Adrenomedullin Improves Cardiac Remodeling and Function in Obese Rats with Hypertension
Abstract
:1. Introduction
2. Results
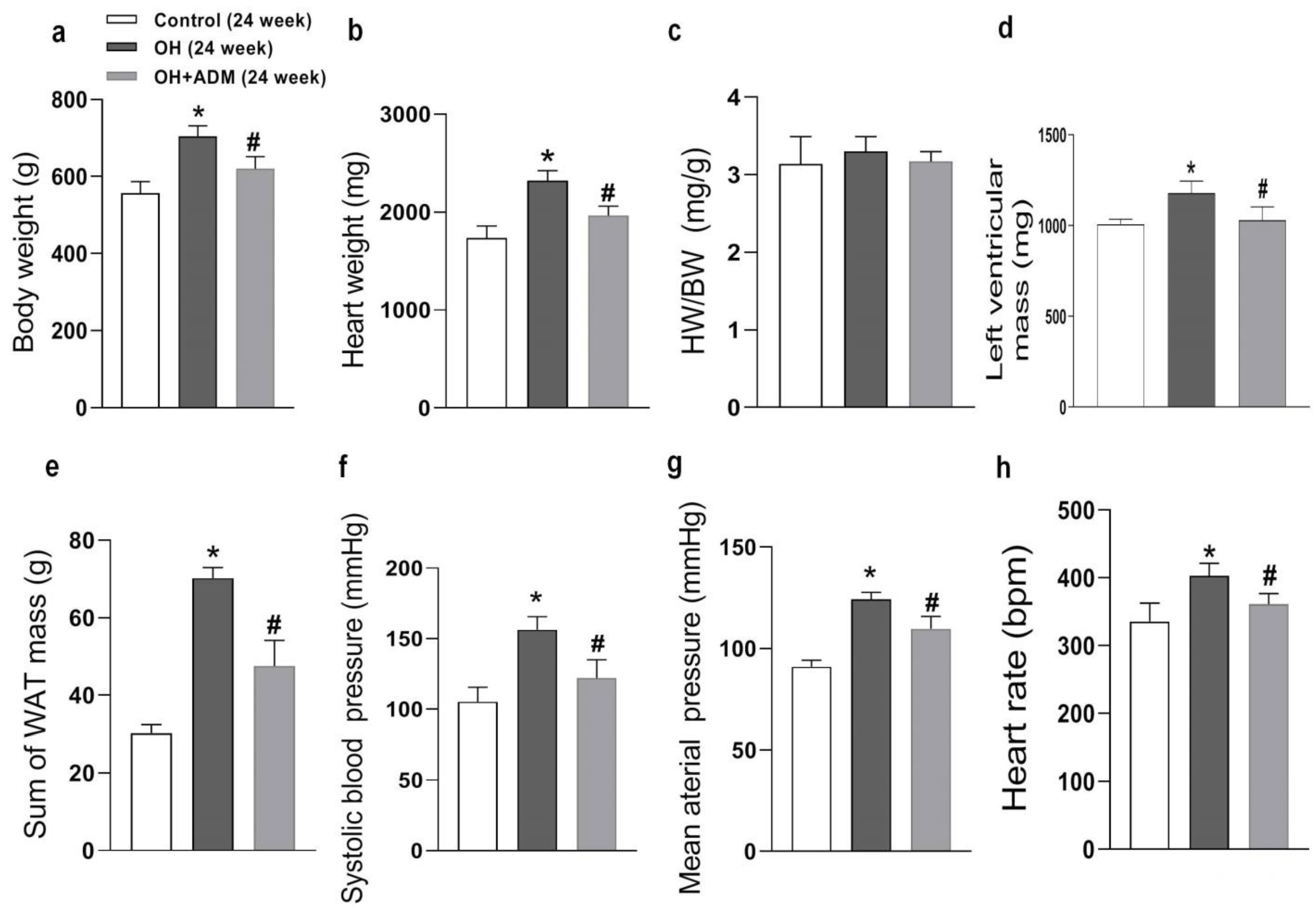
2.1. The Roles of ADM in Body Weight, Hypertension, and Cardiac Parameters in Rats Fed by HFD
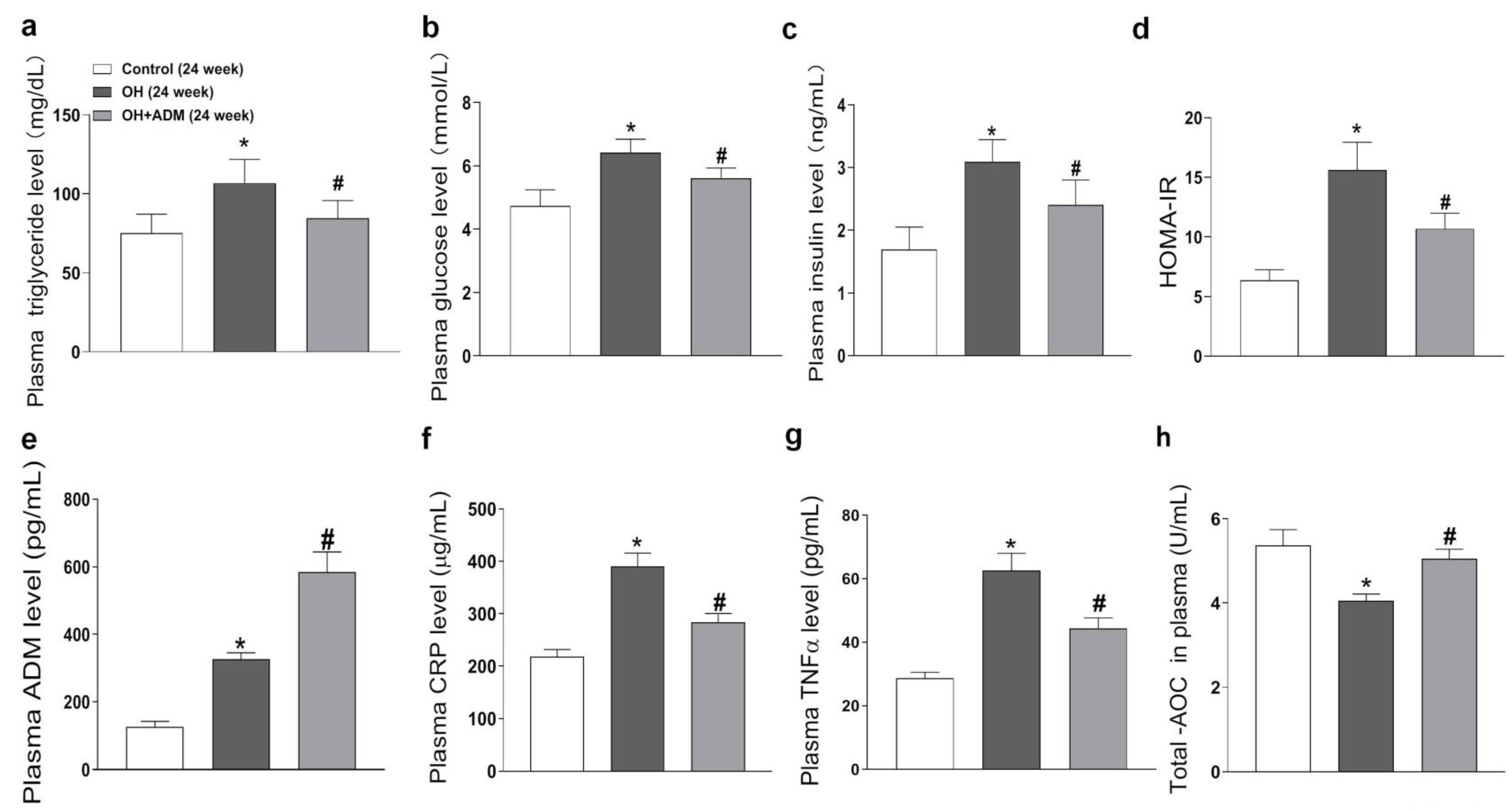
2.2. Plasma Level of ADM and the Effects of ADM on Metabolic Parameters, Plasma CRP, TNF-α, and T-AOC
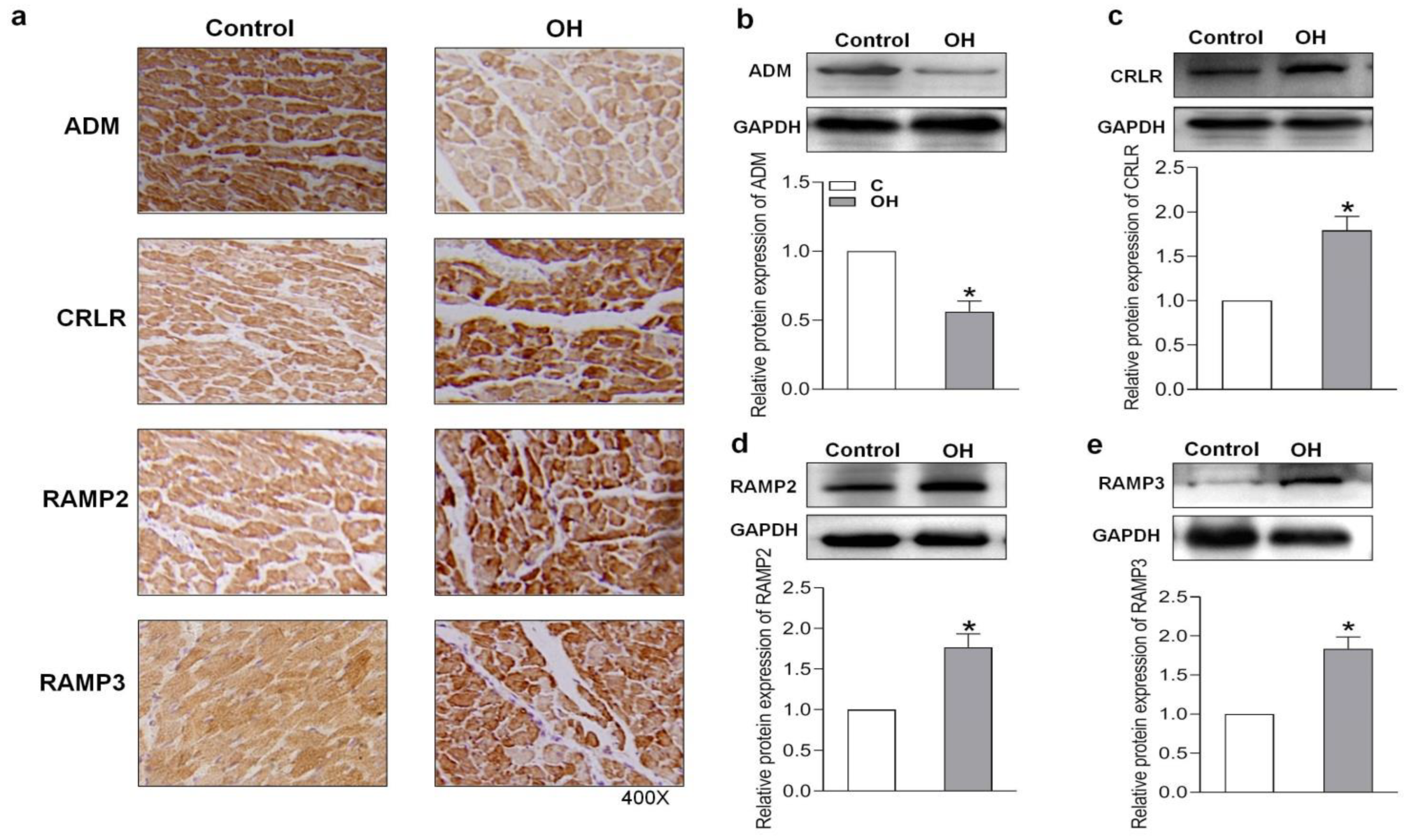
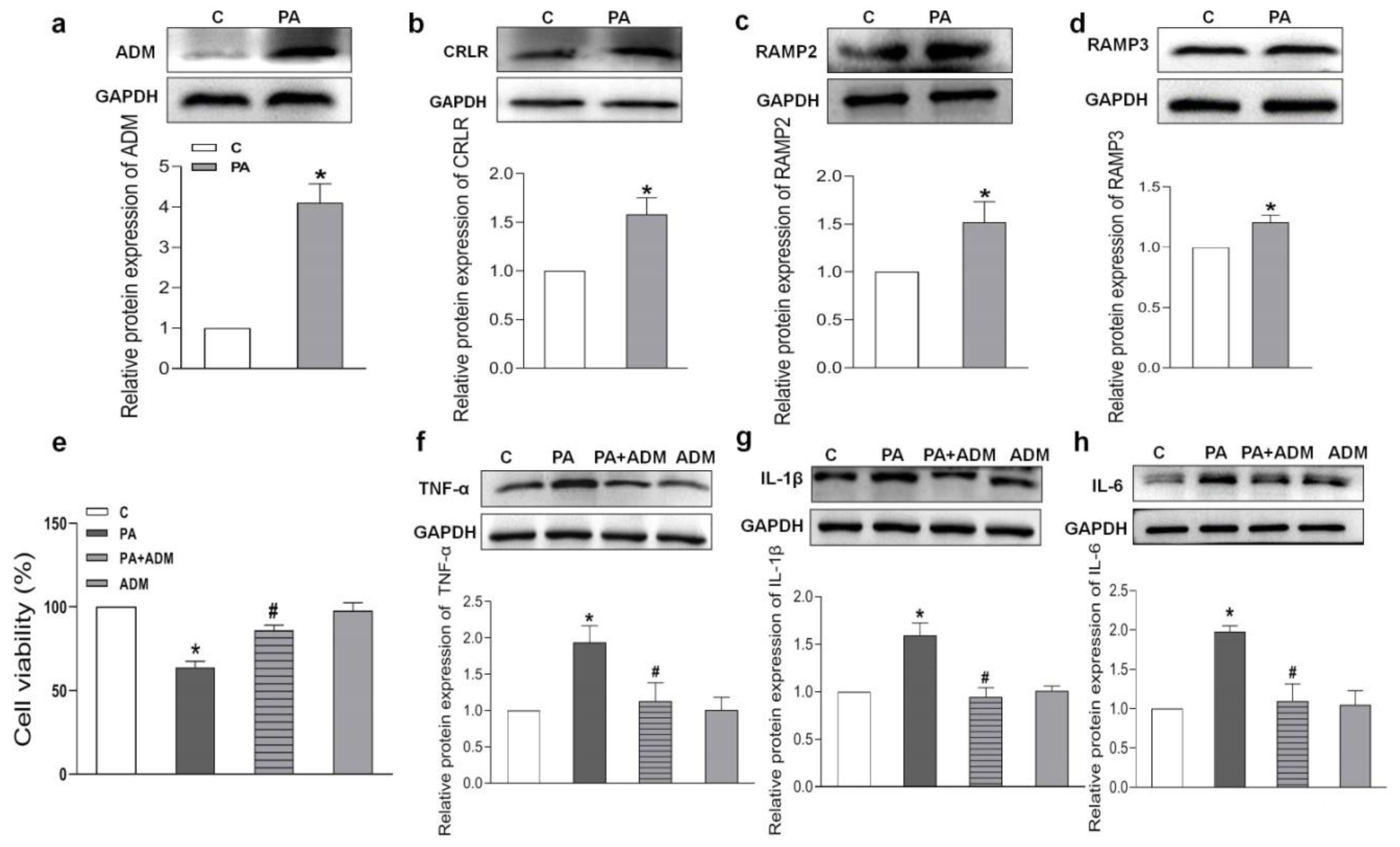
2.3. Endogenous Expression of ADM and Its Receptor System in Hearts of HFD-Induced OH Rats
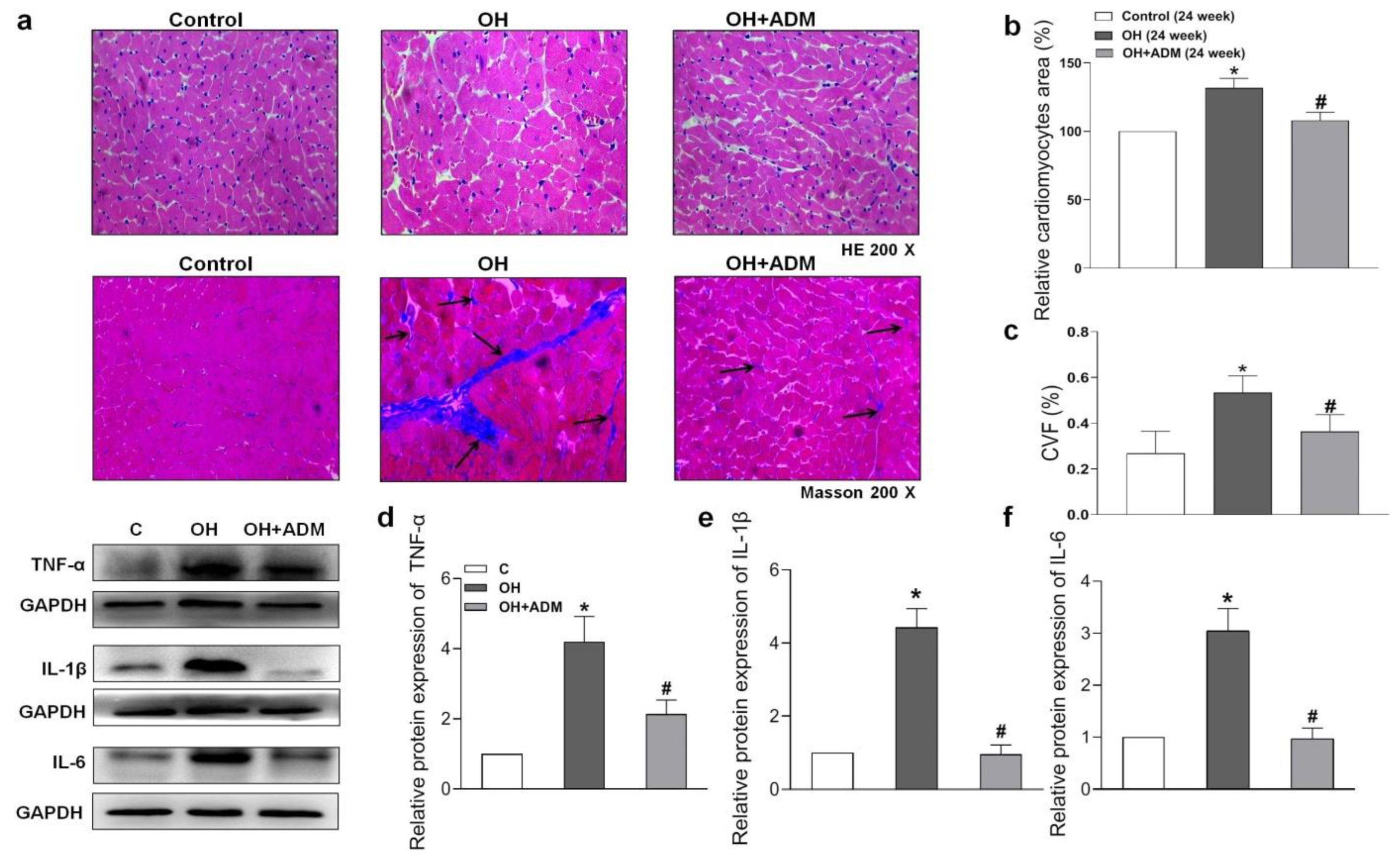
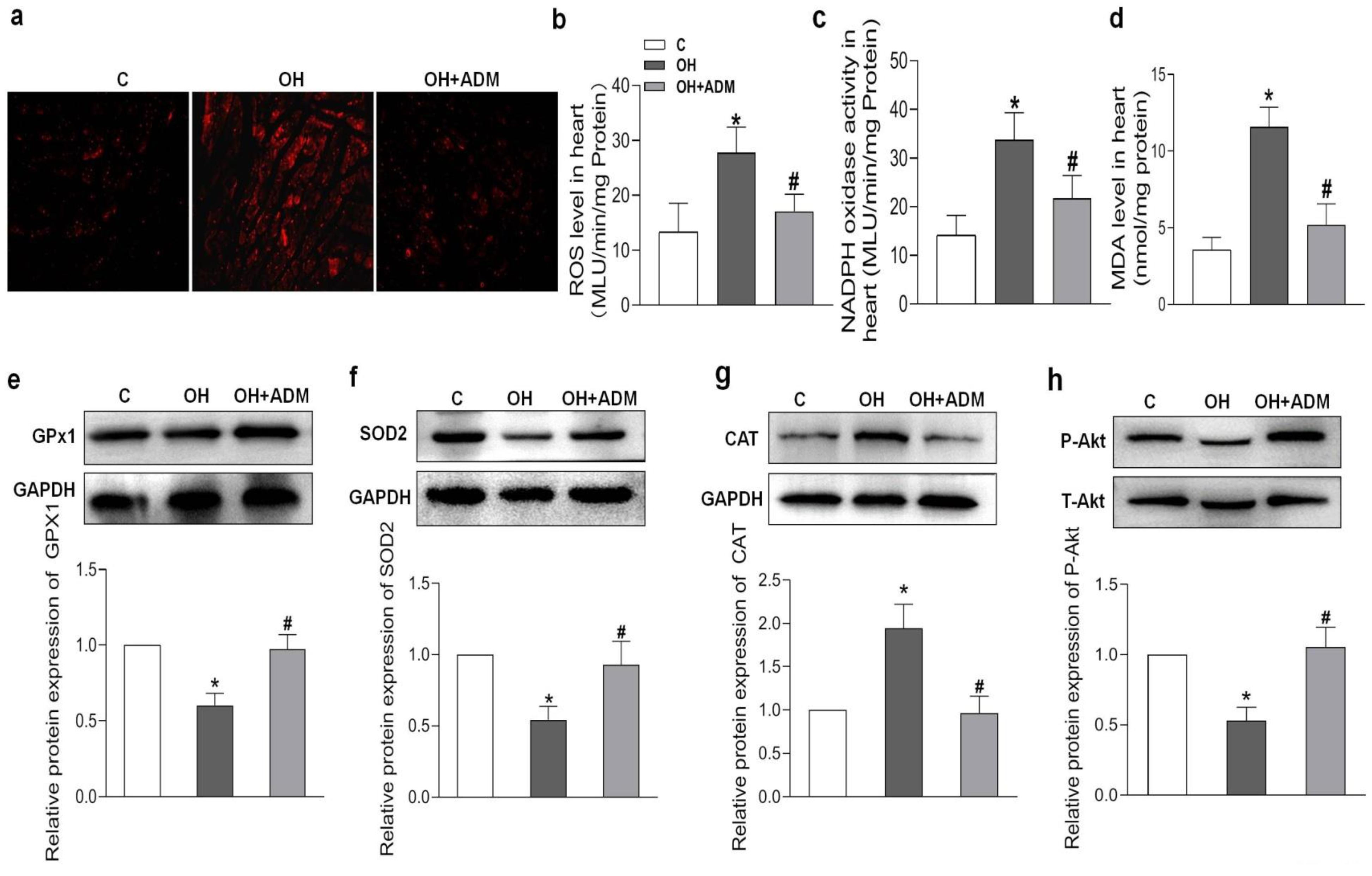
2.4. Effects of ADM on Cardiac Remodeling, Inflammation and Oxidative Stress in HFD-Treated OH Rats
2.5. Endogenous Expression of ADM and Its Receptor System in PA-Treated H9c2 Cells and the Effects of Exogenous ADM Pretreatment on PA-Induced Inflammation in the H9c2 Cell Line
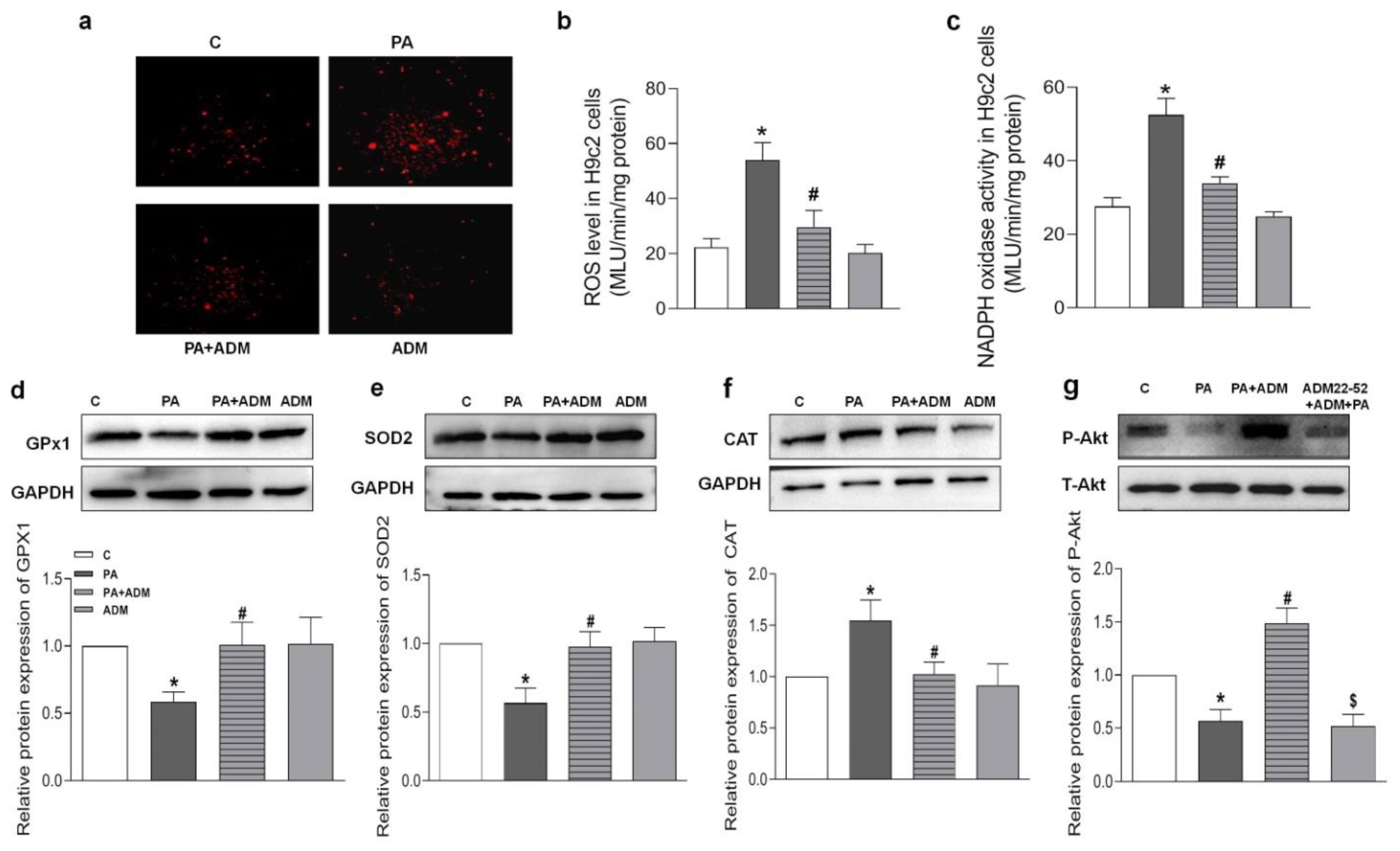
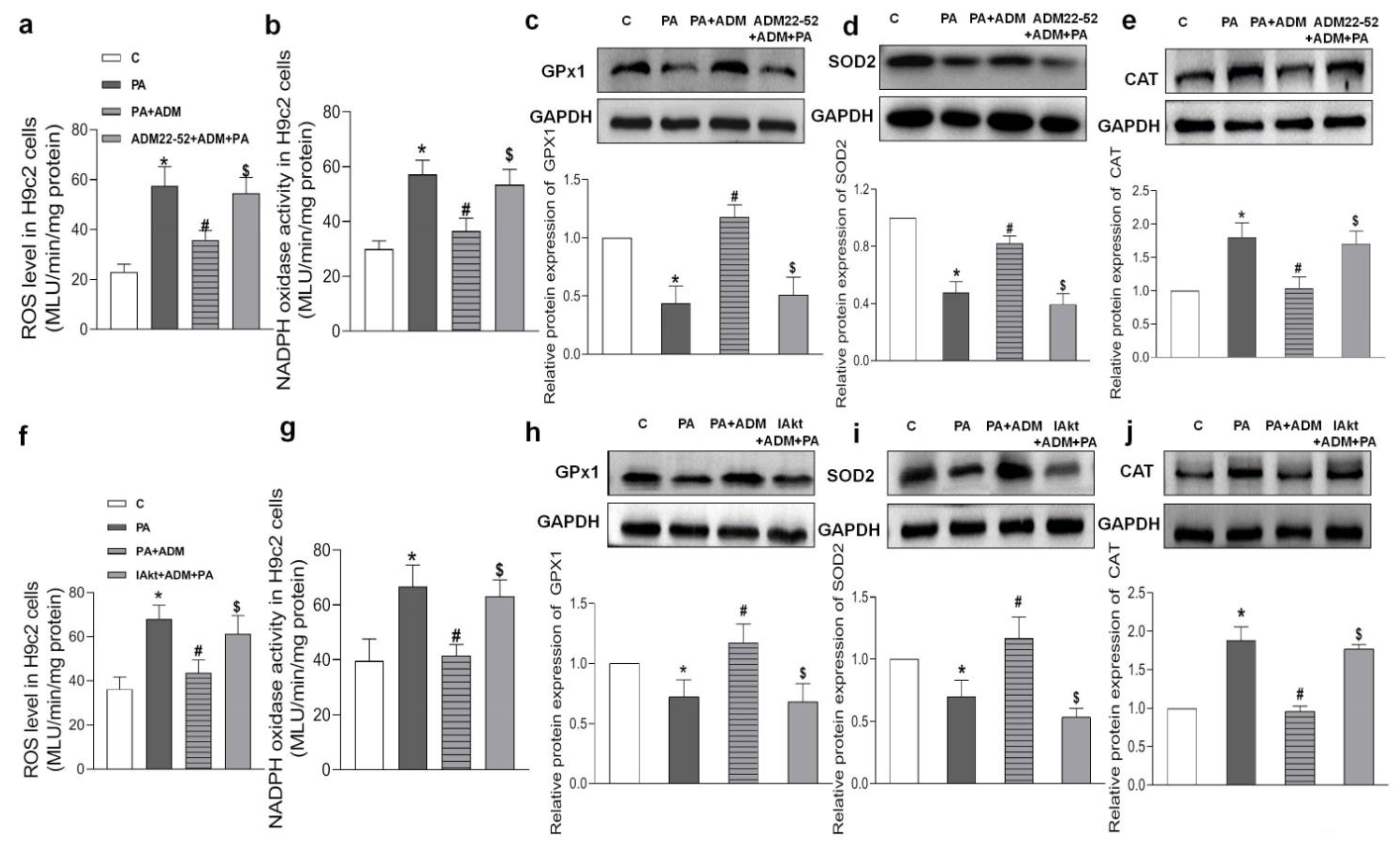
2.6. Effects of ADM Pretreatment on Oxidative Stress in PA-Treated H9c2 Cells
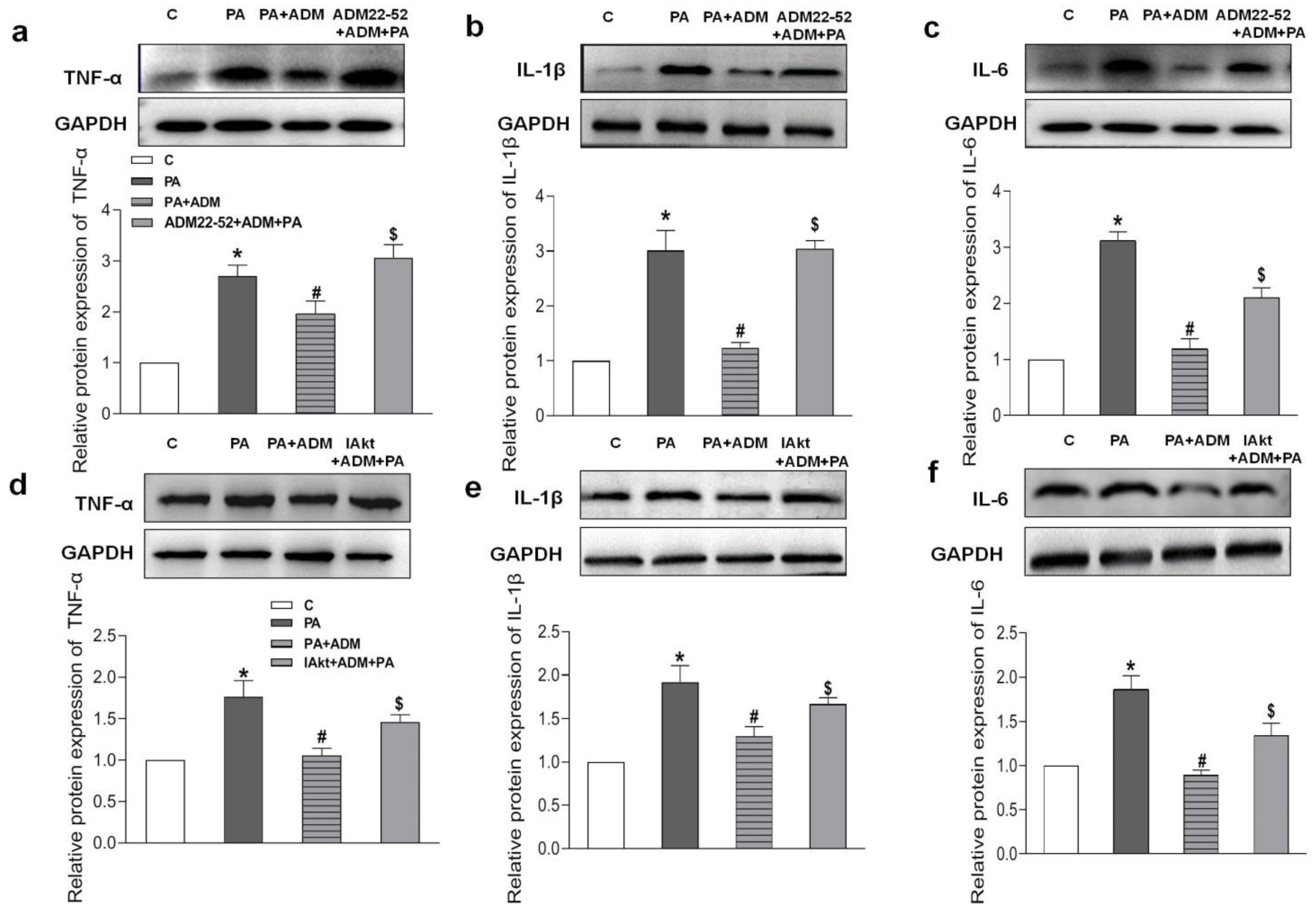
2.7. Roles of ADM22-52 (ADM Receptor Antagonist) and A6730 (Akt Activation Inhibitor) in ADM’s Responses to PA-Caused Inflammation and Oxidative Stress in H9c2 Cells
3. Discussion
4. Methods
4.1. Animals
4.2. Measurement of Heart and SBP
4.3. Echocardiographic Measurement
4.4. Cardiac Sample Preparation and H&E and Masson Staining
4.5. Enzyme-Linked Immunosorbent Assay (ELISA)
4.6. Cell Culture and Treatment
4.7. Cell Viability Assay
4.8. DHE Fluorescence Staining for ROS Level Assay
4.9. Analysis of Inflammation and Oxidative Stress Markers
4.10. Determination of ROS Level and NADPH Oxidase Activity
4.11. Immunohistochemistry
4.12. Western Blot Analysis
4.13. Reagents and Antibodies
4.14. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OH | obesity-related hypertension |
| TNF-α | tumor necrosis factor-α |
| IL-1β | interleukin-1β |
| IL-6 | interleukin-6 |
| ROS | reactive oxygen species |
| Akt | protein kinase B |
| ADM | adrenomedullin |
| CRLR | calcitonin receptor-like receptor |
| RAMP2 | receptor activity modifying protein 2 |
