Neuroprotective Effects of Some Nutraceuticals against Manganese-Induced Parkinson’s Disease in Rats: Possible Modulatory Effects on TLR4/NLRP3/NF-κB, GSK-3β, Nrf2/HO-1, and Apoptotic Pathways
Abstract
:1. Introduction
2. Results
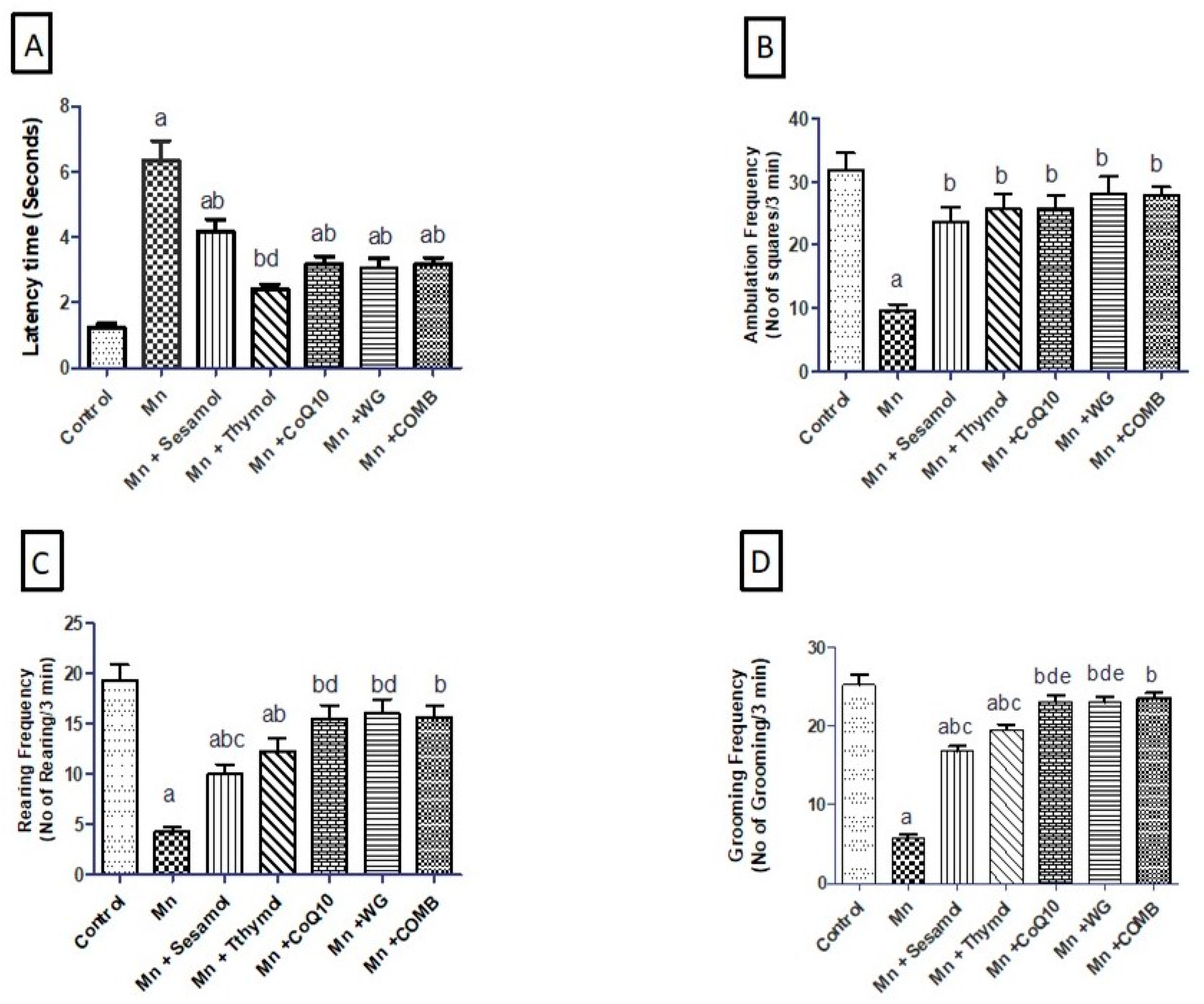
2.1. Effect of Sesamol, Thymol, CoQ10, WG, or Their Combination on MnCl2-Induced Alterations in Motor Functions in Open-Field Test
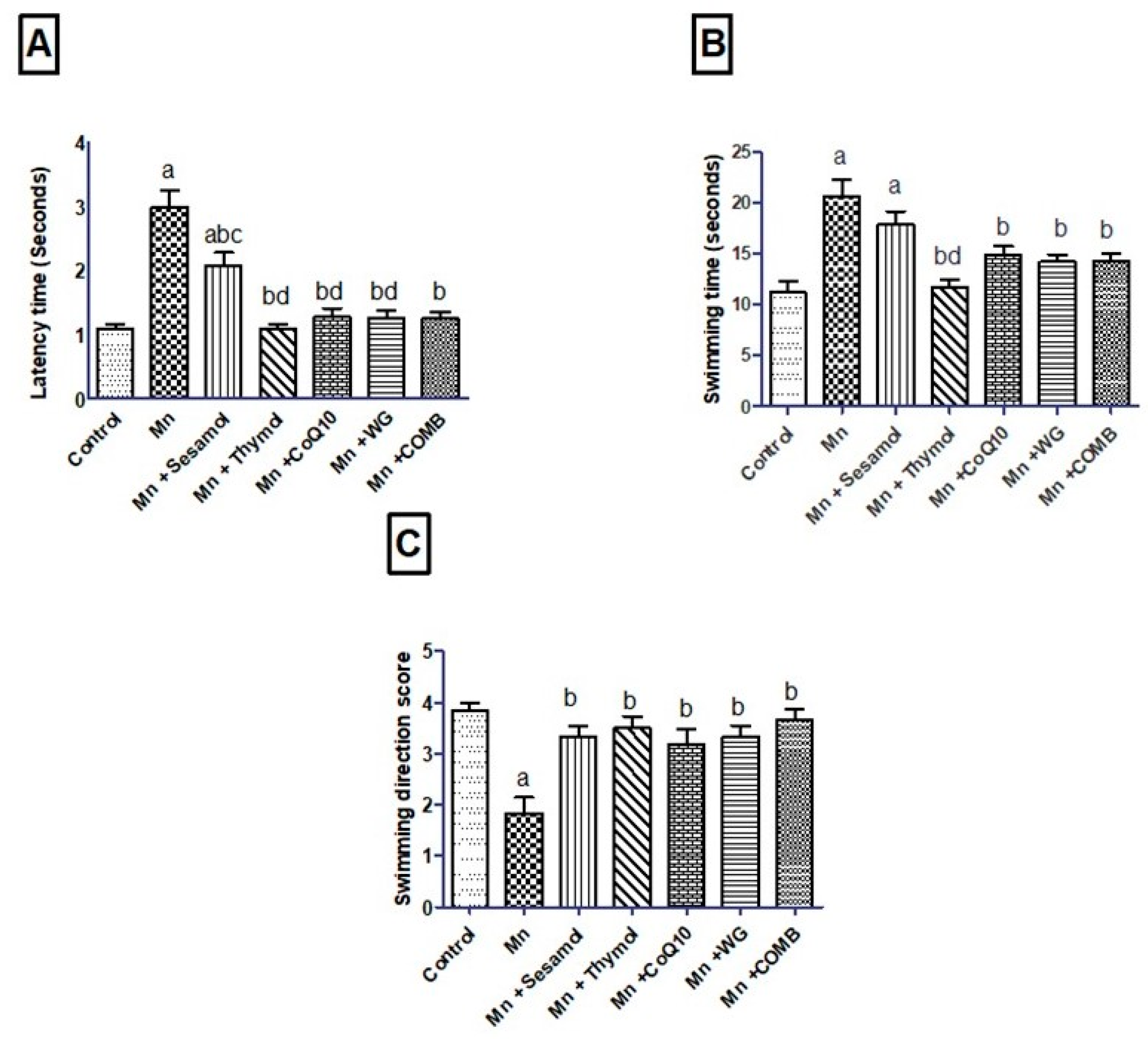
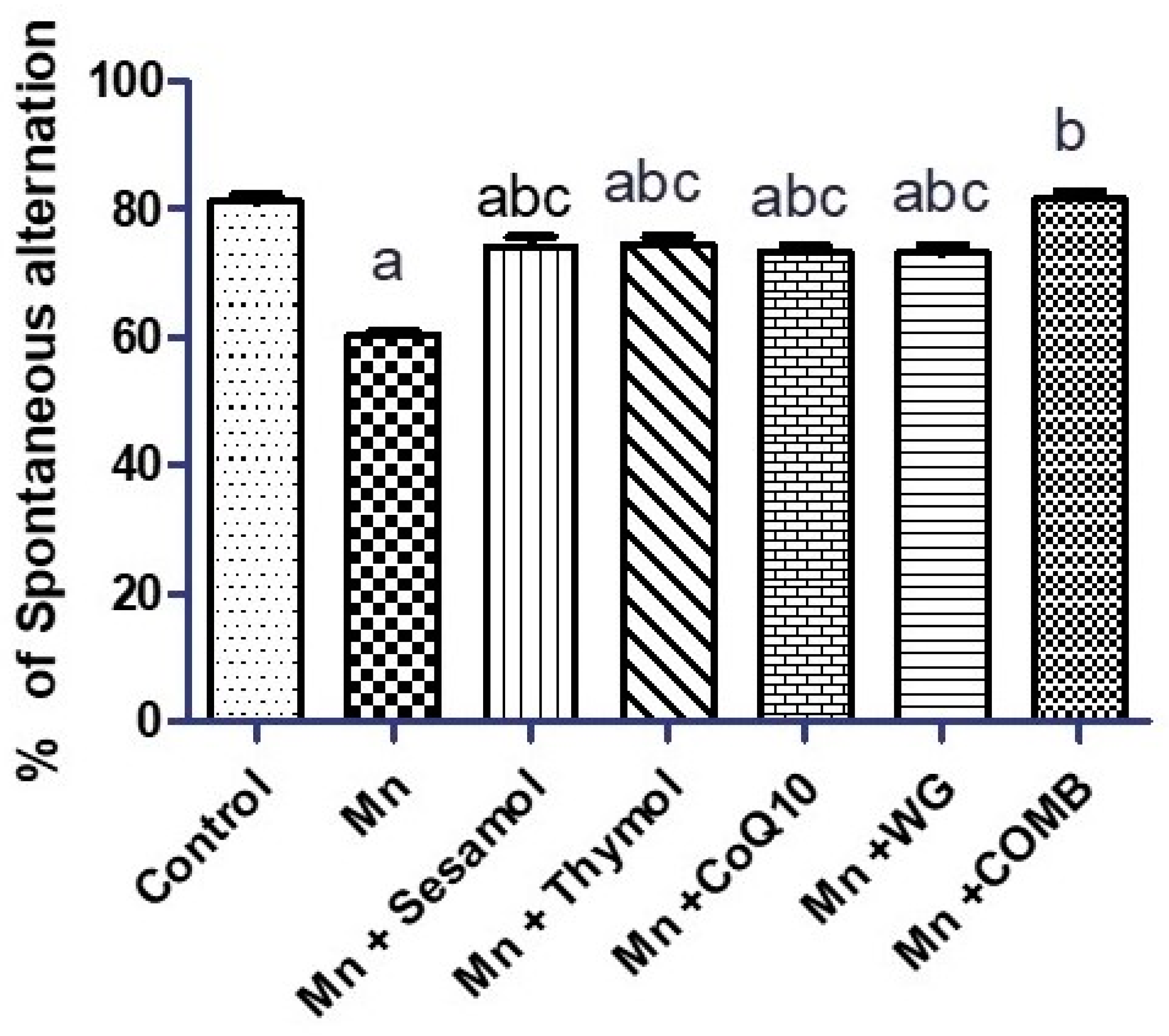
2.2. Effect of Sesamol, Thymol, CoQ10, WG, or Their Combination on MnCl2-Induced Changes in Rats’ Motor, Attention, and Cognitive Functions in Swimming Test and Rats’ Working Memory in the Y-Maze Test
