Exploring the Underlying Mechanism of Ren-Shen-Bai-Du Powder for Treating Inflammatory Bowel Disease Based on Network Pharmacology and Molecular Docking
Abstract
:1. Introduction
2. Results
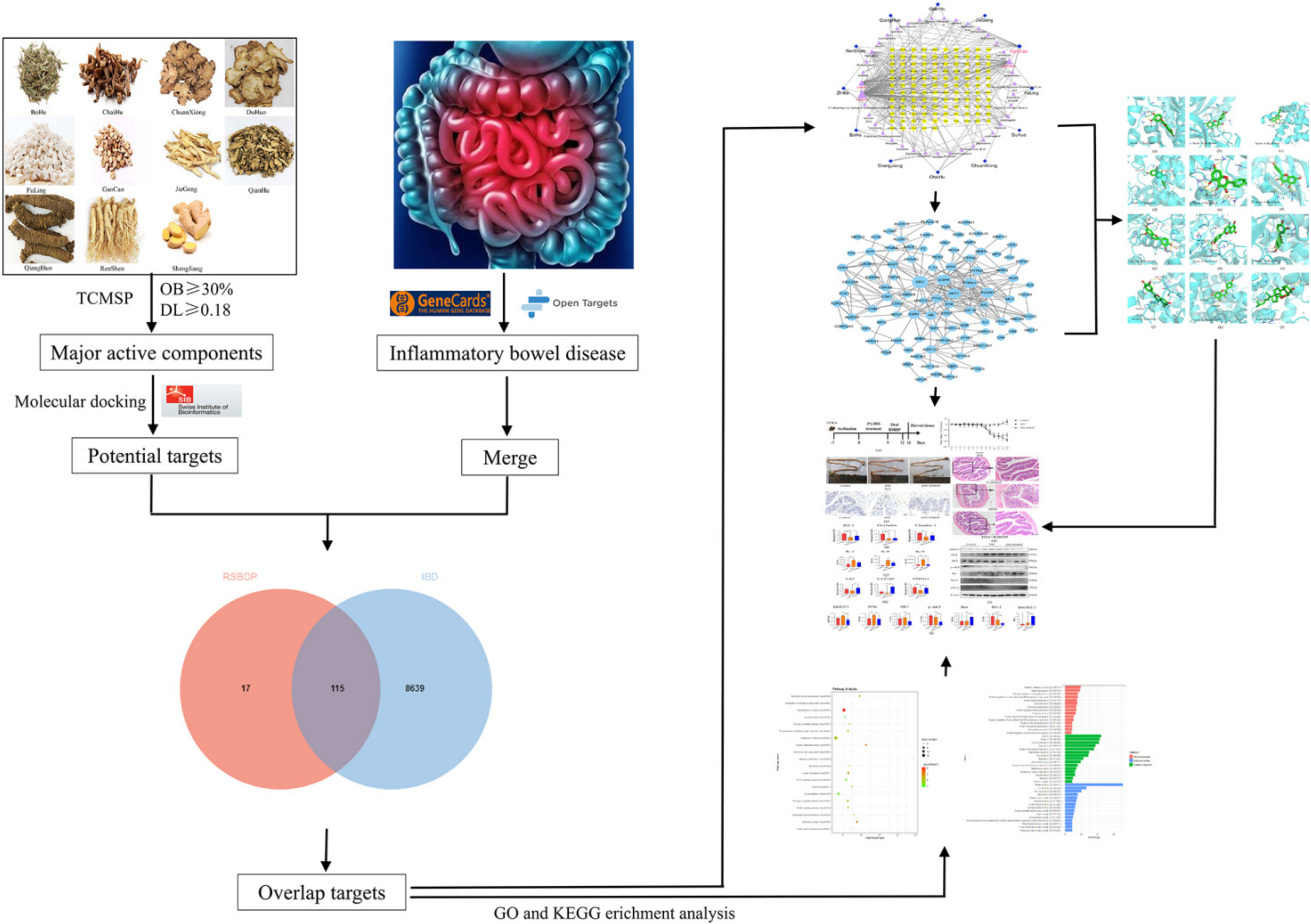
2.1. Screening for Key Active Ingredients of RSBDP and Prediction of Important Targets
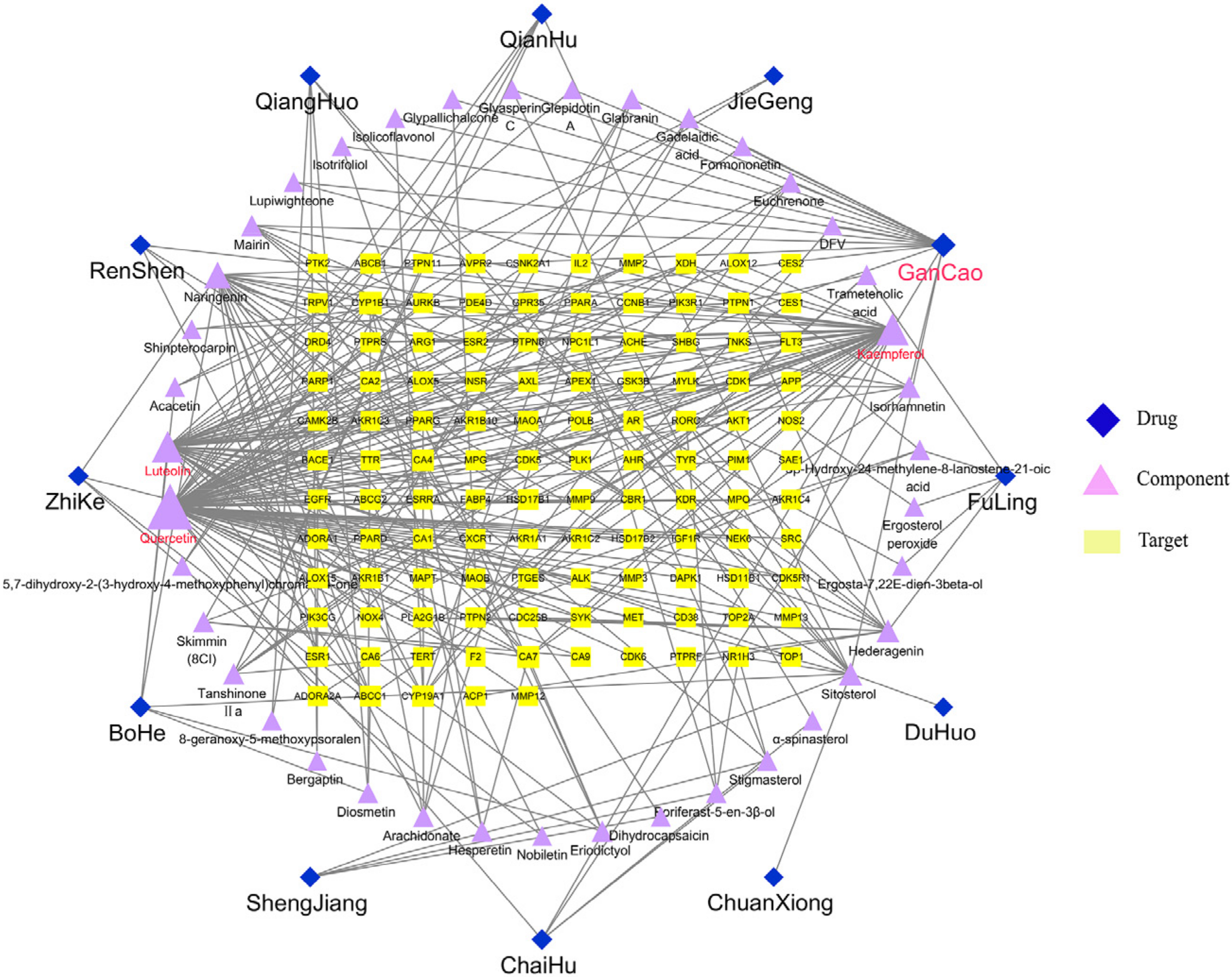
2.2. The Construction of the Drug-Ingredient-Target Relationship Network
2.3. The Construction and Topological Analysis of the PPI Network
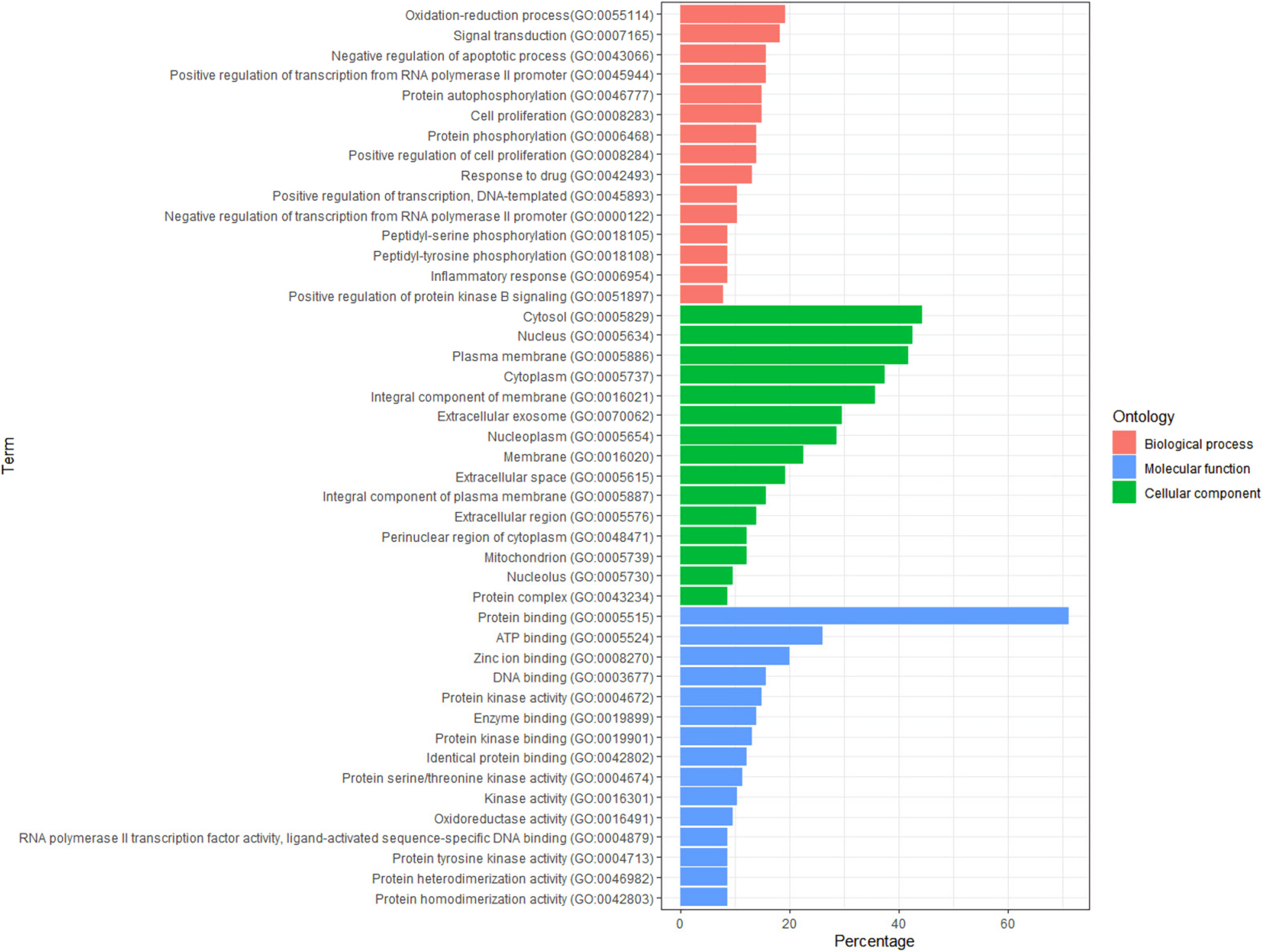
2.4. GO and KEGG Enrichment Analyses
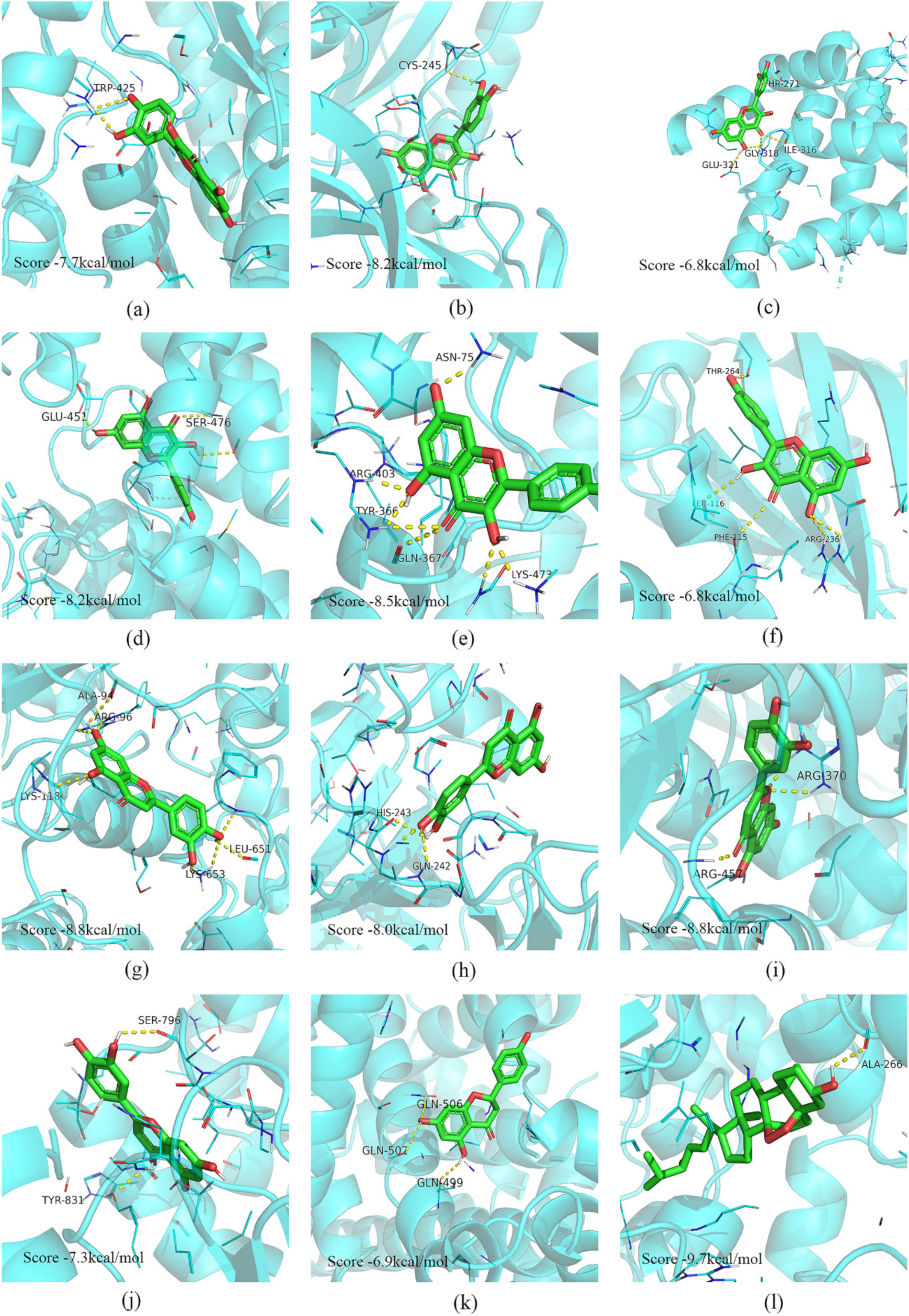
2.5. Molecular Docking of Vital Active Ingredients and Core Targets
2.6. Validation of RSBDP Treatment Effectiveness and Targets
3. Discussion
4. Materials and Methods
4.1. Screening for the Main Active Ingredients of RSBDP
4.2. The Target Prediction of RSBDP Ingredients for IBD Therapy
4.3. The Construction and Analysis of a “Drug-Ingredient-Target” Network
4.4. The Construction and Analysis of the Protein-Protein Interaction Network
4.5. Gene Ontology and Kyoto Encyclopedia of Genes and Genomes Signal Pathway Enrichment Analysis
4.6. Molecular Docking Analysis
4.7. Animal Experiment
4.7.1. Drug
4.7.2. Animals and Experimental Design
4.7.3. Histological Analysis
4.7.4. Enzyme-Linked Immunosorbent Assay
4.7.5. RNA Extraction and Quantitative Real-Time PCR (qRT-PCR)
4.7.6. Western Blotting
4.7.7. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nikolaus, S.; Schulte, B.; Al-Massad, N.; Thieme, F.; Schulte, D.M.; Bethge, J.; Rehman, A.; Tran, F.; Aden, K.; Hasler, R.; et al. Increased Tryptophan Metabolism Is Associated with Activity of Inflammatory Bowel Diseases. Gastroenterology 2017, 153, 1504–1516.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuo, T.; Kamm, M.A.; Colombel, J.F.; Ng, S.C. Urbanization and the gut microbiota in health and inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 440–452. [Google Scholar] [CrossRef] [PubMed]
- Olivera, P.; Danese, S.; Jay, N.; Natoli, G.; Peyrin-Biroulet, L. Big data in IBD: A look into the future. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhang, M.; Dou, D.; Kang, T.; Li, F. Effects of Deoxyschisandrin on Visceral Sensitivity of Mice with Inflammatory Bowel Disease. Evid. Based Complement. Altern. Med. 2019, 2019, 2986097. [Google Scholar] [CrossRef] [Green Version]