| RAMP3 | receptor activity modifying protein 3 |
| HFD | high-fat diet |
| BP | blood pressure |
| LVEF | Left ventricular ejection fraction |
| LVFS | fractional shortening |
| IVVs | left ventricular mass, left ventricular volume at end systole |
| LVIDs | left ventricular internal dimensions at end systole |
| LVPWd | left ventricular posterior wall dimensions at end diastole |
| IVSd | interventricular septal thickness at the end of diastole |
| SBP | systolic blood pressue |
| BW | body weight |
| HW | heart weight |
| WAT | white adipose tissue |
| HW/BW | the ratio between the heart weight and body weight |
| CRP | C-reactionprotein |
| T-AOC | total antioxidant capacity |
| HOMA-IR | homeostasis model assessment of insulin resistance |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| MDA | malondialdehyde. |
| GPx1 | glutathione peroxidase |
| SOD2 | superoxide dismutase 2 |
| CAT | catalase |
| CVF | collagen volume fraction |
| PA | palmitate |
References
- Sarma, S.; Sockalingam, S.; Dash, S. Obesity as a multisystem disease: Trends in obesity rates and obesity-related complications. Diabetes Obes. Metab. 2021, 23 (Suppl. S1), 3–16. [Google Scholar] [CrossRef] [PubMed]
- Seravalle, G.; Grassi, G. Obesity and hypertension. Pharm. Res. 2017, 122, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chrysant, S.G. Pathophysiology and treatment of obesity-related hypertension. J. Clin. Hypertens. 2019, 21, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef]
- Oparil, S.; Acelajado, M.C.; Bakris, G.L.; Berlowitz, D.R.; Cifkova, R.; Dominiczak, A.F.; Grassi, G.; Jordan, J.; Poulter, N.R.; Rodgers, A.; et al. Hypertension. Nat. Rev. Dis. Prim. 2018, 4, 18014. [Google Scholar] [CrossRef] [Green Version]
- Samak, M.; Fatullayev, J.; Sabashnikov, A.; Zeriouh, M.; Schmack, B.; Farag, M.; Popov, A.-F.; Dohmen, P.M.; Choi, Y.-H.; Wahlers, T.; et al. Cardiac Hypertrophy: An Introduction to Molecular and Cellular Basis. Med. Sci. Monit. Basic Res. 2016, 22, 75–79. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Rawish, E.; Nording, H.M.; Langer, H.F. Inflammation in Metabolic and Cardiovascular Disorders—Role of Oxidative Stress. Life 2021, 11, 672. [Google Scholar] [CrossRef]