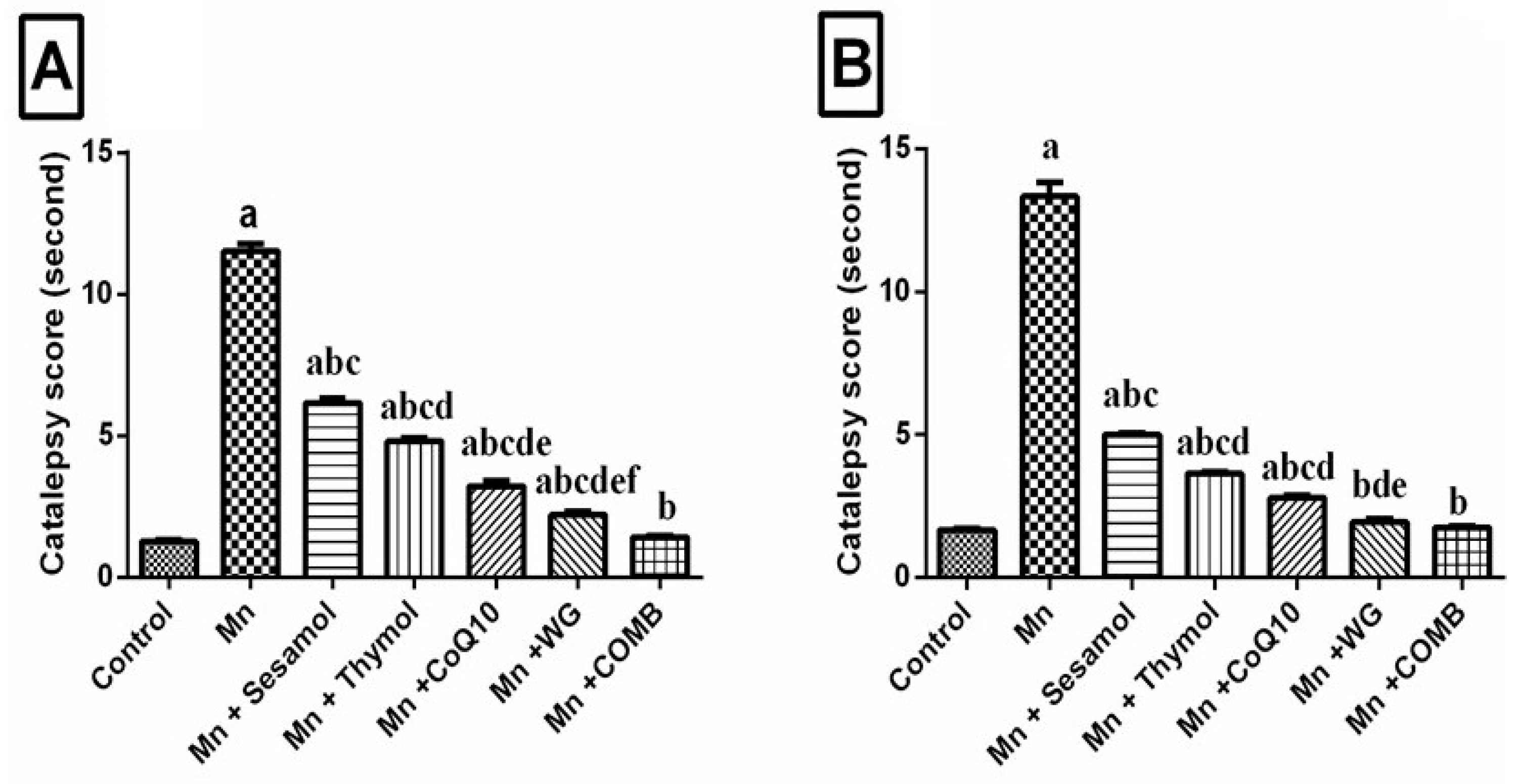
2.3. Effect of Sesamol, Thymol, CoQ10, WG, or Their Combination on MnCl2-Induced Changes in Catalepsy Scores in Both Bar and Grid Tests
2.4. Effect of Sesamol, Thymol, CoQ10, WG, or Their Combination on MnCl2-Induced Changes in Brain Monoamine Neurotransmitter Levels (Dopamine, Norepinephrine, and Serotonin) and ACHE Activity
2.5. Effect of Sesamol, Thymol, CoQ10, WG, or Their Combination on MnCl2-Induced Changes in Cognitive Enhancement Biomarker; BDNF, Neurodegeneration Biomarkers; GABA and Glutamate Levels
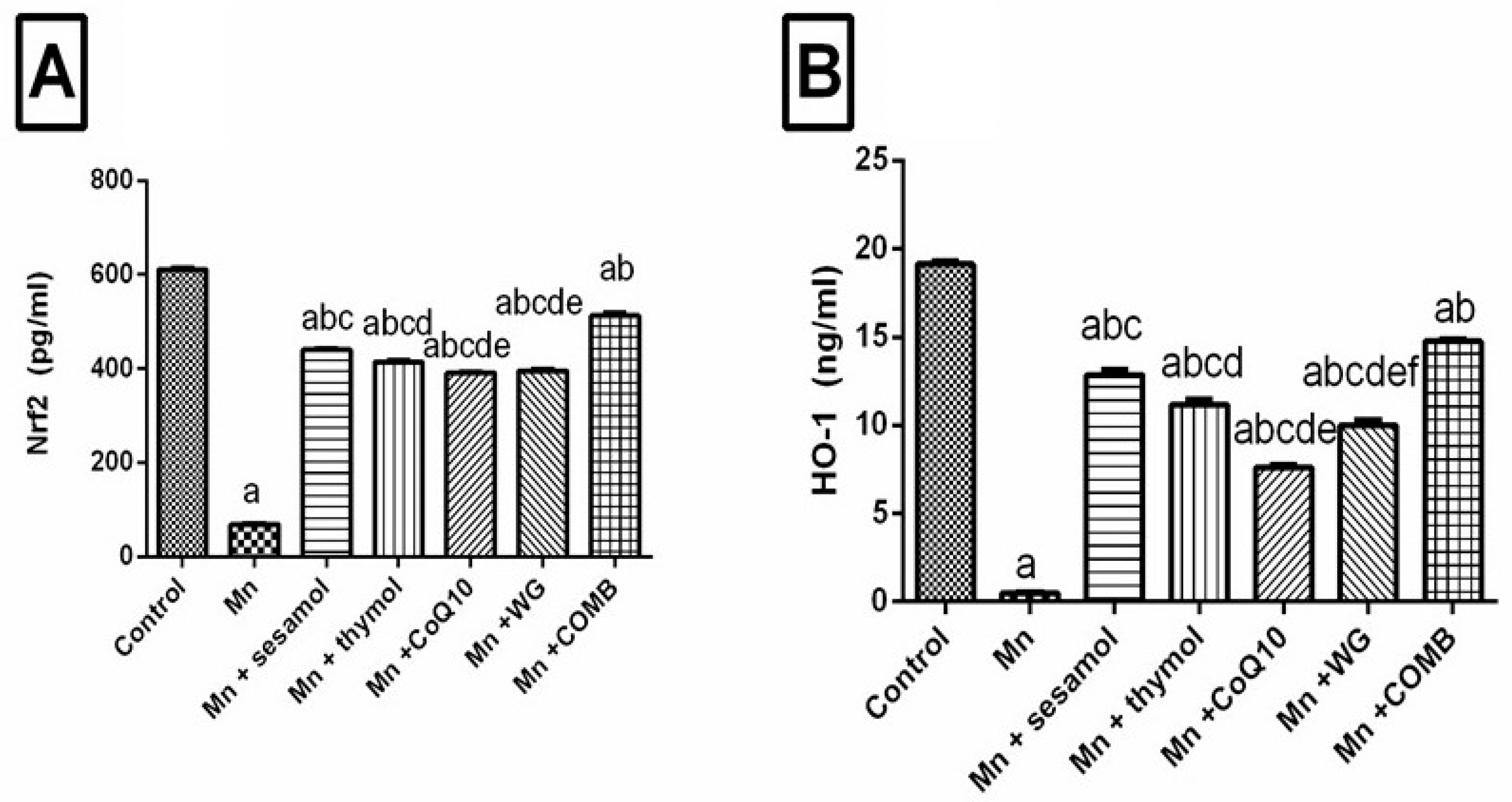
