Physiologically Based Pharmacokinetic (PBPK) Modeling to Predict PET Image Quality of Three Generations EGFR TKI in Advanced-Stage NSCLC Patients
Abstract
:1. Introduction
2. Results
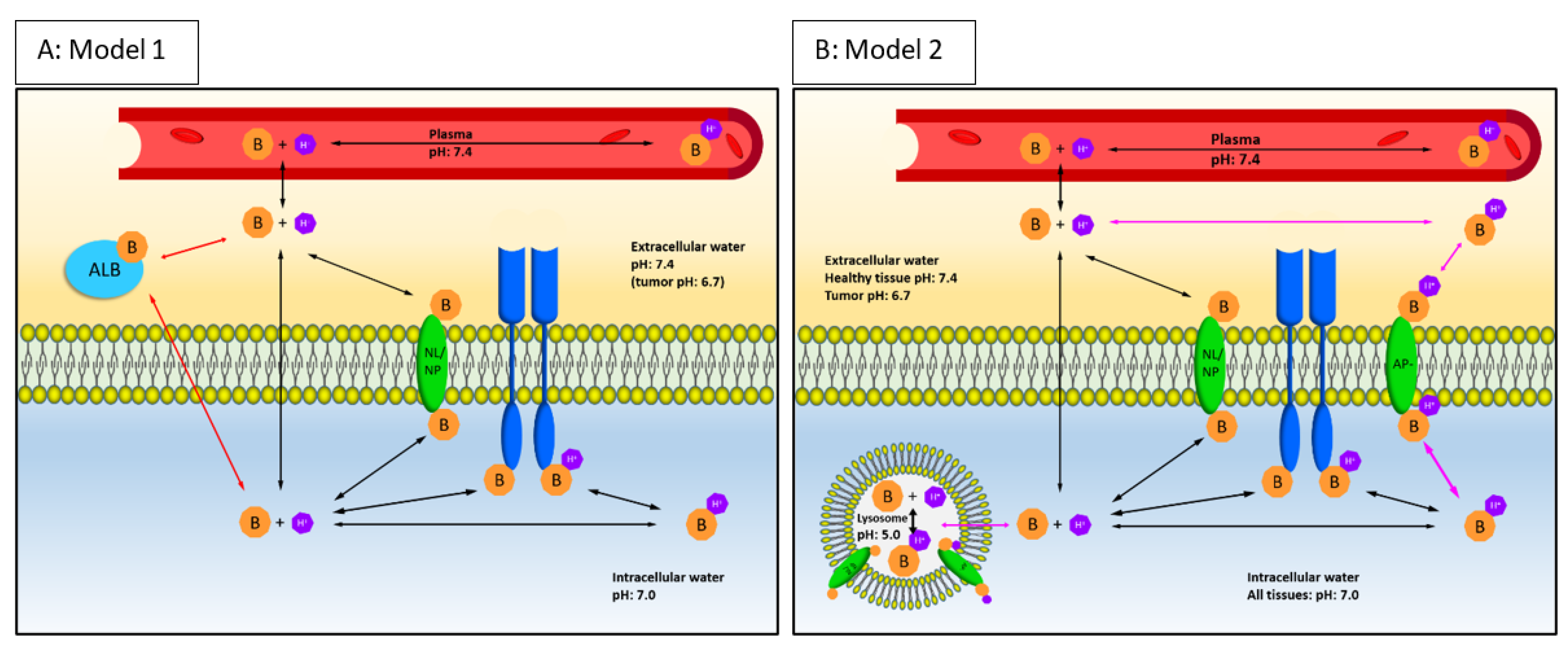
2.1. Components of the Mechanistical PBPK Model
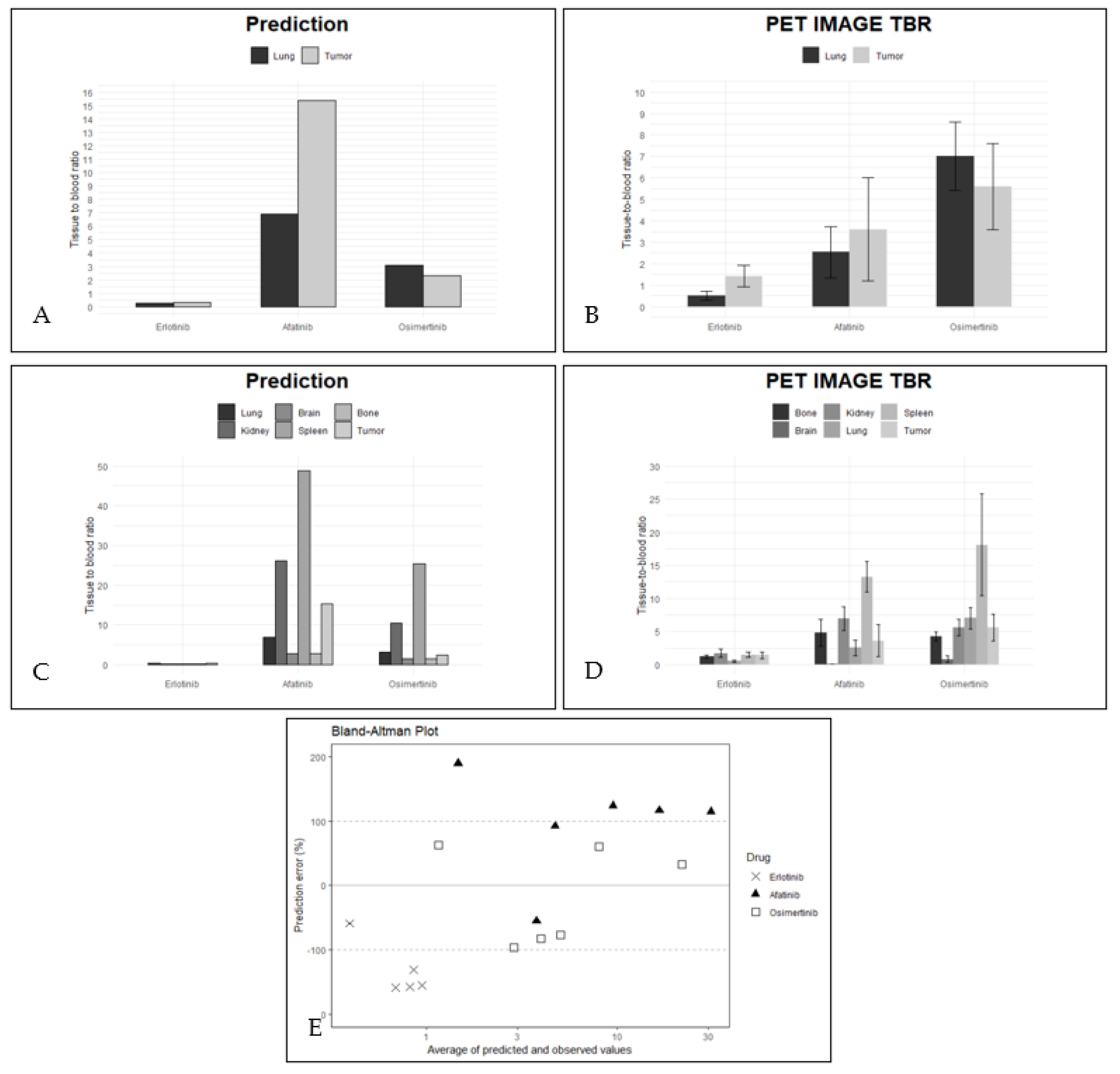
2.2. PBPK Model Validation Using PET Data
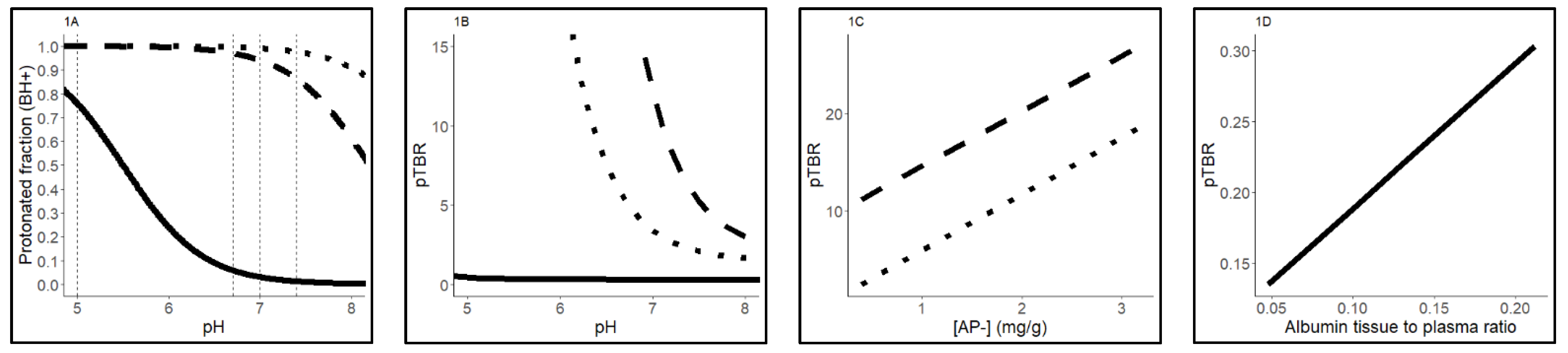
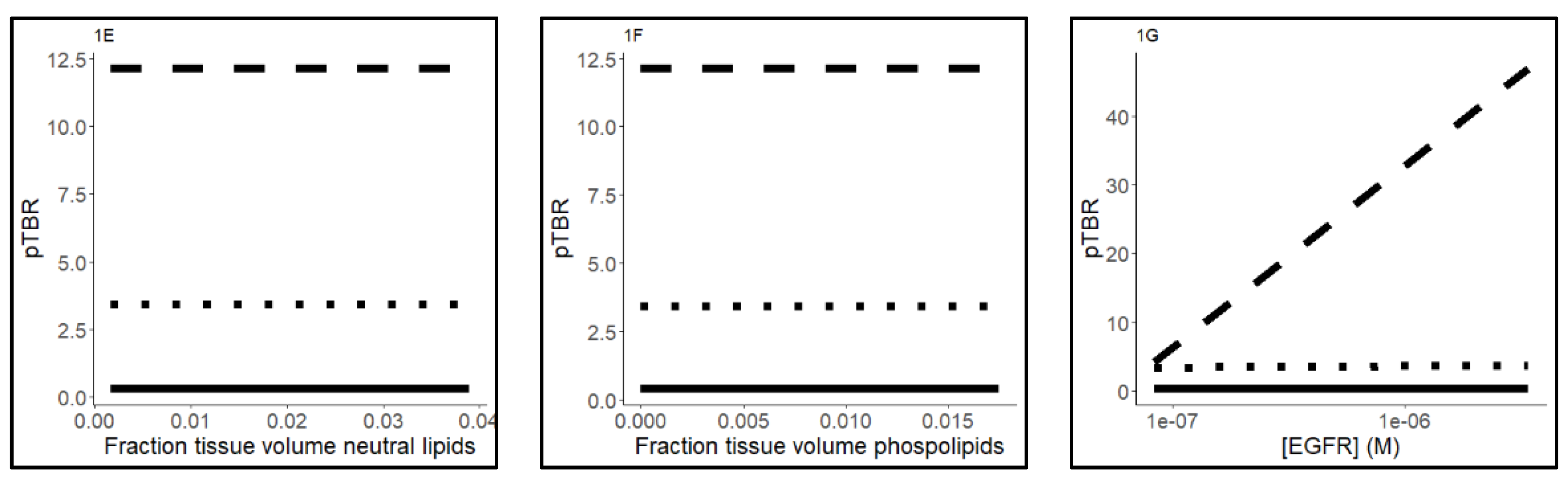
2.3. Sensitivity Analysis
3. Discussion
Future Perspective
4. Materials and Methods
4.1. Overview
4.2. Scan Data
4.3. PBPK Model: Base Model Selection
4.4. Base Models: Physicochemical Drug Distribution
4.5. Extension of the Physicochemical Base Models with EGFR Target Binding
4.6. Extension of the Physicochemical-EGFR Models with Lysosomal Sequestration (Mechanistical PBPK Model)
4.7. Including Hallmarks of NSCLC
4.8. Simulation of Tumor-to-Lung Contrast and Tissue Distribution
4.9. Software and Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Faehling, M.; Schwenk, B.; Kramberg, S.; Eckert, R.; Volckmar, A.L.; Stenzinger, A.; Strater, J. Oncogenic driver mutations, treatment, and EGFR-TKI resistance in a Caucasian population with non-small cell lung cancer: Survival in clinical practice. Oncotarget 2017, 8, 77897–77914. [Google Scholar] [CrossRef] [PubMed]
- Red Brewer, M.; Yun, C.H.; Lai, D.; Lemmon, M.A.; Eck, M.J.; Pao, W. Mechanism for activation of mutated epidermal growth factor receptors in lung cancer. Proc. Natl. Acad. Sci. USA 2013, 110, E3595–E3604. [Google Scholar] [CrossRef]