- Curro, D.; Ianiro, G.; Pecere, S.; Bibbo, S.; Cammarota, G. Probiotics, fibre and herbal medicinal products for functional and inflammatory bowel disorders. Br. J. Pharmacol. 2017, 174, 1426–1449. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.F.; Hsu, J.T.; Wu, K.C.; Hsiao, C.F.; Lin, J.A.; Cheng, Y.H.; Liu, Y.H.; Lee, D.Y.; Chang, H.H.; Cho, D.Y.; et al. A systematic identification of anti-inflammatory active components derived from Mu Dan Pi and their applications in inflammatory bowel disease. Sci. Rep. 2020, 10, 17238. [Google Scholar] [CrossRef]
- Xiong, P.; Chen, L.; Chen, X.; Zhang, P.; Zhong, C.; Liu, X.; Jia, B. Based on “Reverse Flow Carrying Boat” Approach to Explore the Intervention Effect of Renshen Baidu Powder on Intestinal Mucosal Barrier Ulcerative Colitis in Rats. Mod. Tradit. Chin. Med. Mater. Med. World Sci. Technol. 2021, 23, 2285–2293. [Google Scholar]
- Liu, B.; Zheng, X.; Li, J.; Li, X.; Wu, R.; Yang, J.; Liu, W.; Zhao, G. Revealing mechanism of Caulis Sargentodoxae for the treatment of ulcerative colitis based on network pharmacology approach. Biosci. Rep. 2021, 41, BSR20204005. [Google Scholar] [CrossRef]
- Chen, D.; Wu, Y.; Chen, Y.; Chen, Q.; Ye, X.; Xu, S.; Luo, S. Exploration of the molecular targets and mechanisms of suxiao xintong dropping pills for myocardial infarction by network pharmacology method. Biosci. Rep. 2021, 41, BSR20204211. [Google Scholar] [CrossRef]
- Guo, K.; Wang, T.; Luo, E.; Leng, X.; Yao, B. Use of Network Pharmacology and Molecular Docking Technology to Analyze the Mechanism of Action of Velvet Antler in the Treatment of Postmenopausal Osteoporosis. Evid. Based Complement. Altern. Med. 2021, 2021, 7144529. [Google Scholar] [CrossRef]
- Neurath, M.F. Current and emerging therapeutic targets for IBD. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 269–278. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Jia, B.; Deng, H. Treatment of ulcerative colitis based on the Niliu Wanzhou’ method. China J. Tradit. Chin. Med. Pharm. 2019, 34, 527–529. [Google Scholar]
- Riemschneider, S.; Hoffmann, M.; Slanina, U.; Weber, K.; Hauschildt, S.; Lehmann, J. Indol-3-Carbinol and Quercetin Ameliorate Chronic DSS-Induced Colitis in C57BL/6 Mice by AhR-Mediated Anti-Inflammatory Mechanisms. Int. J. Environ. Res. Public Health 2021, 18, 2262. [Google Scholar] [CrossRef]
