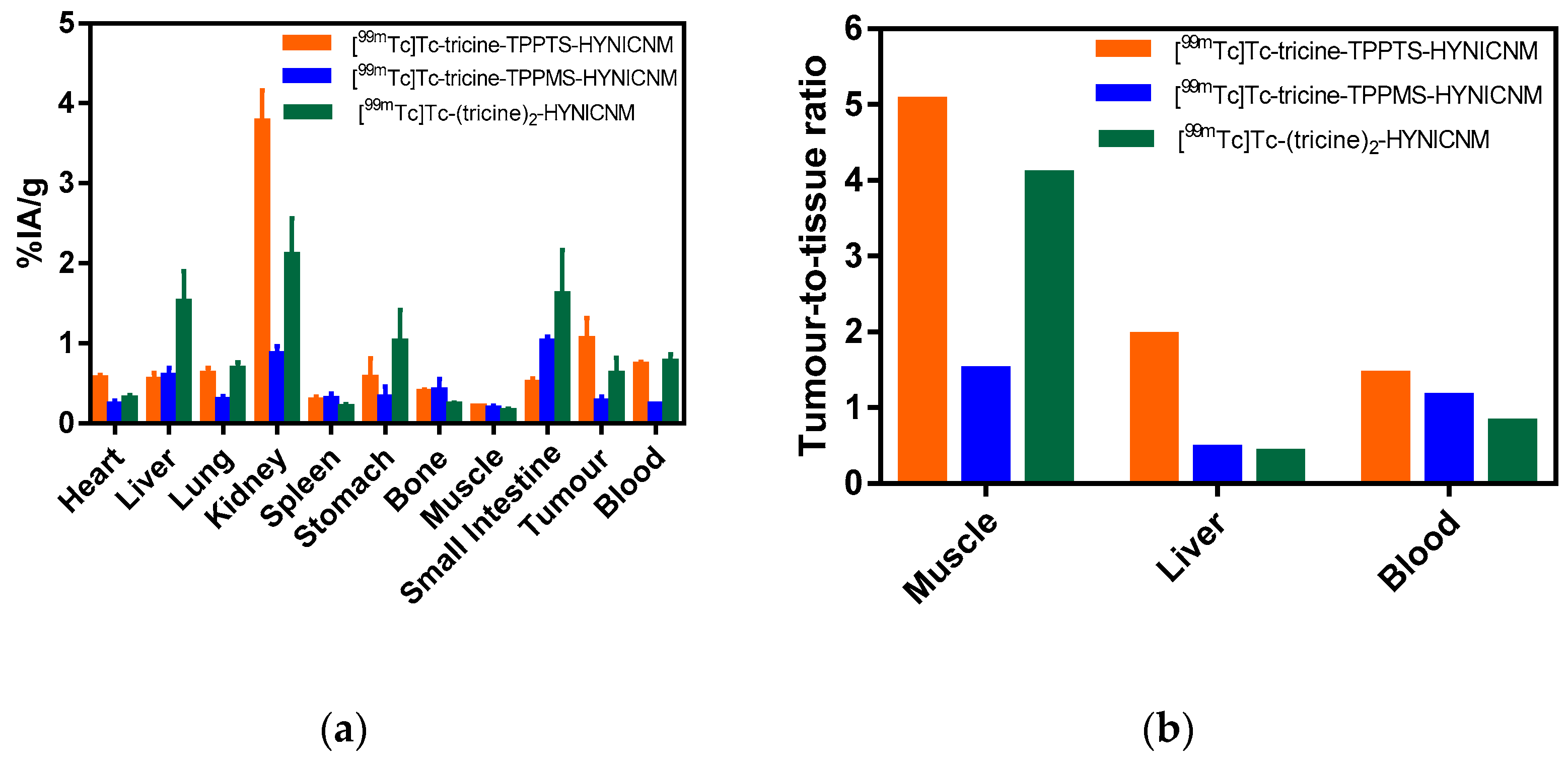
2.5. Biodistribution Studies
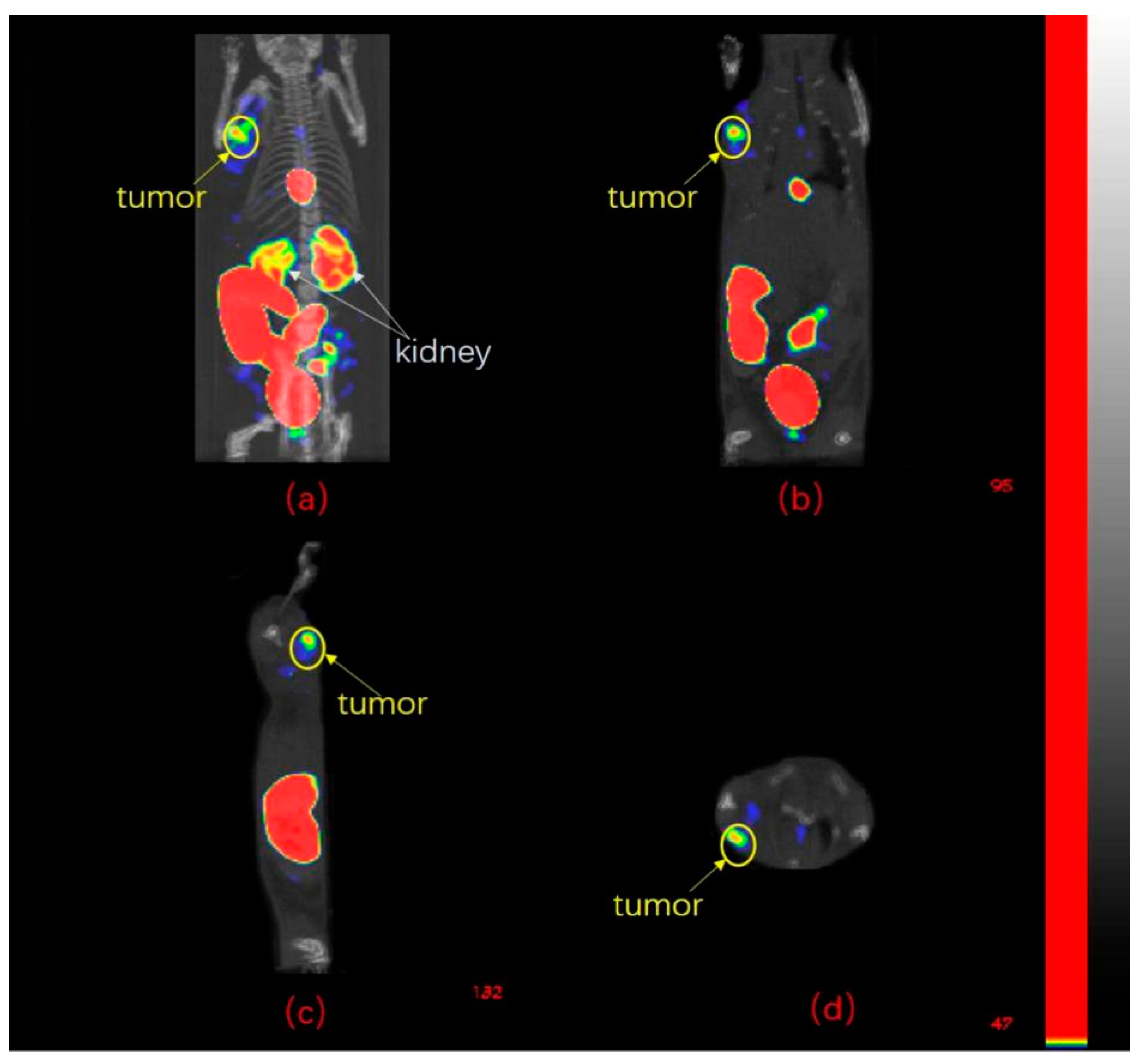
2.6. SPECT/CT Imaging Studies
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis of HYNICNM
4.3. Radiolabeling and Quality Control
4.4. In Vitro Stability Study
4.5. Determination of the Partition Coefficient (log P)
4.6. In Vitro Cellular Uptake
4.7. Biodistribution Studies
4.8. SPECT/CT Imaging Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moeller, B.J.; Richardson, R.A.; Dewhirst, M.W. Hypoxia and radiotherapy: Opportunities for improved outcomes in cancer treatment. Cancer Metastasis Rev. 2007, 26, 241–248. [Google Scholar] [CrossRef]
- Widmer, D.S.; Hoek, K.S.; Cheng, P.F.; Eichhoff, O.M.; Biedermann, T.; Raaijmakers, M.I.G.; Hemmi, S.; Dummer, R.; Levesque, M.P. Hypoxia contributes to melanoma heterogeneity by triggering HIF1α-dependent phenotype switching. J. Invest. Dermatol. 2013, 133, 2436–2443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakkad, S.; Krishnamachary, B.; Jacob, D.; Pacheco-Torres, J.; Goggins, E.; Bharti, S.K.; Penet, M.-F.; Bhujwalla, Z.M. Molecular and functional imaging insights into the role of hypoxia in cancer aggression. Cancer Metastasis Rev. 2019, 38, 51–64. [Google Scholar] [CrossRef]
- Apte, S.; Chin, F.T.; Graves, E.E. Molecular imaging of hypoxia: Strategies for probe design and application. Curr. Org. Synth. 2011, 8, 593–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabral, P.; Cerecetto, H. Radiopharmaceuticals in tumor hypoxia imaging: A review focused on medicinal chemistry aspects. Anticancer Agents Med. Chem. 2017, 17, 318–332. [Google Scholar] [CrossRef]
- Höckel, M.; Knoop, C.; Schlenger, K.; Vorndran, B.; Baussmann, E.; Mitze, M.; Knapstein, P.G.; Vaupel, P. Intratumoral pO2 predicts survival in advanced cancer of the uterine cervix. Radiother. Oncol. 1993, 26, 45–50. [Google Scholar] [CrossRef]
- Vaupel, P.; Schlenger, K.; Knoop, C.; Höckel, M. Oxygenation of human tumors: Evaluation of tissue oxygen distribution in breast cancers by computerized O2 tension measurements. Cancer Res. 1991, 51, 3316–3322. [Google Scholar]
- Wilson, W.R.; Hay, M.P. Targeting hypoxia in cancer therapy. Nat. Rev. Cancer 2011, 11, 393–410. [Google Scholar]