- Eck, M.J.; Yun, C.H. Structural and mechanistic underpinnings of the differential drug sensitivity of EGFR mutations in non-small cell lung cancer. Biochim. Biophys. Acta 2010, 1804, 559–566. [Google Scholar] [CrossRef]
- Yun, C.H.; Boggon, T.J.; Li, Y.; Woo, M.S.; Greulich, H.; Meyerson, M.; Eck, M.J. Structures of lung cancer-derived EGFR mutants and inhibitor complexes: Mechanism of activation and insights into differential inhibitor sensitivity. Cancer Cell 2007, 11, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Fan, J.; Qian, R.; Luo, P.; Zhang, J. Efficacy and safety of osimertinib in treating EGFR-mutated advanced NSCLC: A meta-analysis. Int. J. Cancer 2019, 145, 284–294. [Google Scholar] [CrossRef]
- Hirano, T.; Yasuda, H.; Tani, T.; Hamamoto, J.; Oashi, A.; Ishioka, K.; Arai, D.; Nukaga, S.; Miyawaki, M.; Kawada, I.; et al. In vitro modeling to determine mutation specificity of EGFR tyrosine kinase inhibitors against clinically relevant EGFR mutants in non-small-cell lung cancer. Oncotarget 2015, 6, 38789–38803. [Google Scholar] [CrossRef]
- Wind, S.; Schnell, D.; Ebner, T.; Freiwald, M.; Stopfer, P. Clinical Pharmacokinetics and Pharmacodynamics of Afatinib. Clin. Pharm. 2017, 56, 235–250. [Google Scholar] [CrossRef]
- Freiwald, M.; Schmid, U.; Fleury, A.; Wind, S.; Stopfer, P.; Staab, A. Population pharmacokinetics of afatinib, an irreversible ErbB family blocker, in patients with various solid tumors. Cancer Chemother Pharm. 2014, 73, 759–770. [Google Scholar] [CrossRef] [PubMed]
