CX08005, a Protein Tyrosine Phosphatase 1B Inhibitor, Attenuated Hepatic Lipid Accumulation and Microcirculation Dysfunction Associated with Nonalcoholic Fatty Liver Disease
Abstract
:1. Introduction
2. Results
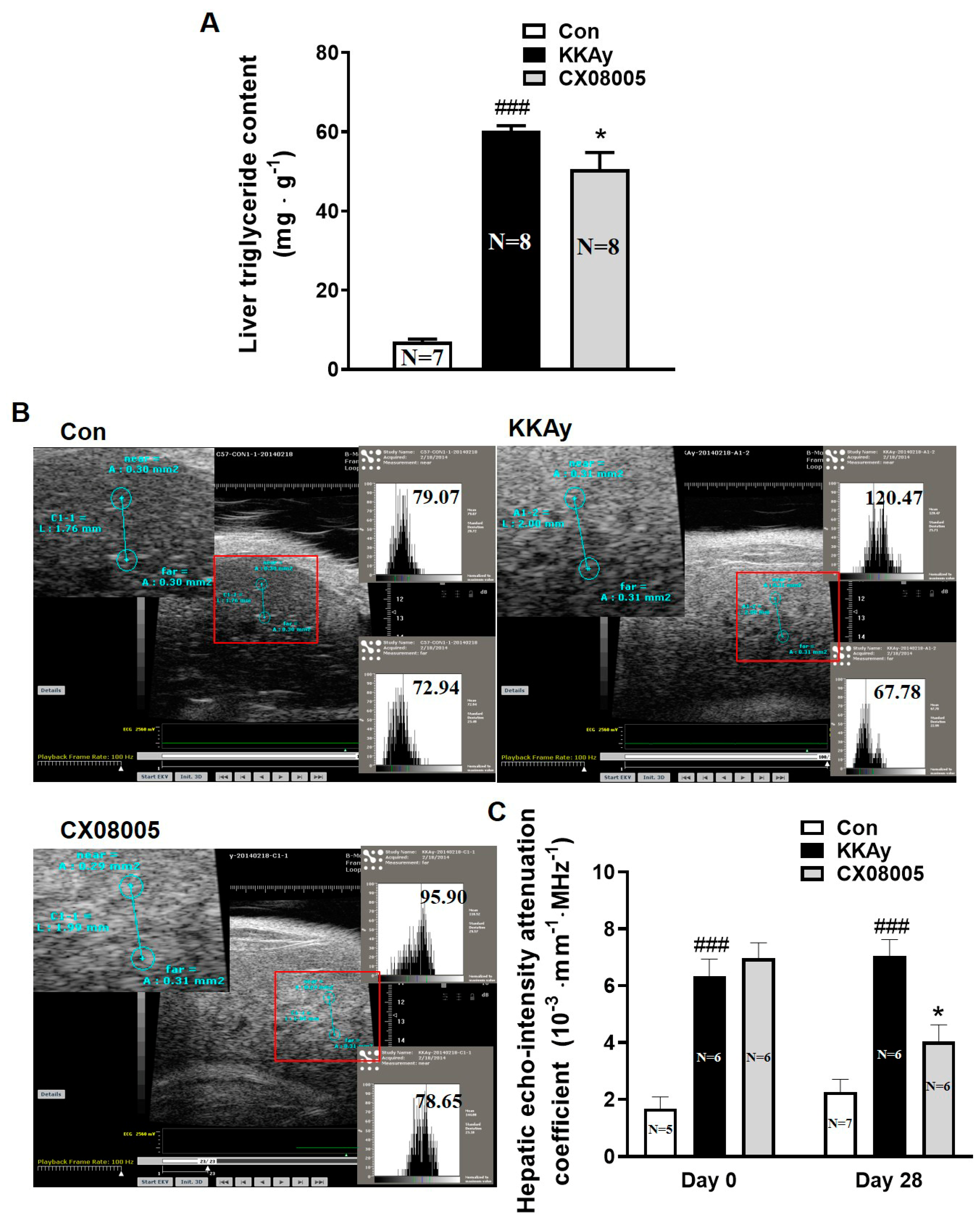
2.1. Attenuated the Hepatic Lipid Accumulation in Mice
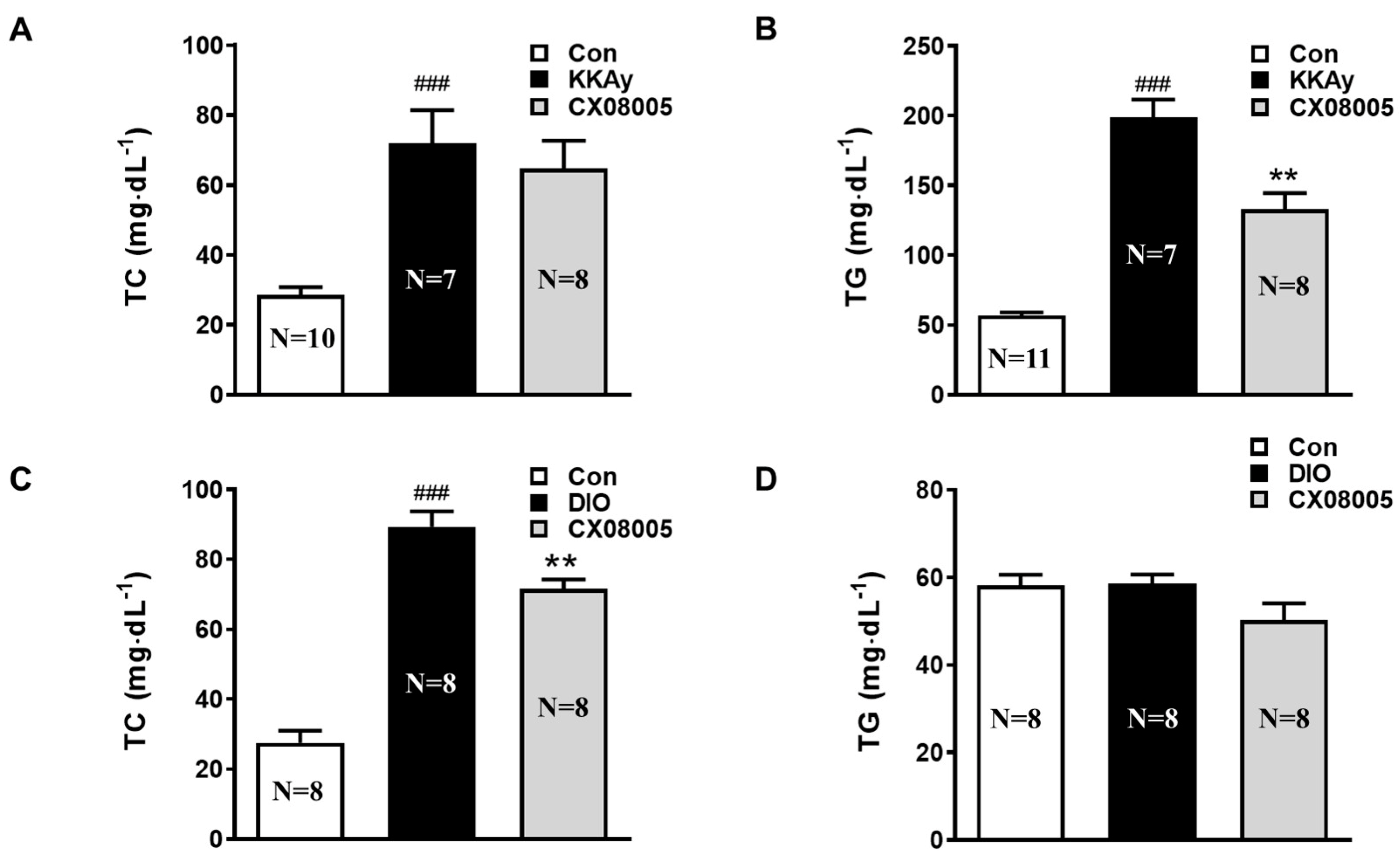
2.2. Improved the Dyslipidemia in Mice
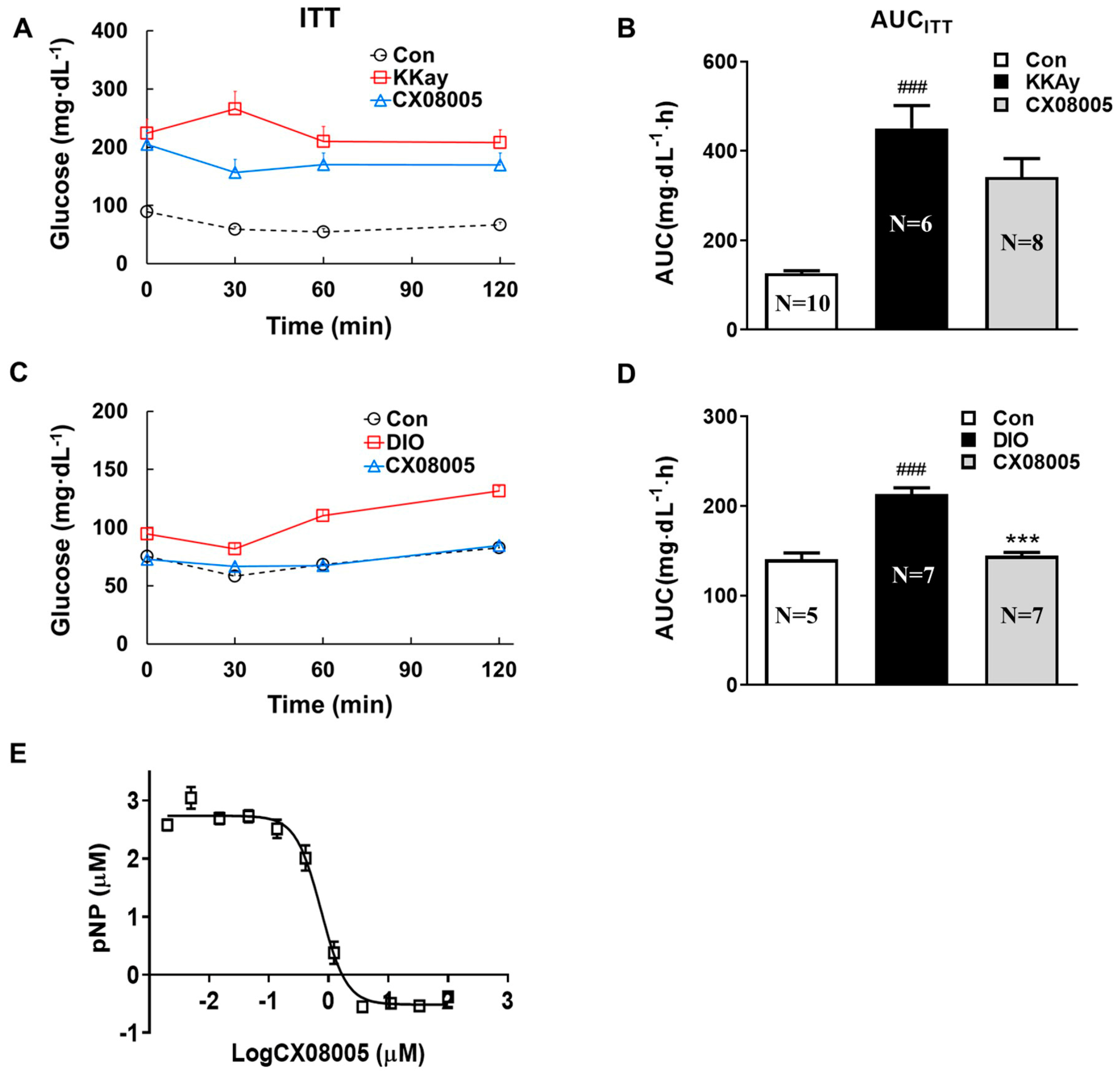
2.3. Effects on Insulin Response in Mice Targeting on PTP1B
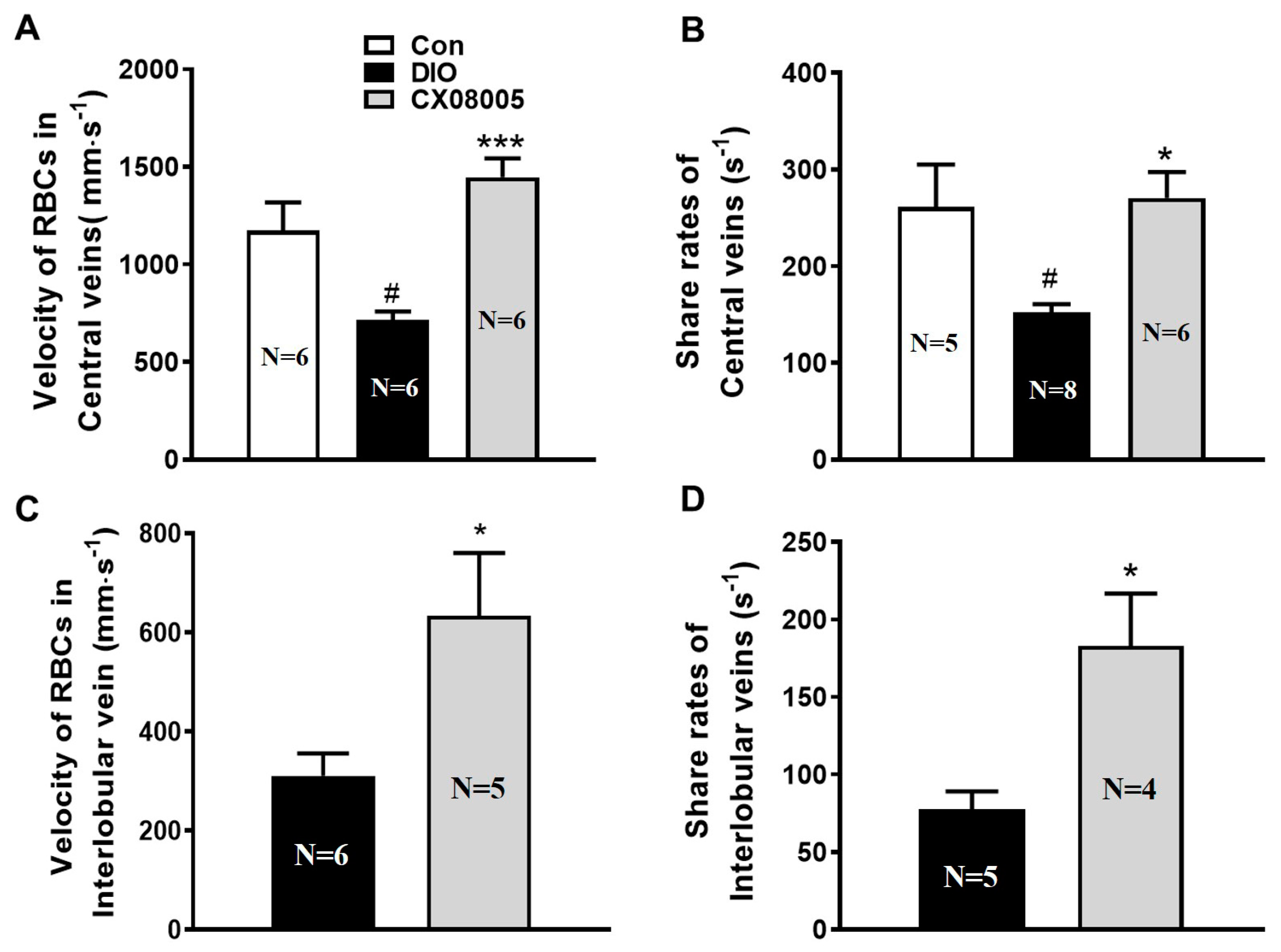
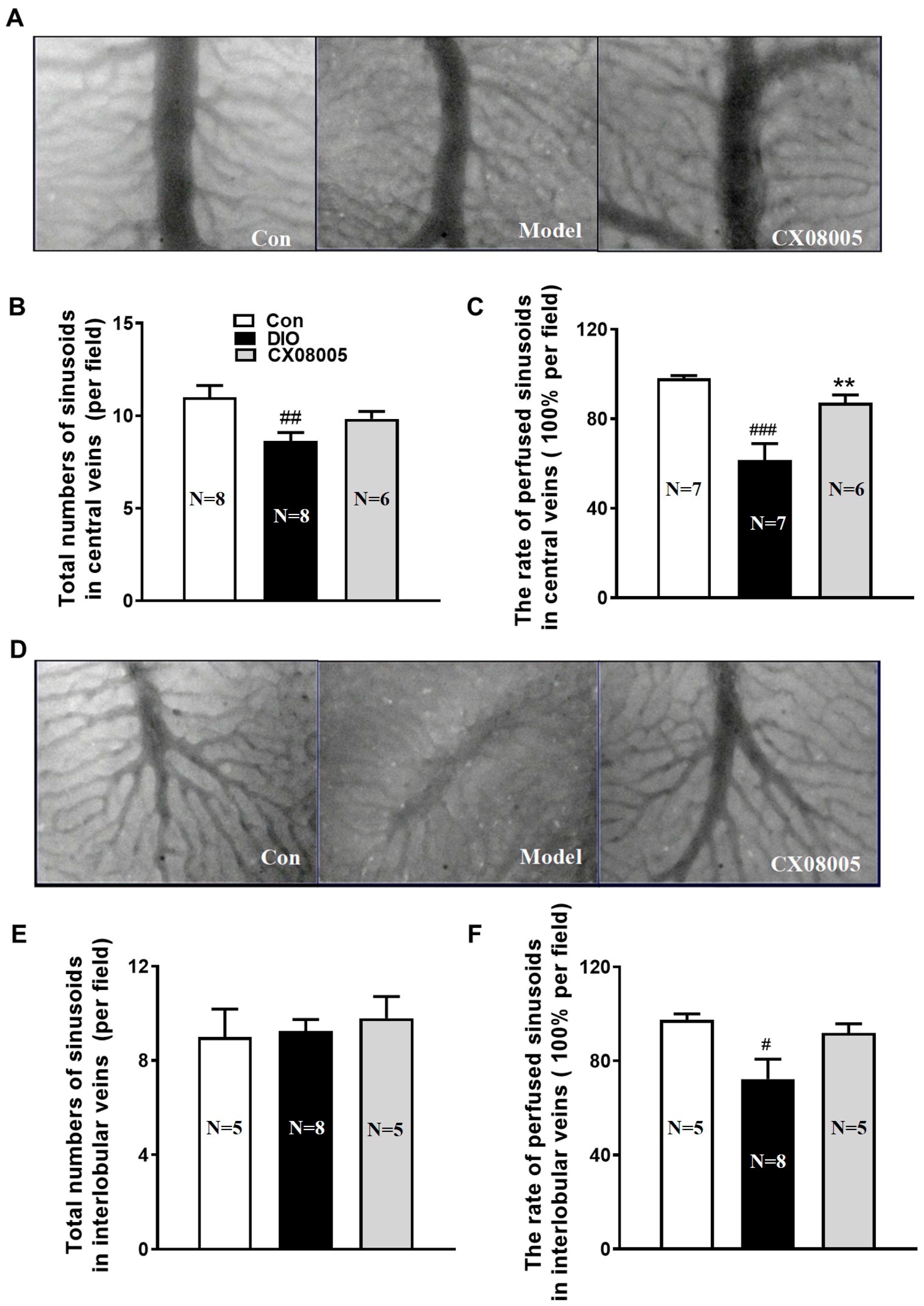
2.4. Ameliorating Hepatic Microcirculation Dysfunction in Mice
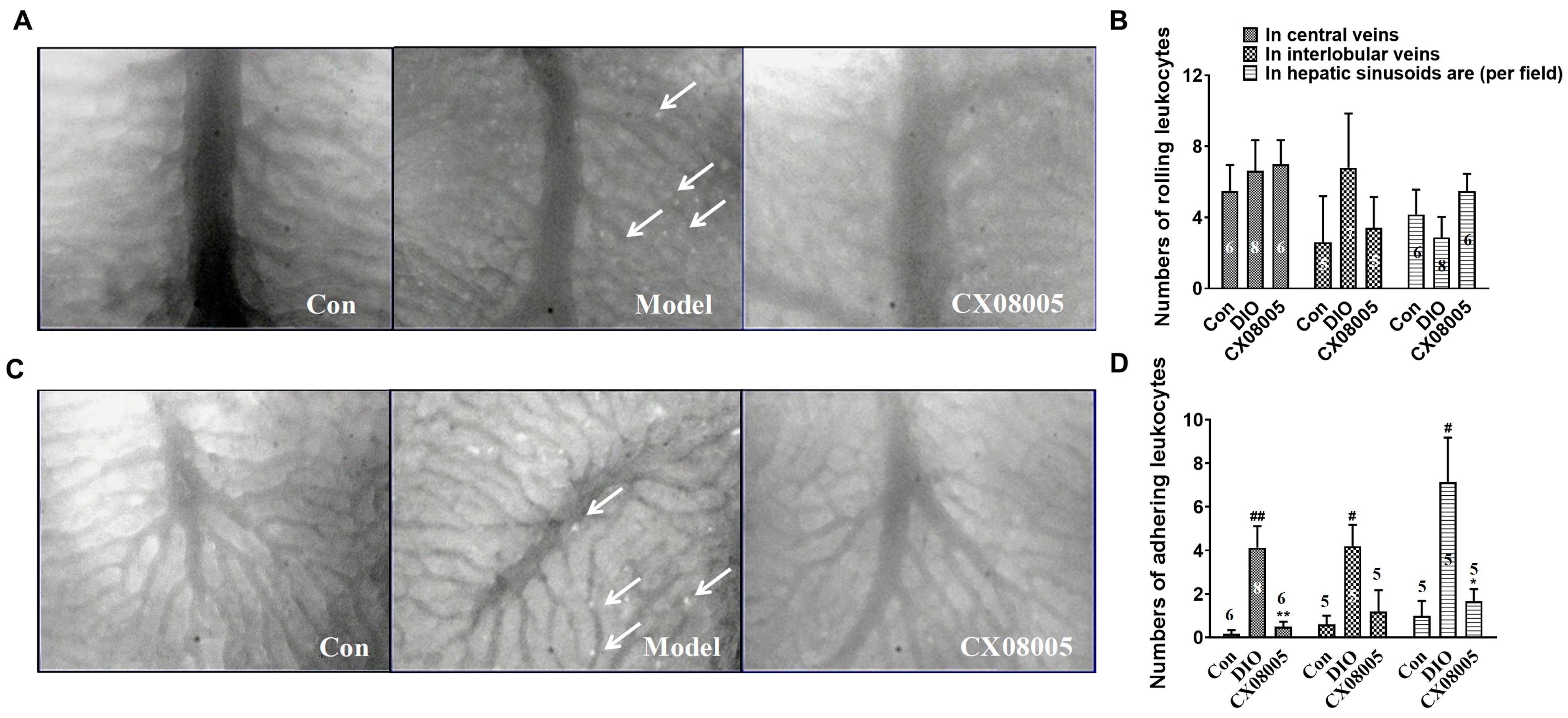
2.5. Attenuating Adhesion of Leukocytes to the Venular Wall in Mice
3. Discussion
4. Materials and Methods
4.1. PTP1B Inhibitory Activity
4.2. Animals and Drug Administration
4.3. Insulin Tolerance Tests
4.4. Determination of Triglycerides and Total Cholesterol
4.5. Hepatic Triglycerides Measurement
4.6. Ultrasound Analyses
4.7. Microcirculation Detection and Parameter Analysis
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Araújo, A.R.; Rosso, N.; Bedogni, G.; Tiribelli, C.; Bellentani, S. Global epidemiology of non-alcoholic fatty liver disease/non-alcoholic steatohepatitis: What we need in the future. Liver Int. 2018, 38 (Suppl. S1), 47–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazarus, J.V.; Mark, H.E.; Villota-Rivas, M.; Palayew, A.; Carrieri, P.; Colombo, M.; Ekstedt, M.; Esmat, G.; George, J.; Marchesini, G.; et al. The global NAFLD policy review and preparedness index: Are countries ready to address this silent public health challenge? J. Hepatol. 