- Ju, S.; Ge, Y.; Li, P.; Tian, X.; Wang, H.; Zheng, X.; Ju, S. Dietary quercetin ameliorates experimental colitis in mouse by remodeling the function of colonic macrophages via a heme oxygenase-1-dependent pathway. Cell Cycle 2018, 17, 53–63. [Google Scholar] [CrossRef] [Green Version]
- Bian, Y.; Dong, Y.; Sun, J.; Sun, M.; Hou, Q.; Lai, Y.; Zhang, B. Protective Effect of Kaempferol on LPS-Induced Inflammation and Barrier Dysfunction in a Coculture Model of Intestinal Epithelial Cells and Intestinal Microvascular Endothelial Cells. J. Agric. Food Chem. 2020, 68, 160–167. [Google Scholar] [CrossRef]
- Lin, T.J.; Yin, S.Y.; Hsiao, P.W.; Yang, N.S.; Wang, I.J. Transcriptomic analysis reveals a controlling mechanism for NLRP3 and IL-17A in dextran sulfate sodium (DSS)-induced colitis. Sci. Rep. 2018, 8, 14927. [Google Scholar] [CrossRef] [Green Version]
- He, W.; Li, Y.; Liu, M.; Yu, H.; Chen, Q.; Chen, Y.; Ruan, J.; Ding, Z.; Zhang, Y.; Wang, T. Citrus aurantium L. and Its Flavonoids Regulate TNBS-Induced Inflammatory Bowel Disease through Anti-Inflammation and Suppressing Isolated Jejunum Contraction. Int. J. Mol. Sci. 2018, 19, 3057. [Google Scholar] [CrossRef]
- Feng, S.; Dai, Z.; Liu, A.; Wang, H.; Chen, J.; Luo, Z.; Yang, C.S. β-Sitosterol and stigmasterol ameliorate dextran sulfate sodium-induced colitis in mice fed a high fat Western-style diet. Food Funct. 2017, 8, 4179–4186. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Ma, Z.; Liang, Q.; Tang, X.; Hu, D.; Tan, H.; Xiao, C.; Gao, Y. Tanshinone IIA ameliorates dextran sulfate sodium-induced inflammatory bowel disease via the pregnane X receptor. Drug Des. Dev. Ther. 2015, 9, 6343–6362. [Google Scholar] [CrossRef]
- Wang, R.; Shen, L.; Li, H.; Peng, H. Eriodictyol attenuates dextran sodium sulphate-induced colitis in mice by regulating the sonic hedgehog signalling pathway. Pharm. Biol. 2021, 59, 974–985. [Google Scholar] [CrossRef]
- Gil, A. Polyunsaturated fatty acids and inflammatory diseases. Biomed. Pharmacother. 2002, 56, 388–396. [Google Scholar] [CrossRef]
- Ren, J.; Yue, B.; Wang, H.; Zhang, B.; Luo, X.; Yu, Z.; Zhang, J.; Ren, Y.; Mani, S.; Wang, Z.; et al. Acacetin Ameliorates Experimental Colitis in Mice via Inhibiting Macrophage Inflammatory Response and Regulating the Composition of Gut Microbiota. Front. Physiol. 2020, 11, 577237. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.B.; Xavier, R.J. Pathway paradigms revealed from the genetics of inflammatory bowel disease. Nature 2020, 578, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Wang, Q.; Ma, Y.; Li, L.; Yu, K.; Zhang, Z.; Chen, G.; Li, X.; Xiao, W.; Xu, P.; et al. Aryl Hydrocarbon Receptor Activation Modulates Intestinal Epithelial Barrier Function by Maintaining Tight Junction Integrity. Int. J. Biol. Sci. 2018, 14, 69–77. [Google Scholar] [CrossRef]