- EMA Assessment Report. Giotrif (INN-Afatinib) European Public Assessment Report. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_Public_assessment_report/human/004124/WC500202024.pdf (accessed on 8 February 2022).
- Nelson, V.; Ziehr, J.; Agulnik, M.; Johnson, M. Afatinib: Emerging next-generation tyrosine kinase inhibitor for NSCLC. Onco Targets 2013, 6, 135–143. [Google Scholar] [CrossRef]
- Planchard, D.; Popat, S.; Kerr, K.; Novello, S.; Smit, E.F.; Faivre-Finn, C.; Mok, T.S.; Reck, M.; Van Schil, P.E.; Hellmann, M.D.; et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv192–iv237. [Google Scholar] [CrossRef] [PubMed]
- van de Stadt, E.A.; Yaqub, M.; Lammertsma, A.A.; Poot, A.J.; Schober, P.R.; Schuit, R.C.; Smit, E.F.; Bahce, I.; Hendrikse, N.H. Quantification of [(18)F]afatinib using PET/CT in NSCLC patients: A feasibility study. EJNMMI Res. 2020, 10, 97. [Google Scholar] [CrossRef]
- van de Stadt, E.A.; Yaqub, M.; Lammertsma, A.A.; Poot, A.J.; Schuit, R.C.; Remmelzwaal, S.; Schwarte, L.A.; Smit, E.F.; Hendrikse, H.; Bahce, I. Identifying advanced stage NSCLC patients who benefit from afatinib therapy using (18)F-afatinib PET/CT imaging. Lung Cancer 2021, 155, 156–162. [Google Scholar] [CrossRef]
- Bahce, I.; Smit, E.F.; Lubberink, M.; van der Veldt, A.A.; Yaqub, M.; Windhorst, A.D.; Schuit, R.C.; Thunnissen, E.; Heideman, D.A.; Postmus, P.E.; et al. Development of [(11)C]erlotinib positron emission tomography for in vivo evaluation of EGF receptor mutational status. Clin. Cancer Res. 2013, 19, 183–193. [Google Scholar] [CrossRef]