2.6. Effect of Sesamol, Thymol, CoQ10, WG, or Their Combination on MnCl2-Induced Changes in Brain Redox Status and Oxidative Stress Biomarkers; Nrf2, HO-1, and TAC
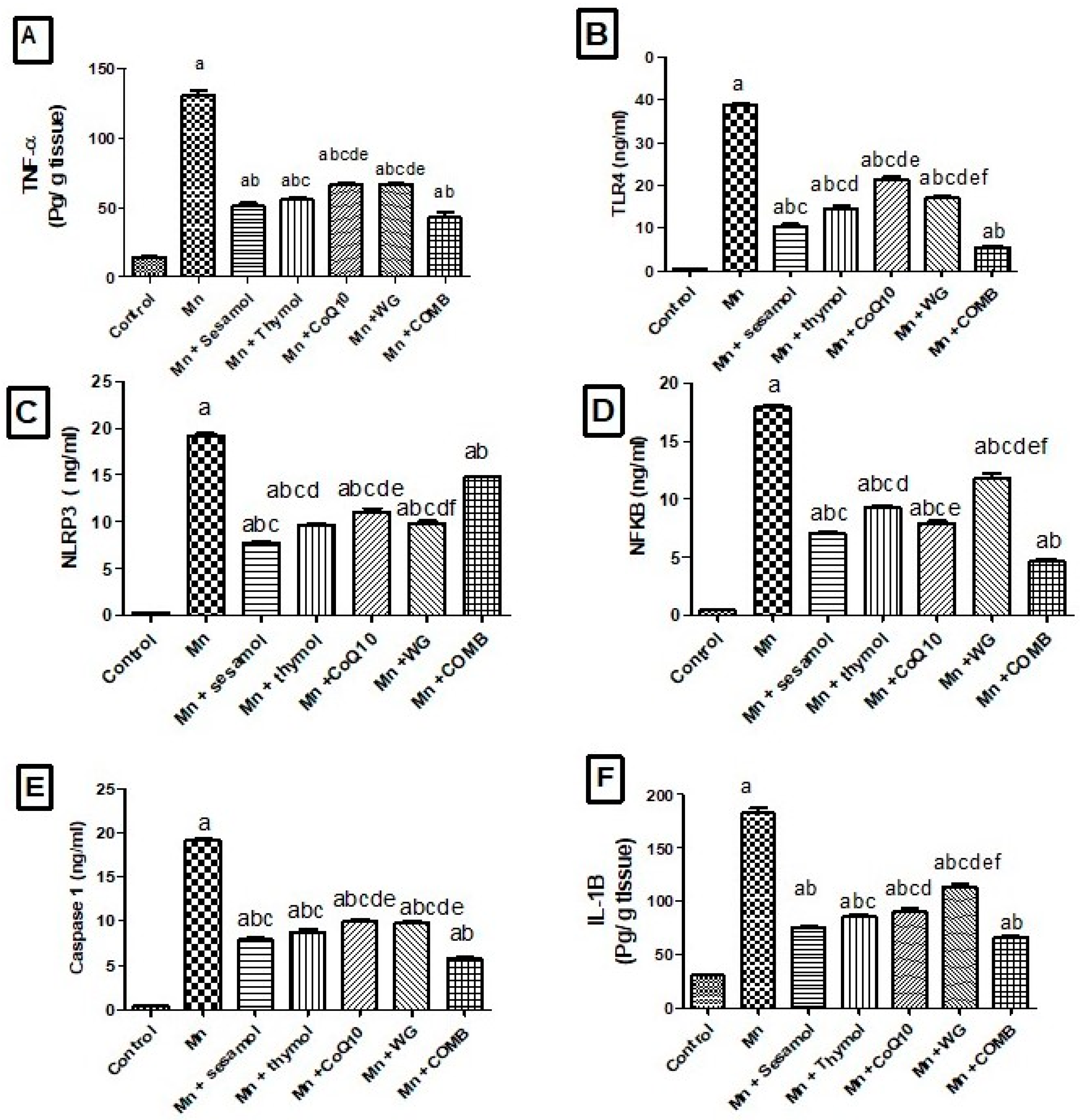
2.7. Effects of Sesamol, Thymol, CoQ10, WG, or Their Combination on MnCl2-Induced Changes in Brain Inflammatory Biomarkers; TLR4, NLRP3, NF-κB, Caspase-1 and Cox-2 and Pro-Inflammatory Cytokines; TNF-α, IL-1β
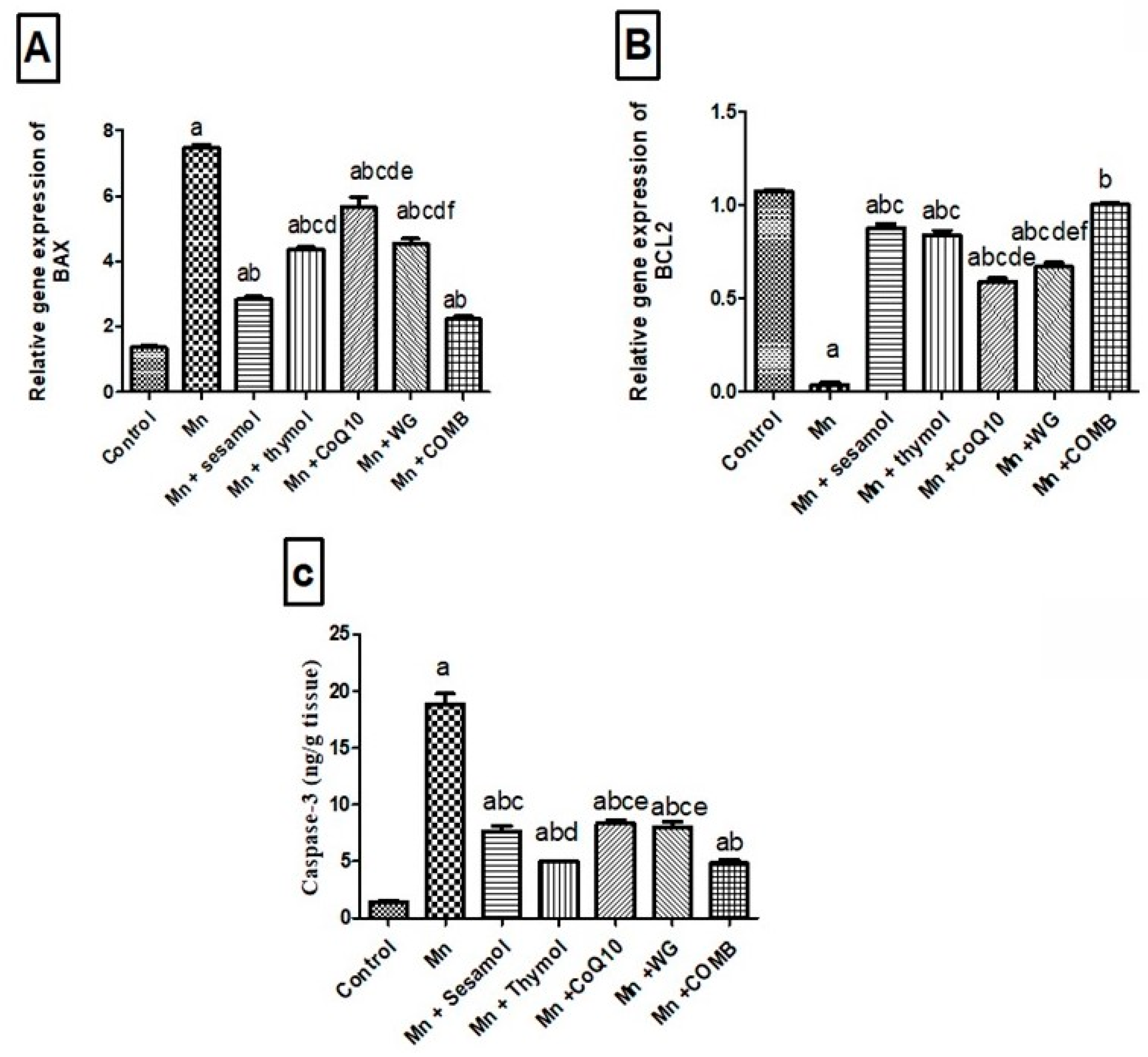
2.8. Effects of Sesamol, Thymol, CoQ10, WG, or Their Combination on MnCl2-Induced Changes in Brain Apoptotic Biomarkers; Bax and Bcl2
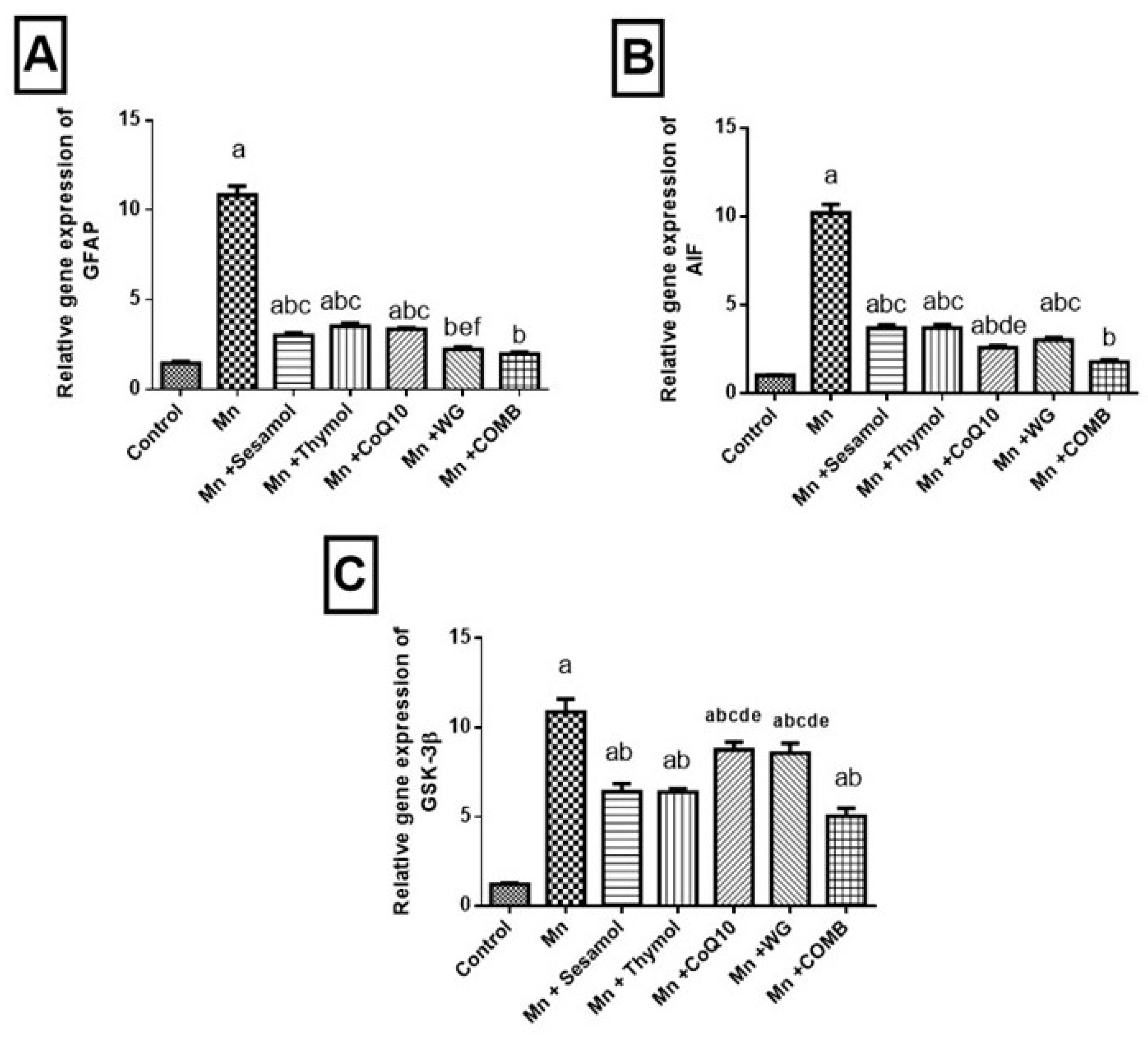
