Treatment of Early Allergic and Late Inflammatory Symptoms of Allergic Rhinitis with Petasites Hybridus Leaf Extract (Ze 339): Results of a Noninterventional Observational Study in Switzerland
Abstract
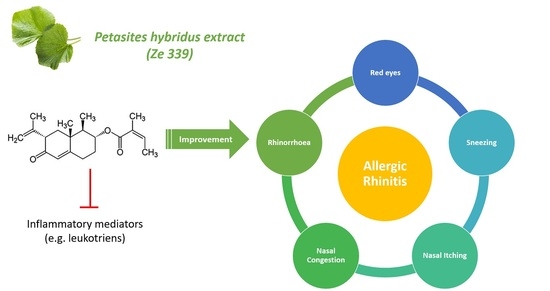
:1. Introduction
2. Results
2.1. Participants, Study Flow and Demographics
2.2. Allergic History of Participants
2.3. Actual Treatment of Allergic Symptoms and its Modalities
2.4. Treatment-Specific Effectiveness
2.5. Duration of Treatment
2.6. Impact on Quality of Life
2.7. Effectiveness on Other Allergic and Atopic Symptoms
2.8. Tolerability
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Ethics
4.3. Study Medication
4.4. Participants
4.4.1. Inclusion Criteria
4.4.2. Exclusion Criteria
4.5. Outcome Measures
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bousquet, J.; Anto, J.M.; Bachert, C.; Baiardini, I.; Bosnic-Anticevich, S.; Walter Canonica, G.; Melen, E.; Palomares, O.; Scadding, G.K.; Togias, A.; et al. Allergic rhinitis. Nat. Rev. Dis. Primers 2020, 6, 95. [Google Scholar] [CrossRef]
- Benninger, M.; Farrar, J.R.; Blaiss, M.; Chipps, B.; Ferguson, B.; Krouse, J.; Marple, B.; Storms, W.; Kaliner, M. Evaluating approved medications to treat allergic rhinitis in the United States: An evidence-based review of efficacy for nasal symptoms by class. Ann. Allergy Asthma Immunol. 2010, 104, 13–29. [Google Scholar] [CrossRef]
- Okano, M. Mechanisms and clinical implications of glucocorticosteroids in the treatment of allergic rhinitis. Clin. Exp. Immunol. 2009, 158, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Ciprandi, G.; Cirillo, I.; Klersy, C.; Marseglia, G.L.; Caimmi, D.; Vizzaccaro, A. Nasal obstruction is the key symptom in hay fever patients. Otolaryngol. Head Neck Surg. 2005, 133, 429–435. [Google Scholar] [CrossRef]
- Brozek, J.L.; Bousquet, J.; Agache, I.; Agarwal, A.; Bachert, C.; Bosnic-Anticevich, S.; Brignardello-Petersen, R.; Canonica, G.W.; Casale, T.; Chavannes, N.H.; et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines-2016 revision. J. Allergy Clin. Immunol. 2017, 140, 950–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Passali, D.; Cingi, C.; Staffa, P.; Passali, F.; Muluk, N.B.; Bellussi, M.L. The International Study of the Allergic Rhinitis Survey: Outcomes from 4 geographical regions. Asia Pac. Allergy 2018, 8, e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bousquet, J.; Van Cauwenberge, P. Allergic Rhinitis and its Impact on Asthma (ARIA) in collaboration with the World Health Organisation. Prim. Care Respir. J. 2002, 11, 18–19. [Google Scholar] [CrossRef] [Green Version]
- Ożarowski, M.; Przystanowicz, J.; Adamczak, A. Phytochemical, pharmacological and clinical studies of Petasites hybridus (L.) P. Gaertn., B. Mey. & Scherb. A review. Herba Pol. 2013, 59, 108–128. [Google Scholar] [CrossRef] [Green Version]
- Neuman, M.G.; Cohen, L.; Opris, M.; Nanau, R.M.; Hyunjin, J. Hepatotoxicity of Pyrrolizidine Alkaloids. J. Pharm. Pharm. Sci. 2015, 18, 825–843. [Google Scholar] [CrossRef] [PubMed]
- Allgaier, C.; Franz, S. Risk assessment on the use of herbal medicinal products containing pyrrolizidine alkaloids. Regul. Toxicol. Pharm. 2015, 73, 494–500. [Google Scholar] [CrossRef]