- Bahce, I.; Yaqub, M.; Errami, H.; Schuit, R.C.; Schober, P.; Thunnissen, E.; Windhorst, A.D.; Lammertsma, A.A.; Smit, E.F.; Hendrikse, N.H. Effects of erlotinib therapy on [(11)C]erlotinib uptake in EGFR mutated, advanced NSCLC. EJNMMI Res. 2016, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Bahce, I.; Yaqub, M.; Smit, E.F.; Lammertsma, A.A.; van Dongen, G.A.; Hendrikse, N.H. Personalizing NSCLC therapy by characterizing tumors using TKI-PET and immuno-PET. Lung Cancer 2017, 107, 1–13. [Google Scholar] [CrossRef]
- Yaqub, M.; Bahce, I.; Voorhoeve, C.; Schuit, R.C.; Windhorst, A.D.; Hoekstra, O.S.; Boellaard, R.; Hendrikse, N.H.; Smit, E.F.; Lammertsma, A.A. Quantitative and Simplified Analysis of 11C-Erlotinib Studies. J. Nucl. Med. 2016, 57, 861–866. [Google Scholar] [CrossRef]
- van de Stadt, E.A. Relationship between biodistribution and tracer kinetics of 11C-erlotinib, 18F-afatinib and 11C-osimertinib and image quality evaluation using pharmacokinetic/pharmacodynamic analysis in advanced stage non-small cell lung cancer patients. Diagnostics 2022, 12, 883. [Google Scholar] [CrossRef]
- Hallifax, D.; Houston, J.B. Saturable uptake of lipophilic amine drugs into isolated hepatocytes: Mechanisms and consequences for quantitative clearance prediction. Drug Metab. Dispos. 2007, 35, 1325–1332. [Google Scholar] [CrossRef] [PubMed]