- Mei, L.; Wang, Y.; Chu, T. 99mTc/Re complexes bearing bisnitroimidazole or mononitroimidazole as potential bioreductive markers for tumor: Synthesis, physicochemical characterization and biological evaluation. Eur. J. Med. Chem. 2012, 58, 50–63. [Google Scholar] [CrossRef]
- Mapelli, P.; Picchio, M. 18F-FAZA PET imaging in tumor hypoxia: A focus on high-grade glioma. Int. J. Biol. Marker. 2020, 35, 42–46. [Google Scholar] [CrossRef]
- Zhang, L.; Yao, X.; Cao, J.; Hong, H.; Zhang, A.; Zhao, R.; Zhang, Y.; Zha, Z.; Liu, Y.; Qiao, J. In vivo ester hydrolysis as a new approach in development of PET tracers for imaging hypoxia. Mol. Pharm. 2019, 16, 1156–1166. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, F.; Zhu, H.; Yang, Z.; Chu, T. Synthesis and bioevaluation of novel [18F]FDG-conjugated 2-nitroimidazole derivatives for tumor-hypoxia imaging. Mol. Pharm. 2019, 16, 2118–2128. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Q.; Zhang, X.; Lin, X.; Duan, X.; Zhang, J. Novel 99mTc labelled complexes with 2-nitroimidazole isocyanide: Design, synthesis and evaluation as potential tumor hypoxia imaging agents. MedChemComm 2018, 9, 988–994. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Ruan, Q.; Zhang, X.R.; Duan, X.J.; Teng, Y.G.; Zhang, J.B. 99mTc labelled complexes with secnidazole xanthate: Synthesis and evaluation as potential radiotracers to target tumor hypoxia. Appl. Radiat. Isot. 2018, 140, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Vats, K.; Mallia, M.B.; Mathur, A.; Sarma, H.D.; Banerjee, S. ‘4 + 1′ Mixed ligand strategy for the preparation of 99mTc-radiopharmaceuticals for hypoxia detecting applications. Chemistryselect 2017, 2, 2910–2916. [Google Scholar] [CrossRef]
- Mallia, M.B.; Mittal, S.; Sarma, H.; Banerjee, S. Modulation of in vivo distribution through chelator: Synthesis and evaluation of a 2-nitroimidazole–dipicolylamine–99mTc(CO)3 complex for detecting tumor hypoxia. Bioorg. Med. Chem. Lett. 2016, 26, 46–50. [Google Scholar]
- Lee, S.T.; Scott, A.M. Hypoxia positron emission tomography imaging with 18F-fluoromisonidazole. Semin. Nucl. Med. 2007, 37, 451–461. [Google Scholar] [CrossRef]
- Fujibayashi, Y.; Cutler, C.; Anderson, C.; Mccarthy, D.; Jones, L.; Sharp, T.; Yonekura, Y.; Welch, M. Comparative studies of Cu-64-ATSM and C-11-Acetate in an acute myocardial infarction model: Ex vivo imaging of hypoxia in rats. Nucl. Med. Biol. 1999, 26, 117–121. [Google Scholar]