- Geremia, A.; Biancheri, P.; Allan, P.; Corazza, G.R.; Di Sabatino, A. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun. Rev. 2014, 13, 3–10. [Google Scholar] [CrossRef]
- Chen, X.; Liu, G.; Yuan, Y.; Wu, G.; Wang, S.; Yuan, L. NEK7 interacts with NLRP3 to modulate the pyroptosis in inflammatory bowel disease via NF-kappaB signaling. Cell Death Dis. 2019, 10, 906. [Google Scholar] [CrossRef]
- Leclair, H.M.; Tardif, N.; Paris, A.; Galibert, M.D.; Corre, S. Role of Flavonoids in the Prevention of AhR-Dependent Resistance During Treatment with BRAF Inhibitors. Int. J. Mol. Sci. 2020, 21, 5025. [Google Scholar] [CrossRef]
- Yang, T.; Feng, Y.L.; Chen, L.; Vaziri, N.D.; Zhao, Y.Y. Dietary natural flavonoids treating cancer by targeting aryl hydrocarbon receptor. Crit. Rev. Toxicol. 2019, 49, 445–460. [Google Scholar] [CrossRef] [Green Version]
- Vondracek, J.; Umannova, L.; Machala, M. Interactions of the aryl hydrocarbon receptor with inflammatory mediators: Beyond CYP1A regulation. Curr. Drug Metab. 2011, 12, 89–103. [Google Scholar] [CrossRef]
- Christmas, P. Role of Cytochrome P450s in Inflammation. Adv. Pharmacol. 2015, 74, 163–192. [Google Scholar] [CrossRef]
- Van der Giessen, J.; van der Woude, C.J.; Peppelenbosch, M.P.; Fuhler, G.M. A Direct Effect of Sex Hormones on Epithelial Barrier Function in Inflammatory Bowel Disease Models. Cells 2019, 8, 261. [Google Scholar] [CrossRef] [Green Version]
- Velica, P.; Davies, N.J.; Rocha, P.P.; Schrewe, H.; Ride, J.P.; Bunce, C.M. Lack of functional and expression homology between human and mouse aldo-keto reductase 1C enzymes: Implications for modelling human cancers. Mol. Cancer 2009, 8, 121. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.; Wei, Z.H.; Li, Y.Y.; Tan, Z.Q.; Lin, C. AKR1C1 Contributes to Cervical Cancer Progression via Regulating TWIST1 Expression. Biochem. Genet. 2021, 59, 516–530. [Google Scholar] [CrossRef]
- Zheng, J.; Yang, Z.; Li, Y.; Yang, L.; Yao, R. Knockdown of AKR1C3 Promoted Sorafenib Sensitivity Through Inhibiting the Phosphorylation of AKT in Hepatocellular Carcinoma. Front. Oncol. 2022, 12, 823491. [Google Scholar] [CrossRef]