2.9. Effect of Sesamol, Thymol, CoQ10, WG, or Their Combination on MnCl2-Induced Changes in Astroglial Injury Biomarker; GFAP, Tissue Injury Biomarker; AIF and Cognitive Impermanent Biomarker; GSK-3β mRNA Expression
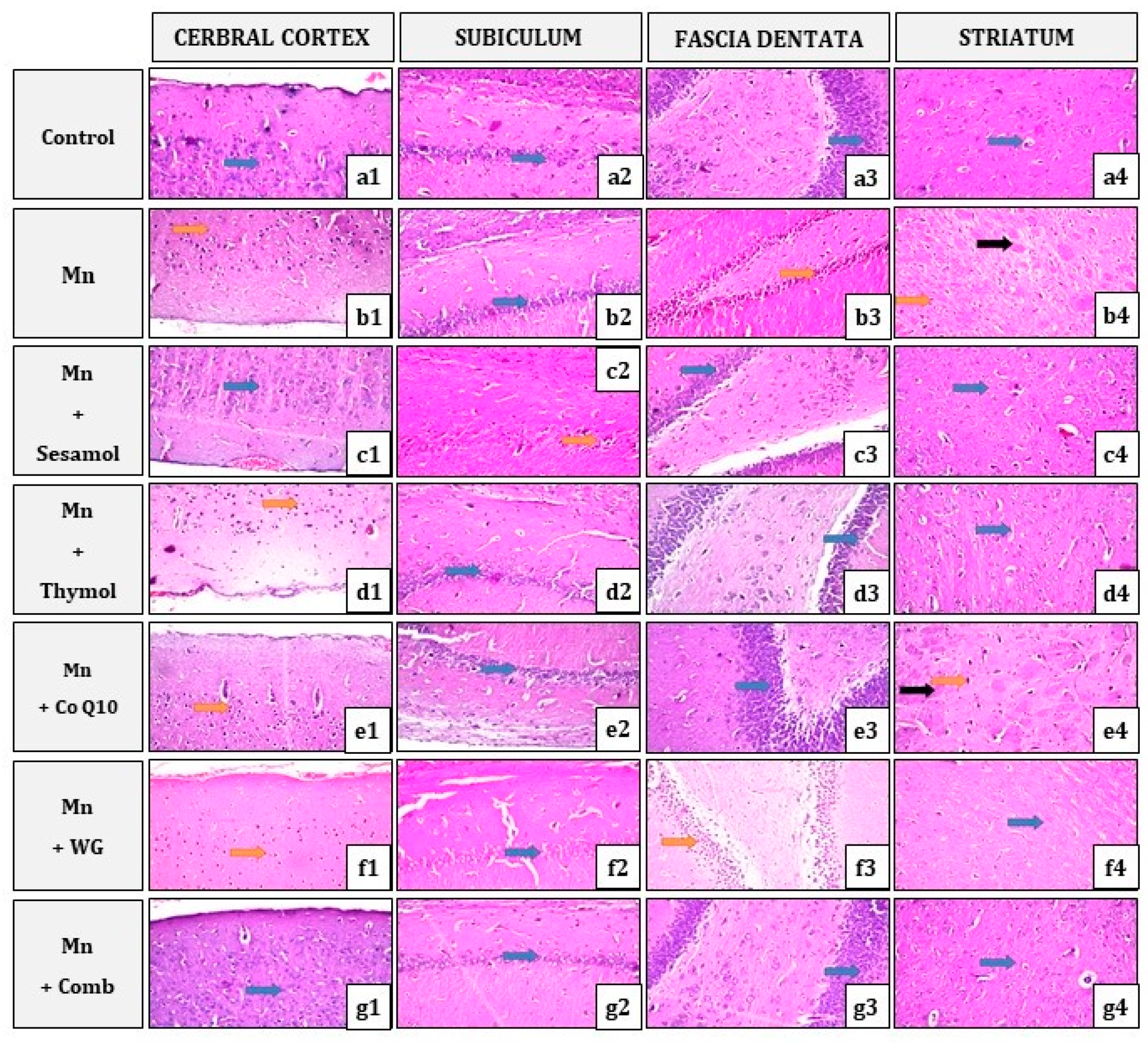
2.10. Effect of Sesamol, Thymol, CoQ10, WG, or Their Combination on MnCl2-Induced Histopathological Alterations in Brain Tissues
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Drugs and Chemicals
4.3. Experimental Design
4.4. Behavioral Tests
4.4.1. Open Field
4.4.2. Swimming Test
Latency Time
Swimming Time
Swimming Direction Score
- Score (4): when the rat swims straight from the starting point to the ramp.