2022, 76, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanwal, F.; Kramer, J.R.; Mapakshi, S.; Natarajan, Y.; Chayanupatkul, M.; Richardson, P.A.; Li, L.; Desiderio, R.; Thrift, A.P.; Asch, S.M.; et al. Risk of Hepatocellular Cancer in Patients with Non-Alcoholic Fatty Liver Disease. Gastroenterology 2018, 155, 1828–1837. [Google Scholar] [CrossRef] [Green Version]
- Collaborators, G.D.a.I. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Canfora, E.E.; Meex, R.C.R.; Venema, K.; Blaak, E.E. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat. Rev. Endocrinol. 2019, 15, 261–273. [Google Scholar] [CrossRef]
- Baron, A.D. Hemodynamic actions of insulin. Am. J. Physiol. 1994, 267, E187–E202. [Google Scholar] [CrossRef] [Green Version]
- Liang, C.; Doherty, J.U.; Faillace, R.; Maekawa, K.; Arnold, S.; Gavras, H.; Hood, W.B., Jr. Insulin infusion in conscious dogs. Effects on systemic and coronary hemodynamics, regional blood flows, and plasma catecholamines. J. Clin. Investig. 1982, 69, 1321–1336. [Google Scholar] [CrossRef] [Green Version]
- Vincent, M.A.; Clerk, L.H.; Lindner, J.R.; Klibanov, A.L.; Clark, M.G.; Rattigan, S.; Barrett, E.J. Microvascular recruitment is an early insulin effect that regulates skeletal muscle glucose uptake in vivo. Diabetes 2004, 53, 1418–1423. [Google Scholar] [CrossRef] [Green Version]
- Postic, C.; Girard, J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: Lessons from genetically engineered mice. J Clin Investig. 2008, 118, 829–838. [Google Scholar] [CrossRef]