- Rasey, J.S.; Hofstrand, P.D.; Chin, L.K.; Tewson, T.J. Characterization of [18F]fluoroetanidazole, a new radiopharmaceutical for detecting tumor hypoxia. J. Nucl. Med. 1999, 40, 1072–1079. [Google Scholar]
- Wu, Y.; Hao, G.; Ramezani, S.; Saha, D.; Zhao, D.; Sun, X.; Sherry, A.D. [68Ga]-HP-DO3A-nitroimidazole: A promising agent for PET detection of tumor hypoxia. Contrast Media Mol. Imaging 2016, 10, 465–472. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.J.; Wallace, S.; Cherif, A.; Li, C.; Gretzer, M.B.; Kim, E.E.; Podoloff, D.A. Development of F-18-labeled fluoroerythronitroimidazole as a PET agent for imaging tumor hypoxia. Radiology 1995, 194, 795–800. [Google Scholar] [CrossRef]
- Grierson, J.R.; Link, J.M.; Mathis, C.A.; Rasey, J.S.; Krohn, K.A. A radiosynthesis of fluorine-18 fluoromisonidazole. J. Nucl. Med. 1989, 30, 343–350. [Google Scholar] [PubMed]
- Nunn, A.; Linder, K.; Strauss, H.W. Nitroimidazoles and imaging hypoxia. Eur. J. Nucl. Med. 1995, 22, 265–280. [Google Scholar]
- Paul, B.; Stuart, G.; Gemma, F. Clinical imaging of hypoxia: Current status and future directions. Free Radical Biol. Med. 2018, 126, 296–312. [Google Scholar]
- Giglio, J.; Rey, A. 99mTc Labelling strategies for the development of potential nitroimidazolic hypoxia imaging agents. Inorganics 2019, 7, 128–143. [Google Scholar] [CrossRef] [Green Version]
- Abrams, M.J.; Juweid, M.; Tenkate, C.I.; Schwartz, D.A.; Fischman, A.J. Technetium-99m-human polyclonal IgG radiolabeled via the hydrazino nicotinamide derivative for imaging focal sites of infection in rats. J. Nucl. Med. 1990, 31, 2022–2028. [Google Scholar]
- Harris, T.D.; Sworin, M.; Williams, N.; Rajopadhye, M.; Damphousse, P.R.; Glowacka, D.; Poirier, M.J.; Yu, K. Synthesis of stable hydrazones of a hydrazinonicotinyl-modified peptide for the preparation of 99mTc-labeled radiopharmaceuticals. Bioconjug. Chem. 1999, 10, 808–814. [Google Scholar]
- Edwards, D.S.; Liu, S.; Barrett, J.A.; Harris, A.R.; Looby, R.J.; Ziegler, M.C.; Heminway, S.J.; Carroll, T.R. New and versatile ternary ligand system for technetium radiopharmaceuticals: Water soluble phosphines and tricine as coligands in labeling a hydrazinonicotinamide-modified cyclic glycoprotein IIb/IIIa receptor antagonist with 99mTc. Bioconjug. Chem. 1997, 8, 146–154. [Google Scholar] [CrossRef]