- de Klerk, D.J.; Honeywell, R.J.; Jansen, G.; Peters, G.J. Transporter and Lysosomal Mediated (Multi)drug Resistance to Tyrosine Kinase Inhibitors and Potential Strategies to Overcome Resistance. Cancers 2018, 10, 503. [Google Scholar] [CrossRef] [PubMed]
- Krchniakova, M.; Skoda, J.; Neradil, J.; Chlapek, P.; Veselska, R. Repurposing Tyrosine Kinase Inhibitors to Overcome Multidrug Resistance in Cancer: A Focus on Transporters and Lysosomal Sequestration. Int. J. Mol. Sci. 2020, 21, 3157. [Google Scholar] [CrossRef] [PubMed]
- Aarons, L. Physiologically based pharmacokinetic modelling: A sound mechanistic basis is needed. Br. J. Clin. Pharm. 2005, 60, 581–583. [Google Scholar] [CrossRef]
- Schmitt, M.V.; Reichel, A.; Liu, X.; Fricker, G.; Lienau, P. Extension of the Mechanistic Tissue Distribution Model of Rodgers and Rowland by Systematic Incorporation of Lysosomal Trapping: Impact on Unbound Partition Coefficient and Volume of Distribution Predictions in the Rat. Drug Metab. Dispos. 2021, 49, 53–61. [Google Scholar] [CrossRef]
- EMA Assessment Report. Tarceva (INN-Erlotinib) Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/tarceva-epar-product-information_en.pdf (accessed on 8 February 2022).
- EMA Assessment Report. Tagrisso (INN-Osimertinib) European Public Assessment Report. Available online: http://www.ema.europa.eu/en/documents/assessment-report/tagrisso-epar-public-assessment-report_en.pdf (accessed on 8 February 2022).