- Carbone, S.; Lavie, C.J.; Elagizi, A.; Arena, R.; Ventura, H.O. The Impact of Obesity in Heart Failure. Heart Fail. Clin. 2020, 16, 71–80. [Google Scholar] [CrossRef]
- Kitamura, K.; Kangawa, K.; Kawamoto, M.; Ichiki, Y.; Nakamura, S.; Matsuo, H.; Eto, T. Adrenomedullin: A novel hypotensive peptide isolated from human pheochromocytoma. Biochem. Biophys. Res. Commun. 1993, 192, 553–560. [Google Scholar] [CrossRef]
- Hay, D.L.; Pioszak, A.A. Receptor Activity-Modifying Proteins (RAMPs): New Insights and Roles. Annu. Rev. Pharm. Toxicol. 2016, 56, 469–487. [Google Scholar] [CrossRef] [Green Version]
- Schonauer, R.; Els-Heindl, S.; Beck-Sickinger, A.G. Adrenomedullin—New perspectives of a potent peptide hormone. J. Pept. Sci. 2017, 23, 472–485. [Google Scholar] [CrossRef] [PubMed]
- Autelitano, D.J.; Ridings, R. Adrenomedullin signalling in cardiomyocytes is dependent upon CRLR and RAMP2 expression. Peptides 2001, 22, 1851–1857. [Google Scholar] [CrossRef]
- Kamitani, S.; Asakawa, M.; Shimekake, Y.; Kuwasako, K.; Nakahara, K.; Sakata, T. The RAMP2/CRLR complex is a functional adrenomedullin receptor in human endothelial and vascular smooth muscle cells. FEBS Lett. 1999, 448, 111–114. [Google Scholar] [CrossRef] [Green Version]
- Owji, A.A.; Smith, D.M.; Coppock, H.A.; Morgan, D.G.; Bhogal, R.; Ghatei, M.A.; Bloom, S.R. An abundant and specific binding site for the novel vasodilator adrenomedullin in the rat. Endocrinology 1995, 136, 2127–2134. [Google Scholar] [CrossRef]
- Tsuruda, T.; Kato, J.; Kuwasako, K.; Kitamura, K. Adrenomedullin: Continuing to explore cardioprotection. Peptides 2019, 111, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Niu, P.; Shindo, T.; Iwata, H.; Ebihara, A.; Suematsu, Y.; Zhang, Y.; Takeda, N.; Iimuro, S.; Hirata, Y.; Nagai, R. Accelerated cardiac hypertrophy and renal damage induced by angiotensin II in adrenomedullin knockout mice. Hypertens. Res. 2003, 26, 731–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshizawa, T.; Takizawa, S.; Shimada, S.; Tokudome, T.; Shindo, T.; Matsumoto, K. Effects of Adrenomedullin on Doxorubicin-Induced Cardiac Damage in Mice. Biol. Pharm. Bull. 2016, 39, 737–746. [Google Scholar] [CrossRef] [Green Version]