- Cersosimo, E.; DeFronzo, R.A. Insulin resistance and endothelial dysfunction: The road map to cardiovascular diseases. Diabetes Metab. Res. Rev. 2006, 22, 423–436. [Google Scholar] [CrossRef]
- Pereira, E.; Silvares, R.R.; Flores, E.E.I.; Rodrigues, K.L.; Daliry, A. Pyridoxamine improves metabolic and microcirculatory complications associated with nonalcoholic fatty liver disease. Microcirculation 2020, 27, e12603. [Google Scholar] [CrossRef] [PubMed]
- Rosenstengel, S.; Stoeppeler, S.; Bahde, R.; Spiegel, H.U.; Palmes, D. Type of steatosis influences microcirculation and fibrogenesis in different rat strains. J. Investig. Surg. 2011, 24, 273–282. [Google Scholar] [CrossRef]
- Schleicher, J.; Guthke, R.; Dahmen, U.; Dirsch, O.; Holzhuetter, H.G.; Schuster, S. A theoretical study of lipid accumulation in the liver-implications for nonalcoholic fatty liver disease. Biochim. Biophys. Acta 2014, 1841, 62–69. [Google Scholar] [CrossRef]
- Seely, B.L.; Staubs, P.A.; Reichart, D.R.; Berhanu, P.; Milarski, K.L.; Saltiel, A.R.; Kusari, J.; Olefsky, J.M. Protein tyrosine phosphatase 1B interacts with the activated insulin receptor. Diabetes 1996, 45, 1379–1385. [Google Scholar] [CrossRef]
- Zabolotny, J.M.; Bence-Hanulec, K.K.; Stricker-Krongrad, A.; Haj, F.; Wang, Y.; Minokoshi, Y.; Kim, Y.B.; Elmquist, J.K.; Tartaglia, L.A.; Kahn, B.B.; et al. PTP1B regulates leptin signal transduction in vivo. Dev. Cell 2002, 2, 489–495. [Google Scholar] [CrossRef] [Green Version]
- Owen, C.; Lees, E.K.; Grant, L.; Zimmer, D.J.; Mody, N.; Bence, K.K.; Delibegović, M. Inducible liver-specific knockdown of protein tyrosine phosphatase 1B improves glucose and lipid homeostasis in adult mice. Diabetologia 2013, 56, 2286–2296. [Google Scholar] [CrossRef] [Green Version]