- Fattahi, S.; Amjadi-Moheb, F.; Tabaripour, R.; Ashrafi, G.H.; Akhavan-Niaki, H. PI3K/AKT/mTOR signaling in gastric cancer: Epigenetics and beyond. Life Sci. 2020, 262, 118513. [Google Scholar] [CrossRef]
- Arab, H.H.; Al-Shorbagy, M.Y.; Saad, M.A. Activation of autophagy and suppression of apoptosis by dapagliflozin attenuates experimental inflammatory bowel disease in rats: Targeting AMPK/mTOR, HMGB1/RAGE and Nrf2/HO-1 pathways. Chem. Biol. Interact. 2021, 335, 109368. [Google Scholar] [CrossRef]
- Ala, M.; Jafari, R.M.; Nematian, H.; Shadboorestan, A.; Dehpour, A.R. Sodium Selenite Modulates IDO1/Kynurenine, TLR4, NF-kappaB and Bcl2/Bax Pathway and Mitigates Acetic Acid-Induced Colitis in Rat. Cell Physiol. Biochem. 2022, 56, 24–35. [Google Scholar] [CrossRef]
- Gao, T.; Wang, T.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Melatonin-mediated MT2 attenuates colitis induced by dextran sodium sulfate via PI3K/AKT/Nrf2/SIRT1/RORalpha/NF-kappaB signaling pathways. Int. Immunopharmacol. 2021, 96, 107779. [Google Scholar] [CrossRef]
- Xi, M.; Wang, X.; Ge, J.; Yin, D. N′-[(3-[benzyloxy]benzylidene]-3,4,5-trihydroxybenzohydrazide (1) protects mice against colitis induced by dextran sulfate sodium through inhibiting NFkappaB/IL-6/STAT3 pathway. Biochem. Biophys. Res. Commun. 2016, 477, 290–296. [Google Scholar] [CrossRef]
- Lugering, A.; Lebiedz, P.; Koch, S.; Kucharzik, T. Apoptosis as a therapeutic tool in IBD? Ann. N. Y. Acad. Sci. 2006, 1072, 62–77. [Google Scholar] [CrossRef]
- Saadatdoust, Z.; Pandurangan, A.K.; Ananda Sadagopan, S.K.; Mohd Esa, N.; Ismail, A.; Mustafa, M.R. Dietary cocoa inhibits colitis associated cancer: A crucial involvement of the IL-6/STAT3 pathway. J. Nutr. Biochem. 2015, 26, 1547–1558. [Google Scholar] [CrossRef]
- Bungsu, I.; Kifli, N.; Ahmad, S.R.; Ghani, H.; Cunningham, A.C. Herbal Plants: The Role of AhR in Mediating Immunomodulation. Front. Immunol. 2021, 12, 697663. [Google Scholar] [CrossRef]
- Chapkin, R.S.; Davidson, L.A.; Park, H.; Jin, U.H.; Fan, Y.Y.; Cheng, Y.; Hensel, M.E.; Landrock, K.K.; Allred, C.; Menon, R.; et al. Role of the Aryl Hydrocarbon Receptor (AhR) in Mediating the Effects of Coffee in the Colon. Mol. Nutr. Food Res. 2021, 65, e2100539. [Google Scholar] [CrossRef]