- Li, L.L.; Peng, C.; Zhang, M.; Liu, Y.; Li, H.; Chen, H.; Sun, Y.; Zhu, C.; Zhang, Y. Mesenchymal stem cells overexpressing adrenomedullin improve heart function through antifibrotic action in rats experiencing heart failure. Mol. Med. Rep. 2018, 17, 1437–1444. [Google Scholar] [CrossRef] [Green Version]
- Geven, C.; van Lier, D.; Blet, A.; Peelen, R.; Elzen, B.T.; Mebazaa, A.; Kox, M.; Pickkers, P. Safety, tolerability and pharmacokinetics/pharmacodynamics of the adrenomedullin antibody adrecizumab in a first-in-human study and during experimental human endotoxaemia in healthy subjects. Br. J. Clin. Pharmacol. 2018, 84, 2129–2141. [Google Scholar] [CrossRef]
- Dai, H.B.; Wang, H.Y.; Wang, F.Z.; Qian, P.; Gao, Q.; Zhou, H.; Zhou, Y.-B. Adrenomedullin ameliorates palmitic acid-induced insulin resistance through PI3K/Akt pathway in adipocytes. Acta Diabetol. 2022, 59, 661–673. [Google Scholar] [CrossRef]
- Hu, W.; Shi, L.; Li, M.Y.; Zhou, P.H.; Qiu, B.; Yin, K.; Zhang, H.-H.; Gao, Y.; Kang, R.; Qin, S.-L.; et al. Adrenomedullin protects Leydig cells against lipopolysaccharide-induced oxidative stress and inflammatory reaction via MAPK/NF-kappaB signalling pathways. Sci. Rep. 2017, 7, 16479. [Google Scholar] [CrossRef] [PubMed]
- Kirisci, M.; Gunes, H.; Kocarslan, A.; Metin, T.O.; Aykan, D.A.; Seyithanoglu, M.; Doganer, A.; Bayrak, G.; Aksu, E. Protective Effects of Adrenomedullin on Rat Cerebral Tissue After Transient Bilateral Common Carotid Artery Occlusion and Reperfusion. Braz. J. Cardiovasc. Surg. 2020, 35, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Xian, X.; Sakurai, T.; Kamiyoshi, A.; Ichikawa-Shindo, Y.; Tanaka, M.; Koyama, T.; Kawate, H.; Yang, L.; Liu, T.; Imai, A.; et al. Vasoprotective Activities of the Adrenomedullin-RAMP2 System in Endothelial Cells. Endocrinology 2017, 158, 1359–1372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kresoja, K.P.; Rommel, K.P.; Wachter, R.; Henger, S.; Besler, C.; Kloting, N.; Schnelle, M.; Hoffmann, A.; Büttner, P.; Ceglarek, U.; et al. Proteomics to improve phenotyping in obese patients with heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2021, 23, 1633–1644. [Google Scholar] [CrossRef]