- Revuelta-Cervantes, J.; Mayoral, R.; Miranda, S.; González-Rodríguez, A.; Fernández, M.; Martín-Sanz, P.; Valverde, A.M. Protein Tyrosine Phosphatase 1B (PTP1B) deficiency accelerates hepatic regeneration in mice. Am. J. Pathol. 2011, 178, 1591–1604. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, J.; Li, J.; Huang, L.; Wu, S.; Liang, W.; Zhong, L.; Ye, J.; Ye, F. A novel protein tyrosine phosphatase 1B inhibitor with therapeutic potential for insulin resistance. Br. J. Pharmacol. 2016, 173, 1939–1949. [Google Scholar] [CrossRef] [Green Version]
- Pereira, E.; Silvares, R.R.; Flores, E.E.I.; Rodrigues, K.L.; Ramos, I.P.; da Silva, I.J.; Machado, M.P.; Miranda, R.A.; Pazos-Moura, C.C.; Gonçalves-de-Albuquerque, C.F.; et al. Hepatic microvascular dysfunction and increased advanced glycation end products are components of non-alcoholic fatty liver disease. PLoS ONE 2017, 12, e0179654. [Google Scholar] [CrossRef]
- Engin, A. The Pathogenesis of Obesity-Associated Adipose Tissue Inflammation. Adv. Exp. Med. Biol. 2017, 960, 221–245. [Google Scholar] [CrossRef] [PubMed]
- Marušić, M.; Paić, M.; Knobloch, M.; Liberati Pršo, A.M. NAFLD, Insulin Resistance, and Diabetes Mellitus Type 2. Can. J. Gastroenterol. Hepatol. 2021, 2021, 6613827. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.S.; Bril, F.; Cusi, K.; Newsome, P.N. Modulation of Insulin Resistance in Nonalcoholic Fatty Liver Disease. Hepatology 2019, 70, 711–724. [Google Scholar] [CrossRef] [Green Version]