- Fouzder, C.; Mukhuty, A.; Kundu, R. Kaempferol inhibits Nrf2 signalling pathway via downregulation of Nrf2 mRNA and induces apoptosis in NSCLC cells. Arch. Biochem. Biophys. 2021, 697, 108700. [Google Scholar] [CrossRef]
- Ru, J.; Li, P.; Wang, J.; Zhou, W.; Li, B.; Huang, C.; Li, P.; Guo, Z.; Tao, W.; Yang, Y.; et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 2014, 6, 13. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Xiao, J.; Suzek, T.O.; Zhang, J.; Wang, J.; Bryant, S.H. PubChem: A public information system for analyzing bioactivities of small molecules. Nucleic Acids Res. 2009, 37, W623–W633. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. Swiss Target Prediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef] [Green Version]
- Fishilevich, S.; Nudel, R.; Rappaport, N.; Hadar, R.; Plaschkes, I.; Iny Stein, T.; Rosen, N.; Kohn, A.; Twik, M.; Safran, M.; et al. GeneHancer: Genome-wide integration of enhancers and target genes in GeneCards. Database 2017, 2017, bax028. [Google Scholar] [CrossRef] [Green Version]
- Carvalho-Silva, D.; Pierleoni, A.; Pignatelli, M.; Ong, C.; Fumis, L.; Karamanis, N.; Carmona, M.; Faulconbridge, A.; Hercules, A.; McAuley, E.; et al. Open Targets Platform: New developments and updates two years on. Nucleic Acids Res. 2019, 47, D1056–D1065. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Franceschini, A.; Szklarczyk, D.; Frankild, S.; Kuhn, M.; Simonovic, M.; Roth, A.; Lin, J.; Minguez, P.; Bork, P.; von Mering, C.; et al. STRING v9.1: Protein-protein interaction networks, with increased coverage and integration. Nucleic Acids Res. 2013, 41, D808–D815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sussman, J.L.; Lin, D.; Jiang, J.; Manning, N.O.; Prilusky, J.; Ritter, O.; Abola, E.E. Protein Data Bank (PDB): Database of three-dimensional structural information of biological macromolecules. Acta Crystallogr. D Biol. Crystallogr. 1998, 54, 1078–1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Drug | Mol ID | Molecule Name | OB (%) | DL |
|---|---|---|---|---|
| ChaiHu | MOL000354 | Isorhamnetin | 49.60 | 0.31 |
| MOL000422 | Kaempferol | 41.88 | 0.24 | |
| MOL000098 | Quercetin | 46.43 | 0.28 | |
| MOL000449 | Stigmasterol | 43.83 | 0.76 | |
| MOL004718 | α-spinasterol | 42.98 | 0.76 | |
| ChuanXiong | MOL000359 | Sitosterol | 36.91 | 0.75 |
| DuHuo | MOL000358 | β-sitosterol | 36.91 | 0.75 |
| FuLing | MOL000287 | 3β-Hydroxy-24-methylene-8-lanostene-21-oic acid | 38.70 | 0.81 |