- Metwalley, K.A.; Farghaly, H.S.; Sherief, T. Plasma adrenomedullin level in children with obesity: Relationship to left ventricular function. World J. Pediatr. 2018, 14, 84–91. [Google Scholar] [CrossRef]
- Suthahar, N.; Meems, L.M.G.; Groothof, D.; Bakker, S.J.L.; Gansevoort, R.T.; van Veldhuisen, D.J.; de Boer, R.A. Relationship between body mass index, cardiovascular biomarkers and incident heart failure. Eur. J. Heart Fail. 2021, 23, 396–402. [Google Scholar] [CrossRef]
- Gaborit, F.S.; Kistorp, C.; Kümler, T.; Hassager, C.; Tønder, N.; Iversen, K.; Kamstrup, P.R.; Faber, J.; Køber, L.; Schou, M. Early Stages of Obesity-related Heart Failure Are Associated with Natriuretic Peptide Deficiency and an Overall Lack of Neurohormonal Activation: The Copenhagen Heart Failure Risk Study. Glob. Heart 2020, 15, 25. [Google Scholar] [CrossRef]
- Letizia, C.; Iacobellis, G.; Caliumi, C.; Leonetti, F.; Cotesta, D.; Ribaudo, M.C.; Petramala, L.; Cianci, R.; Celi, M.; D’Erasmo, E.; et al. Acute hyperinsulinemia is associated with increased plasma adrenomedullin concentrations in uncomplicated obesity. Exp. Clin. Endocrinol. Diabetes 2005, 113, 171–175. [Google Scholar] [CrossRef]
- Dai, H.B.; Wang, F.Z.; Kang, Y.; Sun, J.; Zhou, H.; Gao, Q.; Li, Z.; Qian, P.; Zhu, G.; Zhou, Y. Adrenomedullin Attenuates Inflammation in White Adipose Tissue of Obese Rats Through Receptor-Mediated PKA Pathway. Obesity 2021, 29, 86–97. [Google Scholar] [CrossRef]
- Calcaterra, V.; Regalbuto, C.; Porri, D.; Pelizzo, G.; Mazzon, E.; Vinci, F.; Zuccotti, G.; Fabiano, V.; Cena, H. Inflammation in Obesity-Related Complications in Children: The Protective Effect of Diet and Its Potential Role as a Therapeutic Agent. Biomolecules 2020, 10, 1324. [Google Scholar] [CrossRef]
- Wang, L.F.; Huang, C.C.; Xiao, Y.F.; Guan, X.H.; Wang, X.N.; Cao, Q.; Liu, Y.; Huang, X.; Deng, L.-B.; Deng, K.-Y.; et al. CD38 Deficiency Protects Heart from High Fat Diet-Induced Oxidative Stress Via Activating Sirt3/FOXO3 Pathway. Cell. Physiol. Biochem. 2018, 48, 2350–2363. [Google Scholar] [CrossRef]