- Powell, E.E.; Wong, V.W.; Rinella, M. Non-alcoholic fatty liver disease. Lancet 2021, 397, 2212–2224. [Google Scholar] [CrossRef]
- Nasiri-Ansari, N.; Androutsakos, T.; Flessa, C.M.; Kyrou, I.; Siasos, G.; Randeva, H.S.; Kassi, E.; Papavassiliou, A.G. Endothelial Cell Dysfunction and Nonalcoholic Fatty Liver Disease (NAFLD): A Concise Review. Cells 2022, 11, 2511. [Google Scholar] [CrossRef]
- Vickers, S.P.; Jackson, H.C.; Cheetham, S.C. The utility of animal models to evaluate novel anti-obesity agents. Br. J. Pharmacol. 2011, 164, 1248–1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lutz, T.A. An Overview of Rodent Models of Obesity and Type 2 Diabetes. Methods Mol. Biol. 2020, 2128, 11–24. [Google Scholar] [CrossRef]
- Sakuma, T.; Nakamura, M.; Chiba, T.; Iwanaga, T.; Kan, M.; Kojima, R.; Ao, J.; Ma, Y.; Unozawa, H.; Fujita, N.; et al. A diet-induced murine model for non-alcoholic fatty liver disease with obesity and insulin resistance that rapidly develops steatohepatitis and fibrosis. Lab. Investig. 2022, 102, 1150–1157. [Google Scholar] [CrossRef]
- Zhang, B.; Ding, F.; Chen, T.; Xia, L.H.; Qian, J.; Lv, G.Y. Ultrasound hepatic/renal ratio and hepatic attenuation rate for quantifying liver fat content. World J. Gastroenterol. 2014, 20, 17985–17992. [Google Scholar] [CrossRef]
- Kwon, H.J.; Kim, K.W.; Lee, S.J.; Kim, S.Y.; Lee, J.S.; Kim, H.J.; Song, G.W.; Kim, S.A.; Yu, E.S.; Lee, J.; et al. Value of the ultrasound attenuation index for noninvasive quantitative estimation of hepatic steatosis. J. Ultrasound. Med. 2013, 32, 229–235. [Google Scholar] [CrossRef]
