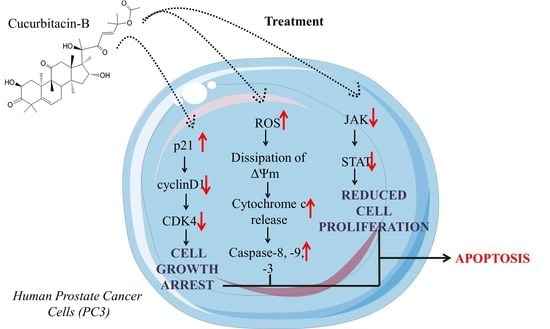
Cucurbitacin-B Exerts Anticancer Effects through Instigation of Apoptosis and Cell Cycle Arrest within Human Prostate Cancer PC3 Cells via Downregulating JAK/STAT Signaling Cascade
Abstract
:1. Introduction
2. Results
2.1. Cur-B Induced Significant Cytotoxic Effects on PC3 Cells
2.2. Cur-B Exposure Altered PC3 Morphology
2.3. Cur-B Instigated Nuclear Condensation
2.4. Cur-B Instigated Intracellular ROS Production
2.5. Cur-B Treatment Induced Dissipation of ΔΨm
2.6. Cur-B Instigated Apoptosis Post Exposure in PC3 Cells
2.7. Cur-B Mediated the Activation of Caspase-8, -9 and -3
2.8. Caspase Inhibitors Mitigated Cur-B-Induced Cytotoxicity
2.9. Cur-B Enhances Apoptosis by Reducing Expression of Anti-Apoptotic Markers
2.10. Cur-B Modulates cyclinD1, CDK4 and p21Cip1 mRNA Expression
2.11. Cur-B Impeded the Expression of JAK1/STAT1 Signaling
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Methods
4.3.1. Evaluation of Cell Viability Post Cur-B Exposure through MTT Assay
4.3.2. Morphological Alterations within Cur-B-Treated Prostate Cancer Cells
4.3.3. Assessment of Nuclear Condensation
4.3.4. Assessment of Cur-B-Instigated Apoptosis
4.3.5. Evaluation of Different Caspases Activity
4.3.6. Assessment of Caspase Inhibitor Pretreatment on Cur-B Exposure
4.3.7. Qualitative Assessment of Mitochondrial Membrane Potential (ΔΨm)
4.3.8. Evaluation of Cur-B-Instigated ROS
4.3.9. Real-Time qPCR Analysis
4.3.10. Statistical Evaluation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Global Cancer Observatory Factsheet, December 2020. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/27-Prostate-fact-sheet.pdf (accessed on 28 February 2022).
- Shukla, S.; Gupta, S. Molecular mechanisms for apigenin-induced cell-cycle arrest and apoptosis of hormone refractory human prostate carcinoma DU145 cells. Mol. Carcinog. 2004, 39, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Bousoik, E.; Montazeri Aliabadi, H. “Do We Know Jack” About JAK? A Closer Look at JAK/STAT Signaling Pathway. Front. Oncol. 2018, 8, 287. [Google Scholar] [CrossRef] [Green Version]
- Rane, S.G.; Reddy, E.P. JAKs, STATs and Src kinases in hematopoiesis. Oncogene 2002, 21, 3334–3358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebersbach, C.; Beier, A.K.; Thomas, C.; Erb, H.H.H. Impact of STAT Proteins in Tumor Progress and Therapy Resistance in Advanced and Metastasized Prostate Cancer. Cancers 2021, 13, 4854. [Google Scholar] [CrossRef]
- Petrylak, D.P.; Tangen, C.M.; Hussain, M.H.; Lara, P.N., Jr.; Jones, J.A.; Taplin, M.E.; Burch, P.A.; Berry, D.; Moinpour, C.; Kohli, M.; et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N. Engl. J. Med. 2004, 351, 1513–1520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahon, K.L.; Henshall, S.M.; Sutherland, R.L.; Horvath, L.G. Pathways of chemotherapy resistance in castration-resistant prostate cancer. Endocr. Relat. Cancer 2011, 18, R103–R123. [Google Scholar] [CrossRef] [PubMed]