- Score (3): when the rat reaches the ramp through either right or left direction.
- Score (2): when the rat reaches the ramp through both right and left directions.
- Score (1): when the rat swims in all directions and in the middle but finally reaches the ramp during the 3 min.
- Score (0): when the rat swims in all directions and floats passively in the water but cannot reach the ramp within 3 min.
4.4.3. Catalepsy Test
4.4.4. Grid Test
4.4.5. Bar Test
4.4.6. Y-Maze Test
4.5. Tissue Sampling and Preparation
4.6. Colorimetric Estimation of Oxidative Stress Biomarkers; Malondialdehyde (MDA), Total Antioxidant Capacity (TAC), and Superoxide Dismutase (SOD)
4.7. Fluorometric Assay of Neurochemical Markers; Norepinephrine (NE), Dopamine (DA), and Serotonin (5 HT)
4.8. Enzyme-Linked Immunosorbent Assays (ELISA)
4.9. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
4.10. Histopathological Examinations
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Disclosure
Abbreviations
References
- Morissette, M.; Samadi, P.; Tahar, A.H.; Bélanger, N.; Di Paolo, T. Striatal Akt/GSK3 signaling pathway in the development of L-Dopa-induced dyskinesias in MPTP monkeys. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2010, 34, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Mínguez-Mínguez, S.; Del Pozo, J.S.G.; Jordán, J. Rasagiline in Parkinson’s disease: A review based on meta-analysis of clinical data. Pharmacol. Res. 2013, 74, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Krashia, P.; Cordella, A.; Nobili, A.; La Barbera, L.; Federici, M.; Leuti, A.; Campanelli, F.; Natale, G.; Marino, G.; Calabrese, V.; et al. Blunting neuroinflammation with resolvin D1 prevents early pathology in a rat model of Parkinson’s disease. Nat. Commun. 2019, 10, 3945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mamais, A.; Kaganovich, A.; Harvey, K. Convergence of signalling pathways in innate immune responses and genetic forms of Parkinson’s disease. Neurobiol. Dis. 2022, 169, 105721. [Google Scholar] [CrossRef] [PubMed]
- Sen Singh, S.; Rai, S.N.; Birla, H.; Zahra, W.; Rathore, A.S.; Singh, S.P. NF-κB-Mediated Neuroinflammation in Parkinson’s Disease and Potential Therapeutic Effect of Polyphenols. Neurotox. Res. 2020, 37, 491–507. [Google Scholar] [CrossRef] [PubMed]
- Bellucci, A.; Bubacco, L.; Longhena, F.; Parrella, E.; Faustini, G.; Porrini, V.; Bono, F.; Missale, C.; Pizzi, M. Nuclear Factor-κB Dysregulation and α-Synuclein Pathology: Critical Interplay in the Pathogenesis of Parkinson’s Disease. Front. Aging Neurosci. 2020, 12, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afonina, I.S.; Zhong, Z.; Karin, M.; Beyaert, R. Limiting inflammation—The negative regulation of NF-B and the NLRP3 inflammasome. Nat. Immunol. 2017, 18, 861–869. [Google Scholar] [CrossRef]
- Li, Y.; Xia, Y.; Yin, S.; Wan, F.; Hu, J.; Kou, L.; Sun, Y.; Wu, J.; Zhou, Q.; Huang, J.; et al. Targeting Microglial α-Synuclein/TLRs/NF-kappaB/NLRP3 Inflammasome Axis in Parkinson’s Disease. Front. Immunol. 2021, 12, 719807. [Google Scholar] [CrossRef]
- Wu, A.G.; Zhou, X.G.; Qiao, G.; Yu, L.; Tang, Y.; Yan, L.; Qiu, W.Q.; Pan, R.; Yu, C.L.; Law, B.Y.; et al. Targeting microglial autophagic degradation in NLRP3 inflammasome-mediated neurodegenerative diseases. Ageing Res. Rev. 2021, 65, 101202. [Google Scholar] [CrossRef]
- Huang, B.; Liu, J.; Meng, T.; Li, Y.; He, D.; Ran, X.; Chen, G.; Guo, W.; Kan, X.; Fu, S.; et al. Polydatin Prevents Lipopolysaccharide (LPS)-Induced Parkinson’s Disease via Regulation of the AKT/GSK3β-Nrf2/NF-κB Signaling Axis. Front. Immunol. 2018, 9, 2527. [Google Scholar] [CrossRef]
- Credle, J.J.; George, J.L.; Wills, J.; Duka, V.; Shah, K.; Lee, Y.C.; Rodriguez, O.; Simkins, T.; Winter, M.; Moechars, D.; et al. GSK-3β dysregulation contributes to parkinson’s-like pathophysiology with associated region-specific phosphorylation and accumulation of tau and α-synuclein. Cell Death Differ. 2015, 22, 838–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olanow, C.W. The pathogenesis of cell death in Parkinson’s disease—2007. Mov. Disord. 2007, 22, 2007. [Google Scholar] [CrossRef] [PubMed]
- de Lau, L.M.L.; Schipper, C.M.A.; Hofman, A.; Koudstaal, P.J.; Breteler, M.M.B. Prognosis of Parkinson Disease. Arch. Neurol. 2005, 62, 1265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaudhuri, K.R.; Schapira, A.H. Non-motor symptoms of Parkinson’s disease: Dopaminergic pathophysiology and treatment. Lancet Neurol. 2009, 8, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, A.B.; Sulsky, S.I. Risk assessment of an essential element: Manganese. J. Toxicol. Environ. Health Part A 2010, 73, 128–155. [Google Scholar] [CrossRef] [PubMed]
- Pfalzer, A.; Bowman, A. Manganese Toxicity in Neurological Disease. Curr. Environ. Health Rep. 2018, 4, 223–228. [Google Scholar] [CrossRef]
- Perl, D.P.; Olanow, C.W. The Neuropathology of Manganese-Induced Parkinsonism. J. Neuropathol. Exp. Neurol. 2007, 66, 675–682. [Google Scholar] [CrossRef] [Green Version]
- Yao, Z.; Bai, Q.; Wang, G. Mechanisms of Oxidative Stress and Therapeutic Targets following Intracerebral Hemorrhage. Oxid. Med. Cell. Longev. 2021, 2021, 8815441. [Google Scholar] [CrossRef]
- Shahpiri, Z.; Bahramsoltani, R.; Hosein Farzaei, M.; Farzaei, F.; Rahimi, R. Phytochemicals as future drugs for Parkinson’s disease: A comprehensive review. Rev. Neurosci. 2016, 27, 651–668. [Google Scholar] [CrossRef]
- Kujawska, M.; Jodynis-Liebert, J. Polyphenols in parkinson’s disease: A systematic review of in vivo studies. Nutrients 2018, 10, 5. [Google Scholar] [CrossRef]
- Geetha, T.; Rohit, B.; Indu Pal, K. Sesamol: An efficient antioxidant with potential therapeutic benefits. Med. Chem. 2009, 5, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Hsu, D.Z.; Chu, P.Y.; Liu, M.Y. The non-peptide chemical 3,4-methylenedioxyphenol blocked lipopolysaccharide (LPS) from binding to LPS-binding protein and inhibited pro-inflammatory cytokines. Innate Immun. 2009, 15, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Nagoor Meeran, M.F.; Javed, H.; Al Taee, H.; Azimullah, S.; Ojha, S.K. Pharmacological properties and molecular mechanisms of thymol: Prospects for its therapeutic potential and pharmaceutical development. Front. Pharmacol. 2017, 8, 380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zahin, M.; Ahmad, I.; Aqil, F. Antioxidant and antimutagenic activity of Carum copticum fruit extracts. Toxicol. Vitr. 2010, 24, 1243–1249. [Google Scholar] [CrossRef] [PubMed]