- Englinger, B.; Kallus, S.; Senkiv, J.; Heilos, D.; Gabler, L.; van Schoonhoven, S.; Terenzi, A.; Moser, P.; Pirker, C.; Timelthaler, G.; et al. Intrinsic fluorescence of the clinically approved multikinase inhibitor nintedanib reveals lysosomal sequestration as resistance mechanism in FGFR-driven lung cancer. J. Exp. Clin. Cancer Res. 2017, 36, 122. [Google Scholar] [CrossRef]
- Amor, D.; Goutal, S.; Marie, S.; Caille, F.; Bauer, M.; Langer, O.; Auvity, S.; Tournier, N. Impact of rifampicin-inhibitable transport on the liver distribution and tissue kinetics of erlotinib assessed with PET imaging in rats. EJNMMI Res. 2018, 8, 81. [Google Scholar] [CrossRef]
- Colclough, N.; Chen, K.; Johnstrom, P.; Strittmatter, N.; Yan, Y.; Wrigley, G.L.; Schou, M.; Goodwin, R.; Varnas, K.; Adua, S.J.; et al. Preclinical Comparison of the Blood-brain barrier Permeability of Osimertinib with Other EGFR TKIs. Clin. Cancer Res. 2021, 27, 189–201. [Google Scholar] [CrossRef]
- Hirasawa, M.; Saleh, M.A.A.; de Lange, E.C.M. The Extension of the LeiCNS-PK3.0 Model in Combination with the "Handshake" Approach to Understand Brain Tumor Pathophysiology. Pharm. Res. 2022, 1–9. [Google Scholar] [CrossRef]
- Zsila, F.; Fitos, I.; Bencze, G.; Keri, G.; Orfi, L. Determination of human serum alpha1-acid glycoprotein and albumin binding of various marketed and preclinical kinase inhibitors. Curr. Med. Chem. 2009, 16, 1964–1977. [Google Scholar] [CrossRef]
- Huang, Z.; Ung, T. Effect of alpha-1-acid glycoprotein binding on pharmacokinetics and pharmacodynamics. Curr. Drug Metab. 2013, 14, 226–238. [Google Scholar] [PubMed]
- Pearce, O.M.T.; Delaine-Smith, R.M.; Maniati, E.; Nichols, S.; Wang, J.; Bohm, S.; Rajeeve, V.; Ullah, D.; Chakravarty, P.; Jones, R.R.; et al. Deconstruction of a Metastatic Tumor Microenvironment Reveals a Common Matrix Response in Human Cancers. Cancer Discov. 2018, 8, 304–319. [Google Scholar] [CrossRef] [PubMed]
- Assmus, F.; Houston, J.B.; Galetin, A. Incorporation of lysosomal sequestration in the mechanistic model for prediction of tissue distribution of basic drugs. Eur. J. Pharm. Sci. 2017, 109, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, M.V.; Lienau, P.; Fricker, G.; Reichel, A. Quantitation of Lysosomal Trapping of Basic Lipophilic Compounds Using In Vitro Assays and In Silico Predictions Based on the Determination of the Full pH Profile of the Endo-/Lysosomal System in Rat Hepatocytes. Drug Metab. Dispos. 2019, 47, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Ling, J.; Johnson, K.A.; Miao, Z.; Rakhit, A.; Pantze, M.P.; Hamilton, M.; Lum, B.L.; Prakash, C. Metabolism and excretion of erlotinib, a small molecule inhibitor of epidermal growth factor receptor tyrosine kinase, in healthy male volunteers. Drug Metab. Dispos. 2006, 34, 420–426. [Google Scholar] [CrossRef]
- Folkman, J. Tumor angiogenesis: Therapeutic implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar] [CrossRef]
- Herbst, R.S.; Onn, A.; Sandler, A. Angiogenesis and lung cancer: Prognostic and therapeutic implications. J. Clin. Oncol. 2005, 23, 3243–3256. [Google Scholar] [CrossRef]
- Ward, C.; Meehan., J.; Gray, M.E.; Murray, A.F.; Argyle, D.J.; Kunkler, I.H.; Langdon, S.P. The impact of tumour pH on cancer progression: Strategies for clinical intervention. Explor. Target Antitumor. 2020, 1, 71–100. [Google Scholar] [CrossRef]