- Mosayebnia, M.; Hajimahdi, Z.; Beiki, D.; Rezaeianpour, M.; Hajiramezanali, M.; Geramifar, P.; Sabzevari, O.; Amini, M.; Hatamabadi, D.; Shahhosseini, S. Design, synthesis, radiolabeling and biological evaluation of new urea-based peptides targeting prostate specific membrane antigen. Bioorg. Chem. 2020, 99, 103743. [Google Scholar] [CrossRef] [PubMed]
- Khodadoust, F.; Ahmadpour, S.; Khamseh, N.; Abedi, S.M.; Hosseinimehr, S.J. An improved 99mTc-HYNIC-(Ser)3 -LTVSPWY peptide with EDDA/tricine as co-ligands for targeting and imaging of HER2 overexpression tumor. Eur. J. Med. Chem. 2017, 144, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wu, Y.; Wang, Z.; Jia, B.; Hu, Z.; Dong, C.; Wang, F. SPECT/CT imaging of the novel HER2-targeted peptide probe 99mTc-HYNIC-H6F in breast cancer mouse models. J. Nucl. Med. 2017, 58, 821–826. [Google Scholar]
- Mogadam, H.Y.; Erfani, M.; Nikpassand, M.; Mokhtary, M. Preparation and assessment of a new radiotracer technetium-99m-6-hydrazinonicotinic acid-tyrosine as a targeting agent in tumor detecting through single photon emission tomography. Bioorg. Chem. 2020, 104, 104181. [Google Scholar] [CrossRef]
- Liu, S.; Edwards, D.S.; Harris, A.R.; Heminway, S.J.; Barrett, J.A. Technetium complexes of a hydrazinonicotinamide-conjugated cyclic peptide and 2-hydrazinopyridine: Synthesis and characterization. Inorg. Chem. 1999, 38, 1326–1335. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Zhang, C.; Yu, Q.; Pang, Y.; Lu, J. Novel 99mTc radiolabeled folate complexes with PEG linkers for FR-positive tumor imaging: Synthesis and biological evaluation. RSC Adv. 2014, 4, 32197–32206. [Google Scholar] [CrossRef]
- Gan, Q.; Song, X.; Zhang, X.; Zhang, J. Preparation and evaluation of 99mTc-labeled HYNIC-palbociclib analogs for cyclin-dependent kinase 4/6-positive tumor imaging. Eur. J. Med. Chem. 2020, 188, 112032. [Google Scholar]
- Ruan, Q.; Zhang, X.; Zhang, J. Radiosynthesis and evaluation of novel [99mTc(I)]+ and [99mTc(I)(CO)3]+ complexes with a 4-nitroimidazole isocyanide for imaging tumor hypoxia. Appl. Organomet. Chem. 2020, 34, e5798. [Google Scholar] [CrossRef]