- Geng, Z.; Fan, W.Y.; Zhou, B.; Ye, C.; Tong, Y.; Zhou, Y.B.; Xiong, X.-Q. FNDC5 attenuates obesity-induced cardiac hypertrophy by inactivating JAK2/STAT3-associated inflammation and oxidative stress. J. Transl. Med. 2019, 17, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez, G.; Araneda, F.; Pena, J.P.; Finkelstein, J.P.; Riquelme, J.A.; Montecinos, L.; Barrientos, G.; Llanos, P.; Pedrozo, Z.; Said, M.; et al. High-Fat-Diet-Induced Obesity Produces Spontaneous Ventricular Arrhythmias and Increases the Activity of Ryanodine Receptors in Mice. Int. J. Mol. Sci. 2018, 19, 533. [Google Scholar] [CrossRef] [Green Version]
- Farhangi, M.A.; Nameni, G.; Hajiluian, G.; Mesgari-Abbasi, M. Cardiac tissue oxidative stress and inflammation after vitamin D administrations in high fat- diet induced obese rats. BMC Cardiovasc. Disord. 2017, 17, 161. [Google Scholar] [CrossRef] [Green Version]
- Iring, A.; Jin, Y.J.; Albarran-Juarez, J.; Siragusa, M.; Wang, S.; Dancs, P.T.; Nakayama, A.; Tonack, S.; Chen, M.; Künne, C.; et al. Shear stress-induced endothelial adrenomedullin signaling regulates vascular tone and blood pressure. J. Clin. Investig. 2019, 129, 2775–2791. [Google Scholar] [CrossRef] [Green Version]
- Si, H.; Zhang, Y.; Song, Y.; Li, L. Overexpression of adrenomedullin protects mesenchymal stem cells against hypoxia and serum deprivationinduced apoptosis via the Akt/GSK3beta and Bcl2 signaling pathways. Int. J. Mol. Med. 2018, 41, 3342–3352. [Google Scholar] [PubMed] [Green Version]
- Shindo, T.; Tanaka, M.; Kamiyoshi, A.; Ichikawa-Shindo, Y.; Kawate, H.; Yamauchi, A.; Sakurai, T. Regulation of cardiovascular development and homeostasis by the adrenomedullin-RAMP system. Peptides 2019, 111, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Silva Figueiredo, P.; Inada, A.C.; Marcelino, G.; Maiara Lopes Cardozo, C.; de Cassia Freitas, K.; de Cassia Avellaneda Guimaraes, R.; Pereira de Castro, A.; Aragão do Nascimento, V.; Aiko Hiane, P. Fatty Acids Consumption: The Role Metabolic Aspects Involved in Obesity and Its Associated Disorders. Nutrients 2017, 9, 1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotchen, T.A. Obesity-related hypertension: Epidemiology, pathophysiology, and clinical management. Am. J. Hypertens. 2010, 23, 1170–1178. [Google Scholar] [CrossRef]