- Schenk, A.; Siewert, B.; Toff, S.; Drewe, J. UPLC TOF MS for sensitive quantification of naturally occurring pyrrolizidine alkaloids in Petasites hybridus extract (Ze 339). J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2015, 997, 23–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wildi, E.; Langer, T.; Schaffner, W.; Buter, K.B. Quantitative analysis of petasin and pyrrolizidine alkaloids in leaves and rhizomes of in situ grown Petasites hybridus plants. Planta Med. 1998, 64, 264–267. [Google Scholar] [CrossRef]
- Giles, M.; Ulbricht, C.; Khalsa, K.P.S.; DeFranko Kirkwood, C.; Park, C.; Basch, E. Butterbur: An evidence-based systematic eview by the natural standard research collaboration. J. Herb. Pharmacother. 2005, 5, 119–143. [Google Scholar] [CrossRef] [PubMed]
- Schapowal, A.; Petasites Study, G. Randomised controlled trial of butterbur and cetirizine for treating seasonal allergic rhinitis. BMJ 2002, 324, 144–146. [Google Scholar] [CrossRef] [Green Version]
- Schapowal, A. Treating intermittent allergic rhinitis: A prospective, randomized, placebo- and antihistamine-controlled study of butterbur extract Ze 339. Phytother. Res. 2005, 19, 530–537. [Google Scholar] [CrossRef]
- Thomet, O.A.; Simon, H.U. Petasins in the treatment of allergic diseases: Results of preclinical and clinical studies. Int. Arch. Allergy Immunol. 2002, 129, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Thomet, O.A.R.; Wiesmann, U.N.; Schapowal, A.; Bizer, C.; Simon, H.U. Role of petasin in the potential anti-inflammatory activity of a plant extract of Petasites hybridus. Biochem. Pharm. 2001, 61, 1041–1047. [Google Scholar] [CrossRef]
- Thomet, O.A.R.; Schapowal, A.; Heinisch, I.V.; Wiesmann, U.N.; Simon, H.U. Anti-inflammatory activity of an extract of Petasites hybridus in allergic rhinitis. Int. Immunopharmacol. 2002, 2, 997–1006. [Google Scholar] [CrossRef]
- Steiert, S.A.; Zissler, U.M.; Chaker, A.M.; Esser-von-Bieren, J.; Dittlein, D.; Guerth, F.; Jakwerth, C.A.; Piontek, G.; Zahner, C.; Drewe, J.; et al. Anti-inflammatory effects of the petasin phyto drug Ze339 are mediated by inhibition of the STAT pathway. Biofactors 2017, 43, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Dumitru, A.F.; Shamji, M.; Wagenmann, M.; Hindersin, S.; Scheckenbach, K.; Greve, J.; Klenzner, T.; Hess, L.; Nebel, S.; Zimmermann, C.; et al. Petasol butenoate complex (Ze 339) relieves allergic rhinitis-induced nasal obstruction more effectively than desloratadine. J. Allergy Clin. Immunol. 2011, 127, 1515–1521. [Google Scholar] [CrossRef] [PubMed]
- Schapowal, A.; Petasites Study, G. Butterbur Ze339 for the treatment of intermittent allergic rhinitis: Dose-dependent efficacy in a prospective, randomized, double-blind, placebo-controlled study. Arch. Otolaryngol. Head Neck Surg. 2004, 130, 1381–1386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Käufeler, R.; Polasek, W.; Brattström, A.; Koetter, U. Efficacy and safety of butterbur herbal extract Ze 339 in seasonal allergic rhinitis: Postmarketing surveillance study. Adv. Ther. 2006, 23, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Linneberg, A.; Dam Petersen, K.; Hahn-Pedersen, J.; Hammerby, E.; Serup-Hansen, N.; Boxall, N. Burden of allergic respiratory disease: A systematic review. Clin. Mol. Allergy 2016, 14, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosario, C.S.; Murrieta-Aguttes, M.; Rosario, N.A. Allergic rhinits: Impact on quality of life of adolescents. Eur. Ann. Allergy Clin. Immunol. 2020. [Google Scholar] [CrossRef] [PubMed]