- Li, Z.; Lin, X.; Zhang, J.; Wang, X.; Jin, Z.; Zhang, W.; Zhang, Y. Kit formulation for preparation and biological evaluation of a novel 99mTc-oxo complex with metronidazole xanthate for imaging tumor hypoxia. Nucl. Med. Biol. 2016, 43, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Arai, M.; Takashi, K.; Setiawan, A.; Kobayashi, M. Hypoxia-selective growth inhibition of cancer cells by furospinosulin-1, a furanosesterterpene isolated from an indonesian marine sponge. ChemMedChem 2010, 5, 1919–1926. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zhou, H.; Li, Z.; Wang, X.; Chu, T. Effect of a second nitroimidazole redox centre on the accumulation of a hypoxia marker: Synthesis and in vitro evaluation of 99mTc-labeled bisnitroimidazole propylene amine oxime complexes. Bioorg. Med. Chem. Lett. 2012, 22, 172–177. [Google Scholar]
- Joyard, Y.; Bischoff, L.; Levacher, V.; Papamicael, C.; Vera, P.; Bohn, P. Synthesis and stability evaluation of new HYNIC derivatives as ligands for technetium-99m. Lett. Org. Chem. 2014, 11, 208–214. [Google Scholar] [CrossRef]
- Liu, S.; Edwards, D.S.; Looby, R.J.; Harris, A.R.; Carroll, T.R. Labeling a hydrazino nicotinamide-modified cyclic IIb/IIIa receptor antagonist with 99mTc using aminocarboxylates as coligands. Bioconjug. Chem. 1996, 7, 63–71. [Google Scholar] [CrossRef]
- Larsen, S.K.; Solomon, H.F.; Caldwell, G.W.; Abrams, M.J. [99mTc]Tricine: A useful precursor complex for the radiolabeling of hydrazinonicotinate protein conjugates. Bioconjug. Chem. 1995, 6, 635–638. [Google Scholar] [CrossRef]
- Joyard, Y.; Joncour, V.L.; Castel, H.; Diouf, C.B.; Bischoff, L.; Papamicaël, C.; Levacher, V.; Vera, P.; Bohn, P. Synthesis and biological evaluation of a novel 99mTc labeled 2-nitroimidazole derivative as a potential agent for imaging tumor hypoxia. Bioorg. Med. Chem. Lett. 2013, 23, 3704–3708. [Google Scholar] [CrossRef]
- Li, N.; Zhu, H.; Chu, T.W.; Yang, Z. Preparation and biological evaluation of 99mTc-N4IPA for single photon emission computerized tomography imaging of hypoxia in mouse tumor. Eur. J. Med. Chem. 2013, 69, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, M.; Motaleb, M.A.; Sakr, T.M. Labeling of ceftriaxone for infective inflammation imaging using 99mTc eluted from 99Mo/99mTc generator based on zirconium molybdate. Appl. Radiat. Isot. 2010, 68, 1959–1963. [Google Scholar] [PubMed]
- Zykov, M.P.; Romanovskii, V.N.; Wester, D.W.; Bartenev, S.A.; Korpusov, G.V.; Filyanin, A.T.; Babain, V.A.; Kodina, G.E.; Strelkov, S.A.; Erofeev, S.P.; et al. Use of extractiongenerator for preparing a 99mTc radiopharmaceutical. Radiochemistry 2001, 43, 297–300. [Google Scholar] [CrossRef]