- Domingo-Domenech, J.; Vidal, S.J.; Rodriguez-Bravo, V.; Castillo-Martin, M.; Quinn, S.A.; Rodriguez-Barrueco, R.; Bonal, D.M.; Charytonowicz, E.; Gladoun, N.; de la Iglesia-Vicente, J.; et al. Suppression of acquired docetaxel resistance in prostate cancer through depletion of notch- and hedgehog-dependent tumor-initiating cells. Cancer Cell 2012, 22, 373–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, P.K. Ethnopharmacology and ethnomedicine-inspired drug development. In Quality Control and Evaluation of Herbal Drugs; Mukherjee, P.K., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 29–51. [Google Scholar] [CrossRef]
- Gao, Y.; Islam, M.S.; Tian, J.; Lui, V.W.; Xiao, D. Inactivation of ATP citrate lyase by Cucurbitacin B: A bioactive compound from cucumber, inhibits prostate cancer growth. Cancer Lett. 2014, 349, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Thoennissen, N.H.; Iwanski, G.B.; Doan, N.B.; Okamoto, R.; Lin, P.; Abbassi, S.; Song, J.H.; Yin, D.; Toh, M.; Xie, W.D.; et al. Cucurbitacin B induces apoptosis by inhibition of the JAK/STAT pathway and potentiates antiproliferative effects of gemcitabine on pancreatic cancer cells. Cancer Res. 2009, 69, 5876–5884. [Google Scholar] [CrossRef] [PubMed]
- NavaneethaKrishnan, S.; Rosales, J.L.; Lee, K.Y. ROS-Mediated Cancer Cell Killing through Dietary Phytochemicals. Oxid. Med. Cell Longev. 2019, 2019, 9051542. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lu, B.; He, M.; Wang, Y.; Wang, Z.; Du, L. Prostate Cancer Incidence and Mortality: Global Status and Temporal Trends in 89 Countries From 2000 to 2019. Front. Public Health 2022, 10, 811044. [Google Scholar] [CrossRef]
- Shukla, S.; Gupta, S. Dietary agents in the chemoprevention of prostate cancer. Nutr. Cancer 2005, 53, 18–32. [Google Scholar] [CrossRef]
- Melchini, A.; Traka, M.H.; Catania, S.; Miceli, N.; Taviano, M.F.; Maimone, P.; Francisco, M.; Mithen, R.F.; Costa, C. Antiproliferative activity of the dietary isothiocyanate erucin, a bioactive compound from cruciferous vegetables, on human prostate cancer cells. Nutr. Cancer 2013, 65, 132–138. [Google Scholar] [CrossRef] [Green Version]
- Lall, R.K.; Syed, D.N.; Adhami, V.M.; Khan, M.I.; Mukhtar, H. Dietary polyphenols in prevention and treatment of prostate cancer. Int. J. Mol. Sci. 2015, 16, 3350–3376. [Google Scholar] [CrossRef]
- Barry, M.A.; Behnke, C.A.; Eastman, A. Activation of programmed cell death (apoptosis) by cisplatin, other anticancer drugs, toxins and hyperthermia. Biochem. Pharmacol. 1990, 40, 2353–2362. [Google Scholar] [CrossRef]
- Favaloro, B.; Allocati, N.; Graziano, V.; Di Ilio, C.; De Laurenzi, V. Role of apoptosis in disease. Aging 2012, 4, 330–349. [Google Scholar] [CrossRef]
- Wong, R.S. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.H.; Thoennissen, N.H.; Goff, C.; Iwanski, G.B.; Forscher, C.; Doan, N.B.; Said, J.W.; Koeffler, H.P. Synergistic effect of low-dose cucurbitacin B and low-dose methotrexate for treatment of human osteosarcoma. Cancer Lett. 2011, 306, 161–170. [Google Scholar] [CrossRef] [Green Version]