| MOL000282 | Ergosta-7,22E-dien-3β-ol | 43.51 | 0.72 | |
| MOL000283 | Ergosterol peroxide | 40.36 | 0.81 | |
| MOL000296 | Hederagenin | 36.91 | 0.75 | |
| MOL000275 | Trametenolic acid | 38.71 | 0.80 | |
| GanCao | MOL001792 | DFV | 32.76 | 0.18 |
| MOL004806 | Euchrenone | 30.29 | 0.57 | |
| MOL000392 | Formononetin | 69.67 | 0.21 | |
| MOL004996 | Gadelaidic acid | 30.70 | 0.20 | |
| MOL004910 | Glabranin | 52.90 | 0.31 | |
| MOL004828 | Glepidotin A | 44.72 | 0.35 | |
| MOL004811 | Glyasperin C | 45.56 | 0.40 | |
| MOL004835 | Glypallichalcone | 61.60 | 0.19 | |
| MOL004949 | Isolicoflavonol | 45.17 | 0.42 | |
| MOL000354 | Isorhamnetin | 49.60 | 0.31 | |
| MOL004814 | Isotrifoliol | 31.94 | 0.42 | |
| MOL000422 | Kaempferol | 41.88 | 0.24 | |
| MOL003656 | Lupiwighteone | 51.64 | 0.37 | |
| MOL000211 | Mairin | 55.38 | 0.78 | |
| MOL004328 | Naringenin | 59.29 | 0.21 | |
| MOL000098 | Quercetin | 46.43 | 0.28 | |
| MOL004891 | Shinpterocarpin | 80.30 | 0.73 | |
| MOL000359 | Sitosterol | 36.91 | 0.75 | |
| JieGeng | MOL001689 | Acacetin | 34.97 | 0.24 |
| MOL000006 | Luteolin | 36.16 | 0.25 | |
| QianHu | MOL005100 | 5,7-dihydroxy-2-(3-hydroxy-4- methoxyphenyl)chroman-4-one | 47.74 | 0.27 |
| MOL000358 | β-sitosterol | 36.91 | 0.75 | |
| MOL000098 | Quercetin | 46.43 | 0.28 | |
| MOL013083 | Skimmin (8CI) | 38.35 | 0.32 | |
| MOL007154 | tanshinone Ⅱa | 49.89 | 0.40 | |
| RenShen | MOL005320 | Arachidonate | 45.57 | 0.20 |
| MOL000358 | β-sitosterol | 36.91 | 0.75 | |
| MOL000422 | kaempferol | 41.88 | 0.24 | |
| MOL000449 | Stigmasterol | 43.83 | 0.76 | |
| ZhiKe | MOL000358 | β-sitosterol | 36.91 | 0.75 |
| MOL002341 | Hesperetin | 70.31 | 0.27 | |
| MOL004328 | Naringenin | 61.67 | 0.52 | |
| MOL005828 | Nobiletin | 61.67 | 0.52 | |
| BoHe | MOL005190 | Eriodictyol | 71.79 | 0.24 |
| ShengJiang | MOL008698 | Dihydrocapsaicin | 47.07 | 0.19 |
| MOL001771 | Poriferast-5-en-3β-ol | 36.91 | 0.75 |
| Ingredients | Betweenness Centrality | Closeness Centrality | Degree |
|---|---|---|---|
| Quercetin | 0.55011787 | 0.49848943 | 76 |
| Kaempferol | 0.15632436 | 0.39568345 | 40 |
| Luteolin | 0.1629187 | 0.3724605 | 37 |
| Naringenin | 0.04758899 | 0.33199195 | 23 |
| Sitosterol | 0.11861359 | 0.3674833 | 13 |
| Targets | Betweenness Centrality | Closeness Centrality | Degree |
|---|---|---|---|
| Cytochrome P450 1B1 (CYP1B1) | 0.03810726 | 0.38461538 | 11 |