- EAACI. Global Atlas of Allergic Rhinitis and Chronic Rhinosinusitis; Akdis, C.A., Hellings, P.W., Agache, I., Eds.; European Academy of Allergy and Clinical Immunology: Zürich, Switzerland, 2015. [Google Scholar]
- Klimek, L.; Sperl, A.; Becker, S.; Mosges, R.; Tomazic, P.V. Current therapeutical strategies for allergic rhinitis. Expert Opin. Pharm. 2019, 20, 83–89. [Google Scholar] [CrossRef]
- Klimek, L.; Mullol, J.; Hellings, P.; Gevaert, P.; Mosges, R.; Fokkens, W. Recent pharmacological developments in the treatment of perennial and persistent allergic rhinitis. Expert Opin. Pharm. 2016, 17, 657–669. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P.; Initiative, S. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. BMJ 2007, 335, 806–808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aydin, A.A.; Zerbes, V.; Parlar, H.; Letzel, T. The medical plant butterbur (Petasites): Analytical and physiological (re)view. J. Pharm. Biomed. Anal. 2013, 75, 220–229. [Google Scholar] [CrossRef]
- FDA. Guidance for Industry: Allergic Rhinitis: Clinical Development Programs for Drug Products-Draft Guidance; U.S. Department of Health and Human Services: Washington, DC, USA, 2018; pp. 1–19.








| Baseline Visit (n = 226) | Visit 2 (n = 205) | Final Visit (n = 131) | |
|---|---|---|---|
| Sex: | |||
| Male | 90 | 83 | 44 |
| Female | 136 | 122 | 87 |
| Age (mean ± SD) | 37.3 ± 17.3 | 37.7 ± 17.5 | 37.1 ± 17.0 |
| Age distribution (years) | Count (Percent) | ||
| <12 | 1 (0.4%) | 1 (0.5) | 0 |
| 12–18 | 39 (17.3%) | 35 (17.1%) | 21 (16.0%) |
| 19–35 | 74 (32.7%) | 64 (31.2%) | 43 (32.8%) |
| 36–60 | 94 (41.6%) | 87 (42.4%) | 58 (44.3%) |
| >60 | 18 (8%) | 18 (8.8%) | 9 (5.9%) |
| n | Percent | ||
|---|---|---|---|
| Diagnosis | Seasonal AR | 90 | 39.8 |
| Perennial AR (possibly including seasonal AR) | 93 | 41.1 | |
| unknown | 43 | 19.0 | |
| Other symptoms | No other symptoms | 128 | 56.6 |
| of atopic disease | (Rhino-) Sinusitis | 50 | 22.1 |
| (allergic) bronchial asthma | 42 | 18.6 | |
| Atopic dermatitis | 30 | 13.3 | |
| Other symptoms | 19 | 8.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blosa, M.; Uricher, J.; Nebel, S.; Zahner, C.; Butterweck, V.; Drewe, J. Treatment of Early Allergic and Late Inflammatory Symptoms of Allergic Rhinitis with Petasites Hybridus Leaf Extract (Ze 339): Results of a Noninterventional Observational Study in Switzerland. Pharmaceuticals 2021, 14, 180. https://doi.org/10.3390/ph14030180
Blosa M, Uricher J, Nebel S, Zahner C, Butterweck V, Drewe J. Treatment of Early Allergic and Late Inflammatory Symptoms of Allergic Rhinitis with Petasites Hybridus Leaf Extract (Ze 339): Results of a Noninterventional Observational Study in Switzerland. Pharmaceuticals. 2021; 14(3):180. https://doi.org/10.3390/ph14030180
Chicago/Turabian StyleBlosa, Maren, Julia Uricher, Sabine Nebel, Catherine Zahner, Veronika Butterweck, and Jürgen Drewe. 2021. "Treatment of Early Allergic and Late Inflammatory Symptoms of Allergic Rhinitis with Petasites Hybridus Leaf Extract (Ze 339): Results of a Noninterventional Observational Study in Switzerland" Pharmaceuticals 14, no. 3: 180. https://doi.org/10.3390/ph14030180