- Shimizu, I.; Minamino, T. Physiological and pathological cardiac hypertrophy. J. Mol. Cell. Cardiol. 2016, 97, 245–262. [Google Scholar] [CrossRef]
- Dick, S.A.; Epelman, S. Chronic Heart Failure and Inflammation: What Do We Really Know? Circ. Res. 2016, 119, 159–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paulus, W.J.; Tschope, C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. Coll. Cardiol. 2013, 62, 263–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez-Sanchez, A.; Madrigal-Santillan, E.; Bautista, M.; Esquivel-Soto, J.; Morales-Gonzalez, A.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sánchez-Rivera, G.; Valadez-Vega, C.; Morales-González, J.A. Inflammation, oxidative stress, and obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maulik, S.K.; Kumar, S. Oxidative stress and cardiac hypertrophy: A review. Toxicol. Mech. Methods 2012, 22, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Chao, L.; Chao, J. Adrenomedullin protects against myocardial apoptosis after ischemia/reperfusion through activation of Akt-GSK signaling. Hypertension 2004, 43, 109–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, P.H.; Hu, W.; Zhang, X.B.; Wang, W.; Zhang, L.J. Protective Effect of Adrenomedullin on Rat Leydig Cells from Lipopolysaccharide-Induced Inflammation and Apoptosis via the PI3K/Akt Signaling Pathway ADM on Rat Leydig Cells from Inflammation and Apoptosis. Mediat. Inflamm. 2016, 2016, 7201549. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.Q.; Wang, L.X.; Yang, C.S.; Chen, S.F.; Xue, Y.Z.; Liu, Y.H. Effects of adrenomedullin on the cell numbers and apoptosis of endothelial progenitor cells. Clin. Investig. Med. 2008, 31, E117–E122. [Google Scholar] [CrossRef] [Green Version]
- Kim, W.; Moon, S.O.; Sung, M.J.; Kim, S.H.; Lee, S.; Kim, H.J.; Koh, G.Y.; Park, S.K. Protective effect of adrenomedullin in mannitol-induced apoptosis. Apoptosis 2002, 7, 527–536. [Google Scholar] [CrossRef]
- Dong, Y.; van der Walt, N.; Pennington, K.A.; Yallampalli, C. Impact of adrenomedullin blockage on lipid metabolism in female mice exposed to high-fat diet. Endocrine 2019, 65, 278–285. [Google Scholar] [CrossRef]