- Williams, J.A.; Ring, B.J.; Cantrell, V.E.; Jones, D.R.; Eckstein, J.; Ruterbories, K.; Hamman, M.A.; Hall, S.D.; Wrighton, S.A. Comparative metabolic capabilities of CYP3A4, CYP3A5, and CYP3A7. Drug Metab. Dispos. 2002, 30, 883–891. [Google Scholar] [CrossRef]
- Abuqayyas, L.; Balthasar, J.P. Application of PBPK modeling to predict monoclonal antibody disposition in plasma and tissues in mouse models of human colorectal cancer. J. Pharm. Pharm. 2012, 39, 683–710. [Google Scholar] [CrossRef]
- Joly-Tonetti, N.; Ondet, T.; Monshouwer, M.; Stamatas, G.N. EGFR inhibitors switch keratinocytes from a proliferative to a differentiative phenotype affecting epidermal development and barrier function. BMC Cancer 2021, 21, 5. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, T.; Leahy, D.; Rowland, M. Physiologically based pharmacokinetic modeling 1: Predicting the tissue distribution of moderate-to-strong bases. J. Pharm. Sci. 2005, 94, 1259–1276. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, T.; Rowland, M. Physiologically based pharmacokinetic modelling 2: Predicting the tissue distribution of acids, very weak bases, neutrals and zwitterions. J. Pharm. Sci. 2006, 95, 1238–1257. [Google Scholar] [CrossRef]
- Graham, H.; Walker, M.; Jones, O.; Yates, J.; Galetin, A.; Aarons, L. Comparison of in-vivo and in-silico methods used for prediction of tissue: Plasma partition coefficients in rat. J. Pharm. Pharm. 2012, 64, 383–396. [Google Scholar] [CrossRef] [PubMed]
- de Araujo, M.E.G.; Liebscher, G.; Hess, M.W.; Huber, L.A. Lysosomal size matters. Traffic 2020, 21, 60–75. [Google Scholar] [CrossRef]
- Liu, L.; Wang, C.; Li, S.; Bai, H.; Wang, J. Tumor immune microenvironment in epidermal growth factor receptor-mutated non-small cell lung cancer before and after epidermal growth factor receptor tyrosine kinase inhibitor treatment: A narrative review. Transl. Lung Cancer Res. 2021, 10, 3823–3839. [Google Scholar] [CrossRef]
| Erlotinib | Afatinib | Osimertinib | |
|---|---|---|---|
| Lung | |||
| EGFR binding | 0.21% | 16.73% | 0.14% |
| Lysosomal trapping | n.a. | 49.19% | 59.16% |
| NL/NP | 1.12% | 0.01% | 0.00% |
| Albumin | 73.01% | n.a. | n.a. |
| AP- | n.a. | 32.63% | 39.74% |
| IW | 14.54% | 1.08% | 0.72% |
| EW | 0.36% | 0.36% | 0.23% |
| Tumor | |||
| EGFR binding | 1.89% | 72.17% | 1.85% |
| Lysosomal trapping | n.a. | 11.99% | 42.79% |
| NL/NP | 1.05% | 0.00% | 0.00% |
| Albumin | 72.32% | n.a. | n.a. |
| AP- | n.a. | 14.63% | 52.91% |
| IW | 13.71% | 0.48% | 0.96% |
| EW | 11.02% | 0.73% | 1.49% |
| Erlotinib | Afatinib | Osimertinib | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Predicted | Observed | Prediction Error (%) | Predicted | Observed | Prediction Error (%) | Predicted | Observed | Prediction Error (%) | |
| Brain | 0.13 | n.a. | n.a. | 2.85 | 0.08 (0.03) | 189.7 | 1.52 | 0.79 (0.5) | 62.7 |
| Lung | 0.28 | 0.51 (0.2) | −58.8 | 6.89 | 2.54 (1.2) | 92.4 | 3.11 | 7.01 (1.6) | −77.1 |
| Spleen | 0.17 | 1.46 (0.4) | −157.8 | 48.72 | 13.23 (2.3) | 114.6 | 25.33 | 18.09 (7.7) | 33.3 |
| Kidney | 0.21 | 1.69 (0.6) | −155.6 | 26.20 | 6.93 (1.8) | 116.3 | 10.48 | 5.61 (2.0) | 60.6 |
| Bone | 0.14 | 1.23 (0.2) | −158.3 | 2.72 | 4.81 (2.0) | −55.3 | 1.48 | 4.24 (0.7) | −96.6 |
| Tumor | 0.30 | 1.42 (0.5) | −131.1 | 15.36 | 3.60 (2.4) | 124.1 | 2.33 | 5.60 (2.0) | −82.4 |