- Özen, T.; Demirtas, I.; Aksit, H. Determination of antioxidant activities of various extracts and essential oil compositions of Thymus praecox subsp. skorpilii var. skorpilii. Food Chem. 2011, 124, 58–64. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y.; Xu, H.; Luo, X.; Yu, J.; Liu, J.; Chang, R.C. Neuroprotection of Coenzyme Q10 in Neurodegenerative Diseases. Curr. Top. Med. Chem. 2016, 16, 858–866. [Google Scholar] [CrossRef] [PubMed]
- Shults, C.W.; Oakes, D.; Kieburtz, K.; Al, E. Effects of Coenzyme Q10 in Early Parkinson Disease: Evidence of Slowing of the Functional Decline. Arch. Neurol. 2002, 59, 1541–1550. [Google Scholar] [CrossRef] [PubMed]
- Falcioni, G. Antioxidant activity of wheat sprouts extract in vitro: Inhibition of DNA oxidative damage. J. Food Sci. 2002, 67, 2918–2922. [Google Scholar] [CrossRef]
- Arya, P.; Kumar, M. Chemoprevention by Triticum aestivum of mouse skin carcinogenesis induced by DMBA and croton oil—Association with oxidative status. Asian Pac. J. Cancer Prev. 2011, 12, 143–148. [Google Scholar]
- Javed, H.; Azimullah, S.; Haque, M.E.; Ojha, S.K. Cannabinoid type 2 (CB2) receptors activation protects against oxidative stress and neuroinflammation associated dopaminergic neurodegeneration in rotenone model of parkinson’s disease. Front. Neurosci. 2016, 10, 321. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Liu, J.; Jiang, L.; Ran, X.; He, D.; Li, Y.; Huang, B.; Wang, W.; Fu, S. Galangin Reduces the Loss of Dopaminergic Neurons in an LPS-Evoked Model of Parkinson’s Disease in Rats. Int. J. Mol. Sci. 2018, 19, 12. [Google Scholar] [CrossRef] [PubMed]
- Bouabid, S.; Delaville, C.; De Deurwaerdère, P.; Lakhdar-Ghazal, N.; Benazzouz, A. Manganese-induced atypical parkinsonism is associated with altered basal ganglia activity and changes in tissue levels of monoamines in the rat. PLoS ONE 2014, 9, e98952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwakye, G.F.; Paoliello, M.M.B.; Mukhopadhyay, S.; Bowman, A.B.; Aschner, M. Manganese-induced parkinsonism and Parkinson’s disease: Shared and distinguishable features. Int. J. Environ. Res. Public Health 2015, 12, 7519–7540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonia Angeline, M.; Sarkar, A.; Anand, K.; Ambasta, R.K.; Kumar, P. Sesamol and naringenin reverse the effect of rotenone-induced PD rat model. Neuroscience 2013, 254, 379–394. [Google Scholar] [CrossRef] [PubMed]
- Attia, H.N.; Maklad, Y.A. Neuroprotective effects of coenzyme Q10 on paraquat-induced Parkinson’s disease in experimental animals. Behav. Pharmacol. 2018, 29, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Nourmohammadi, S.; Yousefi, S.; Manouchehrabadi, M.; Farhadi, M.; Azizi, Z.; Torkaman-Boutorabi, A. Thymol protects against 6-hydroxydopamine-induced neurotoxicity in in vivo and in vitro model of Parkinson’s disease via inhibiting oxidative stress. BMC Complement. Med. Ther. 2022, 22, 40. [Google Scholar] [CrossRef]
- Yousefi Babadi, V.; Sadeghi, L.; Shirani, K.; Malekirad, A.A.; Rezaei, M. The Toxic Effect of Manganese on the Acetylcholinesterase Activity in Rat Brains. J. Toxicol. 2014, 2014, 946372. [Google Scholar] [CrossRef]
- Olanow, C.W.; Brundin, P. Parkinson’s Disease and Alpha Synuclein: Is Parkinson’s Disease a Prion-Like Disorder? Mov. Disord. 2013, 28, 31–40. [Google Scholar] [CrossRef]
- Yan, M.H.; Wanga, Z.; Zhua, Z. Mitochondrial defects and oxidative stress in Alzheimer disease and Parkinson disease. Free Radic. Biol. Med. 2013, 62, 90–101. [Google Scholar] [CrossRef] [Green Version]
- Bramham, C.R.; Messaoudi, E. BDNF function in adult synaptic plasticity: The synaptic consolidation hypothesis. Prog. Neurobiol. 2005, 76, 99–125. [Google Scholar] [CrossRef]
- Abu-Elfotuh, K.; Hamdan, A.M.E.; Abbas, A.N.; Alahmre, A.T.S.; Elewa, M.A.F.; Masoud, R.A.E.; Ali, A.A.; Othman, M.; Kamal, M.M.; Hassan, F.A.M.; et al. Evaluating the neuroprotective activities of vinpocetine, punicalagin, niacin and vitamin E against behavioural and motor disabilities of manganese-induced Parkinson’s disease in Sprague Dawley rats. Biomed. Pharmacother. 2022, 153, 113330. [Google Scholar] [CrossRef] [PubMed]
- Avila, D.S.; Gubert, P.; Fachinetto, R.; Wagner, C.; Aschner, M.; Rocha, J.B.; Soares, F.A. Involvement of striatal lipid peroxidation and inhibition of calcium influx into brain slices in neurobehavioral alterations in a rat model of short-term oral exposure to manganese. Neurotoxicology 2008, 29, 1062–10628. [Google Scholar] [CrossRef] [PubMed]
- Cobley, J.N.; Fiorello, M.L.; Bailey, D.M. 13 Reasons Why the Brain Is Susceptible to Oxidative Stress. Redox Biol. 2018, 15, 490–503. [Google Scholar] [CrossRef] [PubMed]
- Infante, J.; García-Gorostiaga, I.; Sánchez-Juan, P.; Sierra, M.; Martín-Gurpegui, J.L.; Terrazas, J.; Mateo, I.; Rodríguez-Rodríguez, E.; Berciano, J.; Combarros, O. Synergistic effect of two oxidative stress-related genes (heme oxygenase-1 and GSK3β) on the risk of Parkinson’s disease. Eur. J. Neurol. 2010, 17, 760–762. [Google Scholar] [CrossRef]
- Tapias, V.; Escames, G.; López, L.C.; López, A.; Camacho, E.; Carrión, M.D.; Entrena, A.; Gallo, M.A.; Espinosa, A.; Acuña-Castroviejo, D. Melatonin and its brain metabolite N(1)-acetyl-5-methoxykynuramine prevent mitochondrial nitric oxide synthase induction in parkinsonian mice. J. Neurosci. Res. 2009, 87, 3002–3010. [Google Scholar] [CrossRef]
- Ojha, S.K.; Meeran, F.N.; Sheikh, A.; Javed, H.; Ojha, S.K.; Meeran, F.N.; Sheikh, A.; Javed, H. Protective effects of thymol against neurodegeneration in rotenone induced rat model of Parkinson’s disease. In Proceedings of the Annual Meeting of the Japanese Pharmacological Society WCP2018 (The 18th World Congress of Basic and Clinical Pharmacology), Kyoto, Japan, 1–6 July 2018; p. PO2-1-68. [Google Scholar]
- Ren, B.; Yuan, T.; Diao, Z.; Zhang, C.; Liu, Z.; Liu, X. Protective effects of sesamol on systemic oxidative stress-induced cognitive impairments: Via regulation of Nrf2/Keap1 pathway. Food Funct. 2018, 9, 5912–5924. [Google Scholar] [CrossRef]
- Yang, X.; Yang, R.; Zhang, F. Role of Nrf2 in Parkinson’s Disease: Toward New Perspectives. Front. Pharmacol. 2022, 13, 919233. [Google Scholar] [CrossRef]
- Sarkar, S.; Malovic, E.; Harischandra, D.S.; Ngwa, H.A.; Ghosh, A.; Hogan, C.; Rokad, D.; Zenitsky, G.; Jin, H.; Anantharam, V.; et al. Manganese exposure induces neuroinflammation by impairing mitochondrial dynamics in astrocytes. Neurotoxicology 2018, 64, 204–218. [Google Scholar] [CrossRef]