- Wu, N.; Zheng, F.; Li, N.; Han, Y.; Xiong, X.Q.; Wang, J.J.; Chen, Q.; Li, Y.-H.; Zhu, G.-Q.; Zhou, Y.-B. RND3 attenuates oxidative stress and vascular remodeling in spontaneously hypertensive rat via inhibiting ROCK1 signaling. Redox Biol. 2021, 48, 102204. [Google Scholar] [CrossRef]
- Wang, F.Z.; Wei, W.B.; Li, X.; Huo, J.Y.; Jiang, W.Y.; Wang, H.Y.; Qian, P.; Li, Z.-Z.; Zhou, Y.-B. The cardioprotective effect of the sodium-glucose cotransporter 2 inhibitor dapagliflozin in rats with isoproterenol-induced cardiomyopathy. Am. J. Transl. Res. 2021, 13, 10950–10961. [Google Scholar] [PubMed]
| Parameters | Control | OH | OH+ADM |
|---|---|---|---|
| LVVs (μL) | 107 ± 10.8 | 161 ± 12.3 * | 124 ± 12.5 # |
| LVIDs (mm) | 4.76 ± 0.54 | 5.80 ± 0.65 * | 5.09 ± 0.58 # |
| LVPWd (mm) | 1.92 ± 0.06 | 2.29 ± 0.09 * | 2.15 ± 0.16 |
| IVSd (mm) | 1.94 ± 0.08 | 2.26 ± 0.13 * | 2.12 ± 0.07 |
| LVEF (%) | 74.7 ± 3.59 | 59.1 ± 2.48 * | 69.1 ± 1.67 # |
| LVFS (%) | 44.1 ± 2.56 | 35.3 ± 2.09 * | 43.4 ± 1.35 # |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, P.; Wang, Q.; Wang, F.-Z.; Dai, H.-B.; Wang, H.-Y.; Gao, Q.; Zhou, H.; Zhou, Y.-B. Adrenomedullin Improves Cardiac Remodeling and Function in Obese Rats with Hypertension. Pharmaceuticals 2022, 15, 719. https://doi.org/10.3390/ph15060719
Qian P, Wang Q, Wang F-Z, Dai H-B, Wang H-Y, Gao Q, Zhou H, Zhou Y-B. Adrenomedullin Improves Cardiac Remodeling and Function in Obese Rats with Hypertension. Pharmaceuticals. 2022; 15(6):719. https://doi.org/10.3390/ph15060719
Chicago/Turabian StyleQian, Pei, Qian Wang, Fang-Zheng Wang, Hang-Bing Dai, Hong-Yu Wang, Qing Gao, Hong Zhou, and Ye-Bo Zhou. 2022. "Adrenomedullin Improves Cardiac Remodeling and Function in Obese Rats with Hypertension" Pharmaceuticals 15, no. 6: 719. https://doi.org/10.3390/ph15060719
APA StyleQian, P., Wang, Q., Wang, F.-Z., Dai, H.-B., Wang, H.-Y., Gao, Q., Zhou, H., & Zhou, Y.-B. (2022). Adrenomedullin Improves Cardiac Remodeling and Function in Obese Rats with Hypertension. Pharmaceuticals, 15(6), 719. https://doi.org/10.3390/ph15060719