| Tissue-Specific Input Parameters | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fnl | Fnp | Few | Fiw | Flys 2 | Tissue Concentration of AP- (mg/g) 2 | Albumin Tissue to Plasma Ratio 3 | EGFR (nM) | |||||||||||||
| Blood cells | 1.7 × 10−3 | 0.0029 | n.a. | 0.60 | n.a. | 0.50 | n.a. | n.a. | ||||||||||||
| Bone | 0.017 | 0.0017 | 0.1 | 0.35 | n.d. | 0.67 | 0.10 | n.a. | ||||||||||||
| Brain | 0.039 | 0.0015 | 0.16 | 0.61 | 0.014 | 0.40 | 0.048 | n.a. | ||||||||||||
| Kidney | 0.039 1 | 0.012 1 | 0.27 | 0.47 | 0.017 | 2.44 1 | 0.13 | 177 | ||||||||||||
| Lung 3 | 0.0088 1 | 0.0030 1 | 0.34 | 0.43 | 0.015 | 0.57 1 | 0.21 | 31.1 | ||||||||||||
| Tumor | 0.01 | 299 | ||||||||||||||||||
| Spleen | 0.021 1 | 0.017 1 | 0.21 | 0.53 | 0.053 | 3.18 | 0.097 | 54.6 | ||||||||||||
| Plasma 4 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | ||||||||||||
| Lung-specific parameters 2 | ||||||||||||||||||||
| Fnl | Fnp | Few | Fiw | pHew | Flys | pH lysosome | Fcell type | |||||||||||||
| -Alveolar macrophages | 0.00881 | 0.00301 | 0.34 | 0.45 | 7.4 | 0.078 | 4.75 | 0.041 | ||||||||||||
| -Type II cells | 0.03 | 5.1 | 0.083 | |||||||||||||||||
| -Residual cells | 0.01 | 5.1 | 0.88 | |||||||||||||||||
| Tumor-specific input parameters | ||||||||||||||||||||
| Residual cells | 0.008 | 0.0030 | 0.34 | 0.45 | 6.7 | 0.01 | 5.1 | 1 | ||||||||||||
| Compound-specific parameters | ||||||||||||||||||||
| Erlotinib | Afatinib | Osimertinib | References | |||||||||||||||||
| Log P | 3.3 | 3.6 | 3.2 | Colclough et al. (2021) [31] | ||||||||||||||||
| pKa | 5.5 | 8.2 | 9.0 | Colclough et al. (2021) [31] | ||||||||||||||||
| B:P ratio 5 | 0.95 | 1.27 | 0.79 | Van de Stadt et al. (2021) [21] | ||||||||||||||||
| Kd EGFR (nM) | 2164 | 2 | 155 | Joly-Tonetti et al. (2021) [44] | ||||||||||||||||
| Funbound 6 | 0.088 | 0.095 | 0.017 | Colclough et al. (2021) [31] | ||||||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartelink, I.H.; van de Stadt, E.A.; Leeuwerik, A.F.; Thijssen, V.L.J.L.; Hupsel, J.R.I.; van den Nieuwendijk, J.F.; Bahce, I.; Yaqub, M.; Hendrikse, N.H. Physiologically Based Pharmacokinetic (PBPK) Modeling to Predict PET Image Quality of Three Generations EGFR TKI in Advanced-Stage NSCLC Patients. Pharmaceuticals 2022, 15, 796. https://doi.org/10.3390/ph15070796
Bartelink IH, van de Stadt EA, Leeuwerik AF, Thijssen VLJL, Hupsel JRI, van den Nieuwendijk JF, Bahce I, Yaqub M, Hendrikse NH. Physiologically Based Pharmacokinetic (PBPK) Modeling to Predict PET Image Quality of Three Generations EGFR TKI in Advanced-Stage NSCLC Patients. Pharmaceuticals. 2022; 15(7):796. https://doi.org/10.3390/ph15070796
Chicago/Turabian StyleBartelink, I. H., E. A. van de Stadt, A. F. Leeuwerik, V. L. J. L. Thijssen, J. R. I. Hupsel, J. F. van den Nieuwendijk, I. Bahce, M. Yaqub, and N. H. Hendrikse. 2022. "Physiologically Based Pharmacokinetic (PBPK) Modeling to Predict PET Image Quality of Three Generations EGFR TKI in Advanced-Stage NSCLC Patients" Pharmaceuticals 15, no. 7: 796. https://doi.org/10.3390/ph15070796
APA StyleBartelink, I. H., van de Stadt, E. A., Leeuwerik, A. F., Thijssen, V. L. J. L., Hupsel, J. R. I., van den Nieuwendijk, J. F., Bahce, I., Yaqub, M., & Hendrikse, N. H. (2022). Physiologically Based Pharmacokinetic (PBPK) Modeling to Predict PET Image Quality of Three Generations EGFR TKI in Advanced-Stage NSCLC Patients. Pharmaceuticals, 15(7), 796. https://doi.org/10.3390/ph15070796