- Li, M.H.; Zhang, X.L.; Ye, F. Establishment of an ultrasonographic method for evaluating the hepatic lipid accumulation in mice. Yao Xue Xue Bao 2014, 49, 1395–1399. [Google Scholar] [PubMed]
- Yi, Q.; Sun, P.; Li, J.; Kong, S.; Tian, J.; Li, X.; Yang, Y.; Zhang, P.; Liu, Y.; Han, J.; et al. Rho, a Fraction From Rhodiola crenulate, Ameliorates Hepatic Steatosis in Mice Models. Front Physiol. 2018, 9, 222. [Google Scholar] [CrossRef]
- Angulo, P. Nonalcoholic fatty liver disease. N. Engl. J. Med. 2002, 346, 1221–1231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bugianesi, E.; Moscatiello, S.; Ciaravella, M.F.; Marchesini, G. Insulin resistance in nonalcoholic fatty liver disease. Curr. Pharm. Des. 2010, 16, 1941–1951. [Google Scholar] [CrossRef]
- Thiebaut, P.A.; Delile, E.; Coquerel, D.; Brunel, J.M.; Renet, S.; Tamion, F.; Richard, V. Protein tyrosine phosphatase 1B regulates endothelial endoplasmic reticulum stress; role in endothelial dysfunction. Vascul. Pharmacol. 2018, 109, 36–44. [Google Scholar] [CrossRef]
- Maupoint, J.; Besnier, M.; Gomez, E.; Bouhzam, N.; Henry, J.P.; Boyer, O.; Nicol, L.; Mulder, P.; Martinet, J.; Richard, V. Selective Vascular Endothelial Protection Reduces Cardiac Dysfunction in Chronic Heart Failure. Circ. Heart Fail 2016, 9, e002895. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.I.; Ketsawatsomkron, P.; Belin de Chantemele, E.J.; Mintz, J.D.; Muta, K.; Salet, C.; Black, S.M.; Tremblay, M.L.; Fulton, D.J.; Marrero, M.B.; et al. Deletion of protein tyrosine phosphatase 1b improves peripheral insulin resistance and vascular function in obese, leptin-resistant mice via reduced oxidant tone. Circ. Res. 2009, 105, 1013–1022. [Google Scholar] [CrossRef]