- Herrero, M.T.; Estrada, C.; Maatouk, L.; Vyas, S. Inflammation in Parkinson’s disease: Role of glucocorticoids. Front. Neuroanat. 2015, 9, 32. [Google Scholar] [CrossRef] [Green Version]
- Zhao, F.; Cai, T.; Liu, M.; Zheng, G.; Luo, W.; Chen, J. Manganese induces dopaminergic neurodegeneration via microglial activation in a rat model of manganism. Toxicol. Sci. 2009, 107, 156–164. [Google Scholar] [CrossRef]
- Gustot, A.; Gallea, J.I.; Sarroukh, R.; Celej, M.S.; Ruysschaert, J.M.; Raussens, V. Amyloid fibrils are the molecular trigger of inflammation in Parkinson’s disease. Biochem. J. 2015, 471, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Codolo, G.; Plotegher, N.; Pozzobon, T.; Brucale, M.; Tessari, I.; Bubacco, L.; de Bernard, M. Triggering of inflammasome by aggregated α-synuclein, an inflammatory response in synucleinopathies. PLoS ONE 2013, 8, e55375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schroder, K.; Tschopp, J. The Inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franchi, L.; Muñoz-Planillo, R.; Reimer, T.; Eigenbrod, T.; Núñez, G. Inflammasomes as microbial sensors. Eur. J. Immunol. 2010, 40, 611–615. [Google Scholar] [CrossRef]
- Dehay, B.; Bourdenx, M.; Gorry, P.; Przedborski, S.; Vila, M.; Hunot, S.; Singleton, A.; Olanow, C.W.; Merchant, K.M.; Bezard, E.; et al. Targeting α-synuclein for treatment of Parkinson’s disease: Mechanistic and therapeutic considerations. Lancet Neurol. 2015, 14, 855–866. [Google Scholar] [CrossRef] [Green Version]
- Colombo, E.; Farina, C. Astrocytes: Key Regulators of Neuroinflammation. Trends Immunol. 2016, 37, 608–620. [Google Scholar] [CrossRef]
- Javed, H.; Azimullah, S.; Meeran, M.F.N.; Ansari, S.A.; Ojha, S. Neuroprotective Effects of Thymol, a Dietary Monoterpene Against Dopaminergic Neurodegeneration in Rotenone-Induced Rat Model of Parkinson’s Disease. Int. J. Mol. Sci. 2019, 20, 1538. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Yang, Y.; Ying, C.; Li, W.; Ruan, H.; Zhu, X.; You, Y.; Han, Y.; Chen, R.; Wang, Y.; et al. Inhibition of glycogen synthase kinase-3beta protects dopaminergic neurons from MPTP toxicity. Neuropharmacology 2007, 52, 1678–1684. [Google Scholar] [CrossRef]
- Hamdan, A.M.E.; Alharthi, F.H.J.; Alanazi, A.H.; El-Emam, S.Z.; Zaghlool, S.S.; Metwally, K.; Albalawi, S.A.; Abdu, Y.S.; Mansour, R.E.; Salem, H.A.; et al. Neuroprotective Effects of Phytochemicals against Aluminum Chloride-Induced Alzheimer’s Disease through ApoE4/LRP1, Wnt3/β-Catenin/GSK3β, and TLR4/NLRP3 Pathways with Physical and Mental Activities in a Rat Model. Pharmaceuticals 2022, 15, 1008. [Google Scholar] [CrossRef]
- Salehpour, F.; Farajdokht, F.; Cassano, P.; Sadigh-Eteghad, S.; Erfani, M.; Hamblin, M.R.; Salimi, M.M.; Karimi, P.; Rasta, S.H.; Mahmoudi, J. Near-infrared photobiomodulation combined with coenzyme Q10 for depression in a mouse model of restraint stress: Reduction in oxidative stress, neuroinflammation, and apoptosis. Brain Res. Bull. 2019, 144, 213–222. [Google Scholar] [CrossRef]
- Abdelmagid, N.; Abdel Aziz, N.; Shedid, S.; Ahmed, A. Ameliorative Effect of Sugarcane (Blackstrap) Molasses against Gamma Radiation or Manganese- Induced Testicular Toxicity in Male Albino Rats. Egypt. J. Radiat. Sci. Appl. 2019, 32, 219–233. [Google Scholar] [CrossRef]
- Ali, A.A.; Ahmed, H.I.; Khalil, M.G.; Alwakeel, A.I.; Abu-Elfotuh, K. Comparative Study on the Influence of Epigallocatechin-3-gallat e and/or Coenzyme Q10 against Alzheimer’s disease Induced by Aluminiumin Normally-Fed and Protein Malnourished Rats. J. Alzheimers Dis. Park. 2016, 6, 1–10. [Google Scholar]
- Chamanara, M.; Abdollahi, A.; Rezayat, S.M.; Ghazi-Khansari, M.; Dehpour, A.; Nassireslami, E.; Rashidian, A. Thymol reduces acetic acid-induced inflammatory response through inhibition of NF-kB signaling pathway in rat colon tissue. Inflammopharmacology 2019, 27, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Sharma, T.; Airao, V.; Buch, P.; Vaishnav, D.; Parmar, S. Sesamol protects hippocampal CA1 neurons and reduces neuronal infarction in global model of cerebral ischemia in rats. PharmaNutrition 2020, 14, 100217. [Google Scholar] [CrossRef]
- Khalil, M.G.; Ali, A.A.; Hassanin, S.O.; Al-Najjar, A.H.; Ghosh, S.; Mahmoud, M.O. Comparative study on the effect of EGCG and wheat grass together with mental and physical activities against induction of Alzheimer’s disease in both isolated and socialized rats. Phytomed. Plus 2022, 2, 100146. [Google Scholar] [CrossRef]
- Saber, T.M.; Arisha, A.H.; Abo-Elmaaty, A.M.A.; Abdelgawad, F.E.; Metwally, M.M.M.; Saber, T.; Mansour, M.F. Thymol alleviates imidacloprid-induced testicular toxicity by modulating oxidative stress and expression of steroidogenesis and apoptosis-related genes in adult male rats. Ecotoxicol. Environ. Saf. 2021, 221, 112435. [Google Scholar] [CrossRef]
- Bitra, V.R.; Rapaka, D.; Mathala, N.; Akula, A. Effect of wheat grass powder on aluminum induced Alzheimer’s disease in Wistar rats. Asian Pac. J. Trop. Med. 2014, 7, S278–S281. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.A.; Kamal, M.M.; Khalil, M.G.; Ali, S.A.; Elariny, H.A.; Bekhit, A.; Wahid, A. Behavioral, Biochemical and Histopathological effects of Standardised Pomegranate extract with Vinpocetine, Propolis or Cocoa in a rat model of Parkinson’s disease. Exp. Aging Res. 2022, 48, 191–210. [Google Scholar] [CrossRef]
- Abdelsalam, R.M.; Safar, M.M. Neuroprotective effects of vildagliptin in rat rotenone Parkinson’s disease model: Role of RAGE-NFκB and Nrf2-antioxidant signaling pathways. J. Neurochem. 2015, 133, 700–707. [Google Scholar] [CrossRef]
- Alam, M.; Schmidt, W.J. Rotenone destroys dopaminergic neurons and induces parkinsonian symptoms in rats. Behav. Brain Res. 2002, 136, 317–324. [Google Scholar] [CrossRef]
- Zaitone, S.A.; Abo-Elmatty, D.M.; Shaalan, A.A. Acetyl-l-carnitine and α-lipoic acid affect rotenone-induced damage in nigral dopaminergic neurons of rat brain, implication for Parkinson’s disease therapy. Pharmacol. Biochem. Behav. 2012, 100, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Vorhees, C.V.; Klein, K.L.; Scott, W.J. Aspirin-induced psychoteratogenesis in rats as a function of embryonic age. Teratog. Carcinog. Mutagen. 1982, 2, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.A.; Hamed, M.R.; El-Sayed, M. Effect of Protein on Postnatal Neurobehavioural Response to Drugs. Master’s Thesis, Faculty of Pharmacy, Cairo University, Cairo, Egypt, 1992. [Google Scholar]