| Carbonic anhydrase 7 (CA7) | 0.021592 | 0.37931034 | 9 |
| Cytochrome P450 19A1 (CYP19A1) | 0.01553419 | 0.35031847 | 8 |
| Carbonic anhydrase 4 (CA4) | 0.01578918 | 0.37757437 | 8 |
| Protein-tyrosine phosphatase 1 (PTPN1) | 0.03389732 | 0.264 | 6 |
| Estrogen receptor 2 (ESR2) | 0.00985945 | 0.35791757 | 6 |
| Multidrug resistance-associated protein 1 (ABCC1) | 0.01345788 | 0.37585421 | 6 |
| ATP-binding cassette sub-family G member 2 (ABCG2) | 0.01657 | 0.37078652 | 5 |
| Estrogen receptor 1 (ESR1) | 0.01515971 | 0.2519084 | 4 |
| Cyclin-dependent kinase 1 (CDK1) | 0.00317045 | 0.36423841 | 4 |
| Targets | Betweenness Centrality | Closeness Centrality | Degree |
|---|---|---|---|
| Tyrosine kinase Src (SRC) | 0.27275215 | 0.48369565 | 29 |
| Epidermal growth factor receptor (EGFR) | 0.1388039 | 0.44278607 | 22 |
| Serine/threonine-protein kinase AKT (AKT1) | 0.17437856 | 0.45641026 | 21 |
| Phosphoinositide-3-kinase regulatory subunit 1 (PIK3R1) | 0.05651908 | 0.41588785 | 20 |
| Tyrosine-protein phosphatase non-receptor type 11 (PTPN11) | 0.0334887 | 0.38695652 | 19 |
| Estrogen receptor α (ESR1) | 0.15395677 | 0.41588785 | 15 |
| Androgen receptor (AR) | 0.2042839 | 0.41784038 | 12 |
| Aldo-keto reductase 1C3 (AKR1C3) | 0.20965192 | 0.33584906 | 11 |
| Tyrosine-protein phosphatase non-receptor type 1 (PTPN1) | 0.01003103 | 0.35742972 | 11 |
| Cyclin-dependent kinase 1 (CDK1) | 0.07792777 | 0.38864629 | 11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, N.; Liu, Y.; Xiong, P.; Zhang, Y.; Mo, J.; Huang, X.; Zhou, Y. Exploring the Underlying Mechanism of Ren-Shen-Bai-Du Powder for Treating Inflammatory Bowel Disease Based on Network Pharmacology and Molecular Docking. Pharmaceuticals 2022, 15, 1038. https://doi.org/10.3390/ph15091038
Jin N, Liu Y, Xiong P, Zhang Y, Mo J, Huang X, Zhou Y. Exploring the Underlying Mechanism of Ren-Shen-Bai-Du Powder for Treating Inflammatory Bowel Disease Based on Network Pharmacology and Molecular Docking. Pharmaceuticals. 2022; 15(9):1038. https://doi.org/10.3390/ph15091038
Chicago/Turabian StyleJin, Ni, Yao Liu, Peiyu Xiong, Yiyi Zhang, Jingwen Mo, Xiushen Huang, and Yi Zhou. 2022. "Exploring the Underlying Mechanism of Ren-Shen-Bai-Du Powder for Treating Inflammatory Bowel Disease Based on Network Pharmacology and Molecular Docking" Pharmaceuticals 15, no. 9: 1038. https://doi.org/10.3390/ph15091038