- Herren, D.J.; Norman, J.B.; Anderson, R.; Tremblay, M.L.; Huby, A.C.; Belin de Chantemèle, E.J. Deletion of Protein Tyrosine Phosphatase 1B (PTP1B) Enhances Endothelial Cyclooxygenase 2 Expression and Protects Mice from Type 1 Diabetes-Induced Endothelial Dysfunction. PLoS ONE 2015, 10, e0126866. [Google Scholar] [CrossRef] [Green Version]
- Villanova, N.; Moscatiello, S.; Ramilli, S.; Bugianesi, E.; Magalotti, D.; Vanni, E.; Zoli, M.; Marchesini, G. Endothelial dysfunction and cardiovascular risk profile in nonalcoholic fatty liver disease. Hepatology 2005, 42, 473–480. [Google Scholar] [CrossRef]
- Seifalian, A.M.; Chidambaram, V.; Rolles, K.; Davidson, B.R. In vivo demonstration of impaired microcirculation in steatotic human liver grafts. Liver Transpl. Surg. 1998, 4, 71–77. [Google Scholar] [CrossRef]
- McCuskey, R.S.; Ito, Y.; Robertson, G.R.; McCuskey, M.K.; Perry, M.; Farrell, G.C. Hepatic microvascular dysfunction during evolution of dietary steatohepatitis in mice. Hepatology 2004, 40, 386–393. [Google Scholar] [CrossRef]
- Zhang, L.; Bansal, M.B. Role of Kupffer Cells in Driving Hepatic Inflammation and Fibrosis in HIV Infection. Front Immunol. 2020, 11, 1086. [Google Scholar] [CrossRef]
- Higashi, T.; Friedman, S.L.; Hoshida, Y. Hepatic stellate cells as key target in liver fibrosis. Adv. Drug Deliv. Rev. 2017, 121, 27–42. [Google Scholar] [CrossRef]