- Alam, M.; Mayerhofer, A.; Schmidt, W.J. The neurobehavioral changes induced by bilateral rotenone lesion in medial forebrain bundle of rats are reversed by L-DOPA. Behav. Brain Res. 2004, 151, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.D.; Souza, C.M.; Menezes, A.P.; Carmo, M.R.; Fonteles, A.A.; Gurgel, J.P.; Lima, F.A.; Viana, G.S.; Andrade, G.M. Catechin attenuates behavioral neurotoxicity induced by 6-OHDA in rats. Pharmacol. Biochem. Behav. 2013, 110, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elfouly, A.; Awny, M.; Ibrahim, M.K.; Aboelsaad, M.; Tian, J.; Sayed, M. Effects of Long-Acting Testosterone Undecanoate on Behavioral Parameters and Na +, K+-ATPase mRNA Expression in Mice with Alzheimer’s Disease. Neurochem. Res. 2021, 46, 2238–2248. [Google Scholar] [CrossRef] [PubMed]
- Ciarlone, A.E. Further modification of a fluorometric method for analyzing brain amines. Microchem. J. 1978, 23, 9–12. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques, 5th ed.; Churchill Livingstone: London, UK, 2002; pp. 119–135. [Google Scholar]
- Awny, M.M.; Al-Mokaddem, A.K.; Ali, B.M. Mangiferin mitigates di-(2-ethylhexyl) phthalate-induced testicular injury in rats by modulating oxidative stress-mediated signals, inflammatory cascades, apoptotic pathways, and steroidogenesis. Arch. Biochem. Biophys. 2021, 711, 108982. [Google Scholar] [CrossRef]
- Kireev, R.A.; Vara, E.; Viña, J.; Tresguerres, J.A.F. Melatonin and oestrogen treatments were able to improve neuroinflammation and apoptotic processes in dentate gyrus of old ovariectomized female rats. Age 2014, 36, 9707. [Google Scholar] [CrossRef]

| Groups | DA | NE | 5HT | ACHE |
|---|---|---|---|---|
| (ng/g Tissue) | (nmol/g Tissue) | (ng/g Tissue) | (U/L) | |
| Control | 72.2 ± 2.7 | 574.5 ± 7.9 | 11.2 ± 0.3 | 24.9 ± 1.2 |
| Mn | 19.1 ± 0.75 a | 190.3 ± 3.1 a | 3.6 ± 0.2 a | 89.6 ± 2.5 a |
| Mn + Sesamol | 31.8 ± 0.9 abc | 373 ± 12.7 abc | 7.3 ± 0.2 ab | 52.8 ± 0.8 abc |
| Mn + Thymol | 45.1± 0.9 abcd | 372.7 ± 2.5 abc | 6.1 ± 0.3 abc | 55.9 ± 2.2 abc |
| Mn + CoQ10 | 25.6 ± 1.1 ace | 359 ± 5.7 abc | 5.7 ± 0.2 abcd | 59.1 ± 1.1 abc |
| Mn + WG | 35.2 ± 2.1 abcef | 359 ± 5.7 abc | 5.5 ± 0.2 abcd | 59.08 ± 1.0 abc |
| Mn + COMB | 62.0 ± 1.1 ab | 453.9 ± 14.1 ab | 7.9 ± 0.2 ab | 36.5 ± 1.3 ab |
| Groups | BDNF | Glutamate | GABA | COX2 |
|---|---|---|---|---|
| (U/g Tissue) | (ng/g Tissue) | (ng/g Tissue) | (ng/g Tissue) | |
| Control | 156.9 ± 2.6 | 1.1 ± 0.06 | 46.8 ± 0.8 | 11.2 ± 44 |
| Mn | 59.3 ± 2.3 a | 8.4 ± 0.6 a | 11.2 ± 0.4 a | 46.77 ± 0.85 a |
| Mn + Sesamol | 117.8 ± 1.4 abc | 4.1 ± 0.2 ab | 18.2 ± 0.6 abc | 20.5 ± 0.48 abc |
| Mn + Thymol | 119.1 ± 0.38 abc | 4.8 ± 0.1 ab | 21.7 ± 0.6 abcd | 21.7 ± 0.56 abc |
| Mn + CoQ10 | 119.0 ± 1.4 abc | 5.2 ± 0.3 ab | 17.4 ± 0.6 abce | 21.8 ± 69 abc |
| Mn + WG | 101.0 ± 3.2 abcdef | 5.2 ± 0.3 ab | 17.4 ± 0.6 abce | 22.1 ± 0.50 abc |
| Mn + COMB | 135.3 ± 1.8 ab | 3.9 ± 0.1 ab | 29.9 ± 0.7 ab | 29.9 ± 0.47 ab |
| Groups | TAC | SOD | MDA | iNOS |
|---|---|---|---|---|
| (µmol/g Tissue) | (U/g Tissue) | (nmol/g Tissue) | (U/mg Protein) | |
| Control | 46.8± 0.8 | 7.1 ± 0.5 | 8.7 ± 0.6 | 1.6 ± 0.07 |
| Mn | 8.9 ± 0.3 a | 0.4 ± 0.01 a | 115 ± 0.7 a | 43.8 ± 0.9 a |
| Mn + Sesamol | 26.9 ± 0.8 abc | 3.5 ± 0.2 abc | 63.8 ± 3.6 abc | 14.1 ± 0.6 abc |
| Mn + Thymol | 21.7 ± 0.6 abcd | 3.3 ± 0.07 abc | 56.1 ± 1.5 ab | 14.6 ± 0.5 abc |
| Mn + CoQ10 | 22.7 ± 1.8 abc | 2.8 ± 0.08 abc | 81.3 ± 1.4 abcde | 18.3 ± 0.6 abcde |
| Mn + WG | 18.2 ± 0.6 abcd | 2.9 ± 0.1 abc | 79.6 ± 2.3 abcde | 21.2 ± 0.7 abcdef |
| Mn + COMB | 33.2 ± 1.8 ab | 4.9 ± 0.06 ab | 48.3 ± 2.7 ab | 7.2 ± 0.7 ab |
| Group | Dose Regimen | Reference |
|---|---|---|
| I (Control) | Received saline with 1% tween 80 for 5 weeks and served as the vehicle-treated normal control. | |
| II (Mn) | Injected with MnCl2 (10 mg/kg/day, i.p.) for 5 weeks. | [32] |
| III (Mn + Sesamol) | Treated with sesamol (15 mg/kg/day, p.o.) 1 h before Mn (10 mg/kg/day, i.p.) for 5 weeks. | [34] |
| IV (Mn + Thymol) | Treated with thymol (30 mg/kg/day, p.o.) 1 h before Mn (10 mg/kg/day, i.p.) for 5 weeks. | [67] |
| V (Mn + CoQ10) | Treated with CoQ10 (200 mg/kg/day, p.o.) 1 h before Mn (10 mg/kg/day, i.p.) for 5 weeks. | [35] |
| VI (Mn + WG) | Treated with WG (100 mg/kg/day, p.o.) 1 h before Mn (10 mg/kg/day, i.p.) for 5 weeks. | [68] |
| VII (Mn + COMB) | Treated with sesamol (15 mg/kg), thymol (30 mg/kg), CoQ10 (200 mg/kg), and WG (100 mg/kg) as the same aforementioned doses, 1 h before Mn (10 mg/kg/day, i.p.) for 5 weeks. |
| Target | Primer Sequence | Accession Numbers | Reference |
|---|---|---|---|
| Bcl-2 | F: 5′-GGATGACTTCTCTCGTCGCTAC-3′ | NM_016993 | [81] |
| R: 5′-TGACATCTCCCTGTTGACGCT-3′ | |||
| Bax | F: 5′-CACGTCTGCGGGGAGTCA-3′ | NM_017059 | [60] |
| R: 5′-TAGGAAAGGAGGCCATCCCA-3′ | |||
| AIF | F: AGTCCTTATTGTGGGCTTATCAAC | NM_031356 | [82] |
| R:TTGGTCTTCTTTAATAGTCTTGTAGGC | |||
| GFAP | F: ACAGACTTTCTCCAACCTCCAG | NM_017009 | [82] |
| R: CCTTCTGACACGGATTTGGT | |||
| GSK-3β | F: 5′-AGCCTATATCCATTCCTTGG-3′ | NM_032080 | [41] |
| R: 5′-CCTCGGACCAGCTGCTTT-3′ | |||
| GAPDH | F: 5’-GGGCAGCCCAGAACATCA-3’ | NM_017008 | [81] |
| R: 5’-TGACCTTG CCCACAGCCT-3’ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu-Elfotuh, K.; Hamdan, A.M.E.; Mohammed, A.A.; Atwa, A.M.; Kozman, M.R.; Ibrahim, A.M.; Motawea, S.M.; Selim, H.M.R.M.; Tohamy, S.T.K.; Nour El-Din, M.; et al. Neuroprotective Effects of Some Nutraceuticals against Manganese-Induced Parkinson’s Disease in Rats: Possible Modulatory Effects on TLR4/NLRP3/NF-κB, GSK-3β, Nrf2/HO-1, and Apoptotic Pathways. Pharmaceuticals 2022, 15, 1554. https://doi.org/10.3390/ph15121554
Abu-Elfotuh K, Hamdan AME, Mohammed AA, Atwa AM, Kozman MR, Ibrahim AM, Motawea SM, Selim HMRM, Tohamy STK, Nour El-Din M, et al. Neuroprotective Effects of Some Nutraceuticals against Manganese-Induced Parkinson’s Disease in Rats: Possible Modulatory Effects on TLR4/NLRP3/NF-κB, GSK-3β, Nrf2/HO-1, and Apoptotic Pathways. Pharmaceuticals. 2022; 15(12):1554. https://doi.org/10.3390/ph15121554
Chicago/Turabian StyleAbu-Elfotuh, Karema, Ahmed Mohsen Elsaid Hamdan, Asmaa A. Mohammed, Ahmed M. Atwa, Magy R. Kozman, Amany M. Ibrahim, Shaimaa M. Motawea, Heba Mohammed Refat M. Selim, Sally Tohamy Kamal Tohamy, Mahmoud Nour El-Din, and et al. 2022. "Neuroprotective Effects of Some Nutraceuticals against Manganese-Induced Parkinson’s Disease in Rats: Possible Modulatory Effects on TLR4/NLRP3/NF-κB, GSK-3β, Nrf2/HO-1, and Apoptotic Pathways" Pharmaceuticals 15, no. 12: 1554. https://doi.org/10.3390/ph15121554