- Tang, D.G.; Li, L.; Chopra, D.P.; Porter, A.T. Extended survivability of prostate cancer cells in the absence of trophic factors: Increased proliferation, evasion of apoptosis, and the role of apoptosis proteins. Cancer Res. 1998, 58, 3466–3479. [Google Scholar]
- Jing, S.; Zou, H.; Wu, Z.; Ren, L.; Zhang, T.; Zhang, J.; Wei, Z. Cucurbitacins: Bioactivities and synergistic effect with small-molecule drugs. J. Funct. Foods 2020, 72, 104042. [Google Scholar] [CrossRef]
- Liu, M.M.; Zhou, N.; Jiang, N.; Lu, K.M.; Wu, C.F.; Bao, J.K. Neuroprotective Effects of Oligosaccharides From Periplaneta Americana on Parkinson's Disease Models In Vitro and In Vivo. Front. Pharmacol 2022, 13, 936818. [Google Scholar] [CrossRef]
- Liskova, A.; Kubatka, P.; Samec, M.; Zubor, P.; Mlyncek, M.; Bielik, T.; Samuel, S.M.; Zulli, A.; Kwon, T.K.; Büsselberg, D. Dietary Phytochemicals Targeting Cancer Stem Cells. Molecules 2019, 24, 899. [Google Scholar] [CrossRef] [Green Version]
- Ribble, D.; Goldstein, N.B.; Norris, D.A.; Shellman, Y.G. A simple technique for quantifying apoptosis in 96-well plates. BMC Biotechnol. 2005, 5, 12. [Google Scholar] [CrossRef] [Green Version]
- Ying, T.H.; Yang, S.F.; Tsai, S.J.; Hsieh, S.C.; Huang, Y.C.; Bau, D.T.; Hsieh, Y.H. Fisetin induces apoptosis in human cervical cancer HeLa cells through ERK1/2-mediated activation of caspase-8-/caspase-3-dependent pathway. Arch. Toxicol. 2012, 86, 263–273. [Google Scholar] [CrossRef]
- Porter, A.G.; Jänicke, R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999, 6, 99–104. [Google Scholar] [CrossRef]
- Wang, C.; Youle, R.J. The role of mitochondria in apoptosis. Annu. Rev. Genet. 2009, 43, 95–118. [Google Scholar] [CrossRef] [Green Version]
- Benítez-Rangel, E.; Olguín-Albuerne, M.; López-Méndez, M.C.; Domínguez-Macouzet, G.; Guerrero-Hernández, A.; Morán, J. Caspase-3 Activation Correlates With the Initial Mitochondrial Membrane Depolarization in Neonatal Cerebellar Granule Neurons. Front. Cell Dev. Biol. 2020, 8, 544. [Google Scholar] [CrossRef]
- Chien, C.C.; Wu, M.S.; Chou, S.W.; Jargalsaikhan, G.; Chen, Y.C. Roles of reactive oxygen species, mitochondrial membrane potential, and p53 in evodiamine-induced apoptosis and G2/M arrest of human anaplastic thyroid carcinoma cells. Chin. Med. 2021, 16, 134. [Google Scholar] [CrossRef]
- Montalto, F.I.; De Amicis, F. Cyclin D1 in Cancer: A Molecular Connection for Cell Cycle Control, Adhesion and Invasion in Tumor and Stroma. Cells 2020, 9, 2648. [Google Scholar] [CrossRef]
- Gizard, F.; Robillard, R.; Barbier, O.; Quatannens, B.; Faucompré, A.; Révillion, F.; Peyrat, J.P.; Staels, B.; Hum, D.W. TReP-132 controls cell proliferation by regulating the expression of the cyclin-dependent kinase inhibitors p21WAF1/Cip1 and p27Kip1. Mol. Cell Biol. 2005, 25, 4335–4348. [Google Scholar] [CrossRef]
- Kale, J.; Osterlund, E.J.; Andrews, D.W. BCL-2 family proteins: Changing partners in the dance towards death. Cell Death Differ. 2018, 25, 65–80. [Google Scholar] [CrossRef] [Green Version]
- Kriesel, J.D.; Jones, B.B.; Hwang, I.P.; Dahms, K.M.; Spruance, S.L. Signal transducers and activators of transcription (Stat) are detectable in mouse trigeminal ganglion neurons. J. Interferon Cytokine Res. 2001, 21, 445–450. [Google Scholar] [CrossRef]