- Liu, R.; Mathieu, C.; Berthelet, J.; Zhang, W.; Dupret, J.M.; Rodrigues Lima, F. Human Protein Tyrosine Phosphatase 1B (PTP1B): From Structure to Clinical Inhibitor Perspectives. Int. J. Mol. Sci. 2022, 23, 7027. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Yamagishi, Y.; Horie, Y.; Kato, S.; Kajihara, M.; Tamai, H.; Granger, D.N.; Ishii, H. Ethanol modulates gut ischemia/reperfusion-induced liver injury in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 282, G640–G646. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.X.; Wang, F.; Liu, Y.Y.; Zeng, Q.J.; Sun, K.; Xue, X.; Li, X.; Yang, J.Y.; An, L.H.; Hu, B.H.; et al. Effect of notoginsenoside R1 on hepatic microcirculation disturbance induced by gut ischemia and reperfusion. World J. Gastroenterol. 2008, 14, 29–37. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Zhang, X.; Tian, J.; Li, J.; Li, X.; Wu, S.; Liu, Y.; Han, J.; Ye, F. CX08005, a Protein Tyrosine Phosphatase 1B Inhibitor, Attenuated Hepatic Lipid Accumulation and Microcirculation Dysfunction Associated with Nonalcoholic Fatty Liver Disease. Pharmaceuticals 2023, 16, 106. https://doi.org/10.3390/ph16010106
Li J, Zhang X, Tian J, Li J, Li X, Wu S, Liu Y, Han J, Ye F. CX08005, a Protein Tyrosine Phosphatase 1B Inhibitor, Attenuated Hepatic Lipid Accumulation and Microcirculation Dysfunction Associated with Nonalcoholic Fatty Liver Disease. Pharmaceuticals. 2023; 16(1):106. https://doi.org/10.3390/ph16010106
Chicago/Turabian StyleLi, Jiang, Xiaolin Zhang, Jinying Tian, Juan Li, Xuechen Li, Song Wu, Yuying Liu, Jingyan Han, and Fei Ye. 2023. "CX08005, a Protein Tyrosine Phosphatase 1B Inhibitor, Attenuated Hepatic Lipid Accumulation and Microcirculation Dysfunction Associated with Nonalcoholic Fatty Liver Disease" Pharmaceuticals 16, no. 1: 106. https://doi.org/10.3390/ph16010106