- Zhang, Y.; Chu, T.; Gao, X.; Liu, X.; Yang, Z.; Guo, Z.; Wang, X. Synthesis and preliminary biological evaluation of the 99mTc labeled nitrobenzoimidazole and nitrotriazole as tumor hypoxia markers. Bioorg. Med. Chem. Lett. 2006, 16, 1831–1833. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ruan, Q.; Duan, X.; Gan, Q.; Song, X.; Fang, S.; Lin, X.; Du, J.; Zhang, J. Novel 99mTc-labeled glucose derivative for single photon emission computed tomography: A promising tumor imaging agent. Mol. Pharm. 2018, 15, 3417–3424. [Google Scholar] [CrossRef]
| Tissue | 30 min | 120 min | 240 min |
|---|---|---|---|
| Heart | 1.64 ± 0.18 | 0.56 ± 0.04 | 0.45 ± 0.09 |
| Liver | 1.30 ± 0.09 | 0.54 ± 0.09 | 0.50 ± 0.06 |
| Lung | 2.49 ± 0.25 | 0.62 ± 0.07 | 0.48 ± 0.06 |
| Kidney | 5.49 ± 0.27 | 3.77 ± 0.39 | 3.93 ± 0.49 |
| Spleen | 0.78 ± 0.12 | 0.28 ± 0.05 | 0.27 ± 0.05 |
| Stomach | 0.79 ± 0.17 | 0.57 ± 0.24 | 0.55 ± 0.18 |
| Bone | 0.60 ± 0.16 | 0.39 ± 0.03 | 0.38 ± 0.03 |
| Muscle | 0.85 ± 0.10 | 0.21 ± 0.01 | 0.20 ± 0.02 |
| Small Intestine | 1.83 ± 0.34 | 0.50 ± 0.06 | 0.43 ± 0.11 |
| Tumor | 2.04 ± 0.22 | 1.05 ± 0.27 | 0.84 ± 0.24 |
| Blood | 3.51 ± 0.20 | 0.73 ± 0.04 | 0.50 ± 0.05 |
| Thyroid(%IA) | 0.06 ± 0.01 | 0.02 ± 0.01 | 0.03 ± 0.01 |
| Tumor/Liver | 1.56 | 1.95 | 1.66 |
| Tumor/Blood | 0.58 | 1.44 | 1.68 |
| Tumor/Muscle | 2.41 | 5.05 | 4.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruan, Q.; Gan, Q.; Zhang, X.; Fang, S.; Zhang, J. Preparation and Bioevaluation of Novel 99mTc-Labeled Complexes with a 2-Nitroimidazole HYNIC Derivative for Imaging Tumor Hypoxia. Pharmaceuticals 2021, 14, 158. https://doi.org/10.3390/ph14020158
Ruan Q, Gan Q, Zhang X, Fang S, Zhang J. Preparation and Bioevaluation of Novel 99mTc-Labeled Complexes with a 2-Nitroimidazole HYNIC Derivative for Imaging Tumor Hypoxia. Pharmaceuticals. 2021; 14(2):158. https://doi.org/10.3390/ph14020158
Chicago/Turabian StyleRuan, Qing, Qianqian Gan, Xuran Zhang, Si’an Fang, and Junbo Zhang. 2021. "Preparation and Bioevaluation of Novel 99mTc-Labeled Complexes with a 2-Nitroimidazole HYNIC Derivative for Imaging Tumor Hypoxia" Pharmaceuticals 14, no. 2: 158. https://doi.org/10.3390/ph14020158