- Yang, C.B.; Pei, W.J.; Zhao, J.; Cheng, Y.Y.; Zheng, X.H.; Rong, J.H. Bornyl caffeate induces apoptosis in human breast cancer MCF-7 cells via the ROS- and JNK-mediated pathways. Acta Pharmacol. Sin. 2014, 35, 113–123. [Google Scholar] [CrossRef] [Green Version]
- Mohapatra, P.; Satapathy, S.R.; Das, D.; Siddharth, S.; Choudhuri, T.; Kundu, C.N. Resveratrol mediated cell death in cigarette smoke transformed breast epithelial cells is through induction of p21Waf1/Cip1 and inhibition of long patch base excision repair pathway. Toxicol. Appl. Pharmacol. 2014, 275, 221–231. [Google Scholar] [CrossRef]
- Alafnan, A.; Hussain, T.; Rizvi, S.M.D.; Moin, A.; Alamri, A. Prostate Apoptotic Induction and NFκB Suppression by Dammarolic Acid: Mechanistic Insight into Onco-Therapeutic Action of an Aglycone Asiaticoside. Curr. Issues Mol. Biol. 2021, 43, 932–940. [Google Scholar] [CrossRef]
- Mishra, T.; Arya, R.K.; Meena, S.; Joshi, P.; Pal, M.; Meena, B.; Upreti, D.K.; Rana, T.S.; Datta, D. Isolation, Characterization and Anticancer Potential of Cytotoxic Triterpenes from Betula utilis Bark. PLoS ONE 2016, 11, e0159430. [Google Scholar] [CrossRef]
- Qi, H.; Siu, S.O.; Chen, Y.; Han, Y.; Chu, I.K.; Tong, Y.; Lau, A.S.; Rong, J. Senkyunolides reduce hydrogen peroxide-induced oxidative damage in human liver HepG2 cells via induction of heme oxygenase-1. Chem. Biol. Interact. 2010, 183, 380–389. [Google Scholar] [CrossRef] [PubMed]







| Gene Name | Forward Sequence | Reverse Sequence |
|---|---|---|
| GAPDH | CGACCACTTTGTCAAGCTCA | CCCCTCTTCAAGGGGTCTAC |
| Bax | GCTGGACATTGGACTTCCTC | CTCAGCCCATCTTCTTCCAG |
| Bad | CCTCAGGCCTATGCAAAAAG | AAACCCAAAACTTCCGATGG |
| Bcl2 | ATTGGGAAGTTTCAAATCAGC | TGCATTCTTGGACGAGGG |
| cyclinD1 | CTTCCTCTCCAAAATGCCAG | AGAGATGGAAGGGGGAAAGA |
| CDK4 | CCTGGCCAGAATCTACAGCTA | ACATCTCGAGGCCAGTCATC |
| p21Cip1 | TGTCCGTCAGAACCCATG | GTGGGAAGGTAGAGCTTGG |
| JAK1 | ATCCTTCGCACAGACAACATC | GCATTCCTGAGCCTTCTTGG |
| STAT1 | ATGGCAGTCTGGCGGCTGAATT | CCAAACCAGGCTGGCACAATTG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alafnan, A.; Alamri, A.; Hussain, T.; Rizvi, S.M.D. Cucurbitacin-B Exerts Anticancer Effects through Instigation of Apoptosis and Cell Cycle Arrest within Human Prostate Cancer PC3 Cells via Downregulating JAK/STAT Signaling Cascade. Pharmaceuticals 2022, 15, 1229. https://doi.org/10.3390/ph15101229
Alafnan A, Alamri A, Hussain T, Rizvi SMD. Cucurbitacin-B Exerts Anticancer Effects through Instigation of Apoptosis and Cell Cycle Arrest within Human Prostate Cancer PC3 Cells via Downregulating JAK/STAT Signaling Cascade. Pharmaceuticals. 2022; 15(10):1229. https://doi.org/10.3390/ph15101229
Chicago/Turabian StyleAlafnan, Ahmed, Abdulwahab Alamri, Talib Hussain, and Syed Mohd Danish Rizvi. 2022. "Cucurbitacin-B Exerts Anticancer Effects through Instigation of Apoptosis and Cell Cycle Arrest within Human Prostate Cancer PC3 Cells via Downregulating JAK/STAT Signaling Cascade" Pharmaceuticals 15, no. 10: 1229. https://doi.org/10.3390/ph15101229