Association of ABCB1 Polymorphisms with Efficacy and Adverse Drug Reactions of Valproic Acid in Children with Epilepsy
Abstract
:1. Introduction
2. Results
2.1. Clinical Characteristics of Children with Epilepsy
2.2. The Association of ABCB1 Polymorphisms with VPA Responsiveness in Children with Epilepsy
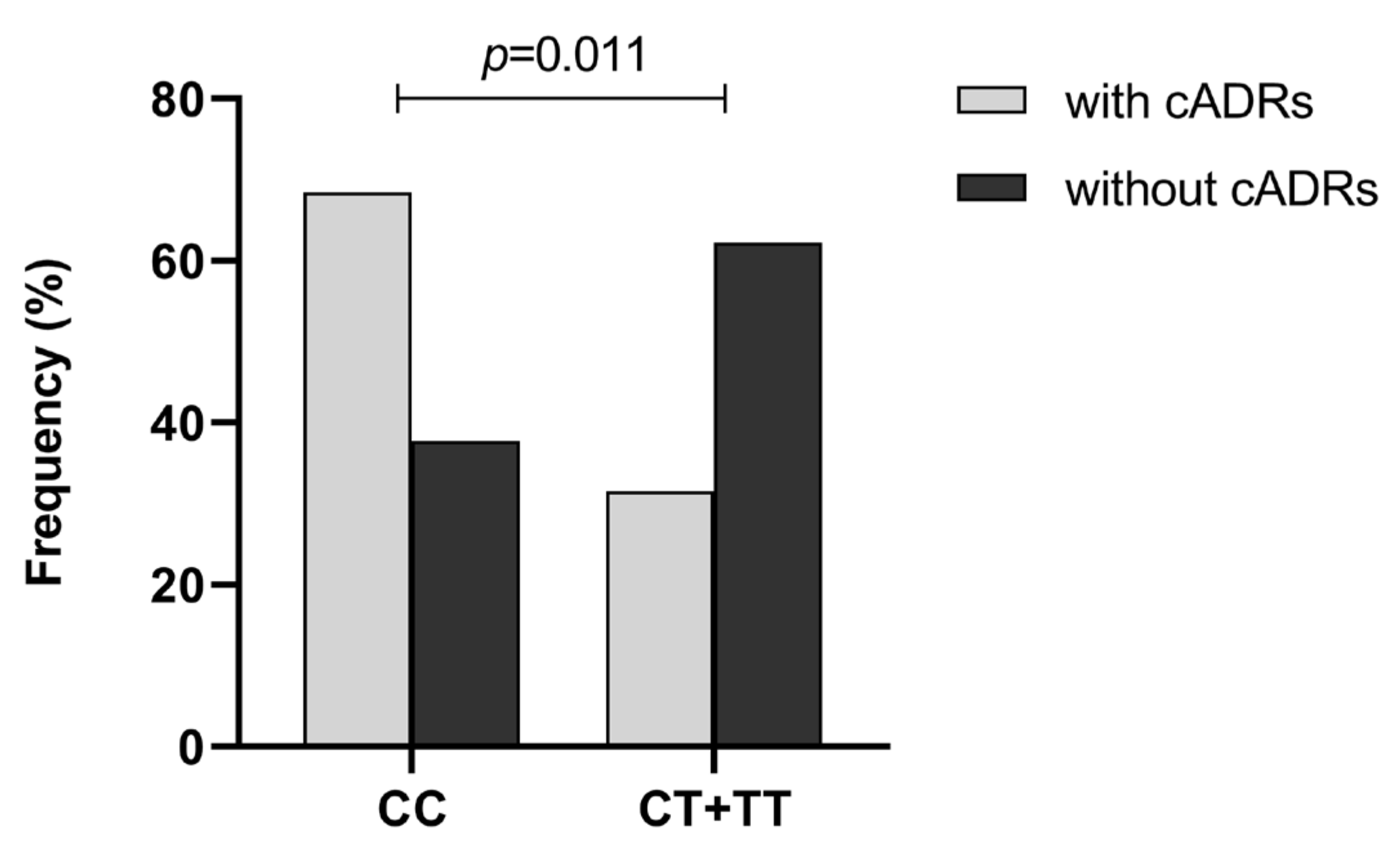
2.3. The Association of ABCB1 Polymorphisms with VPA-Induced ADRs in Children with Epilepsy
2.4. The Association between ABCB1 Polymorphisms and Serum Concentrations of VPA
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Determination of VPA Serum Concentration
4.3. Drug Response and ADRs Evaluation
4.4. SNP Selection and Genotyping
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Thijs, R.D.; Surges, R.; O’Brien, T.J.; Sander, J.W. Epilepsy in adults. Lancet 2019, 393, 689–701. [Google Scholar] [CrossRef]
- Ding, D.; Zhou, D.; Sander, J.W.; Wang, W.Z.; Li, S.C.; Hong, Z. Epilepsy in China: Major progress in the past two decades. Lancet Neurol. 2021, 20, 316–326. [Google Scholar] [CrossRef]
- Kwan, P.; Brodie, M.J. Early identification of refractory epilepsy. N. Engl. J. Med. 2000, 342, 314–319. [Google Scholar] [CrossRef]
- Devinsky, O.; Vezzani, A.; O’Brien, T.J.; Jette, N.; Scheffer, I.E.; de Curtis, M.; Perucca, P. Epilepsy. Nat. Rev. Dis. Primers 2018, 4, 24. [Google Scholar] [CrossRef]
- Pohlmann-Eden, B.; Weaver, D.F. The puzzle(s) of pharmacoresistant epilepsy. Epilepsia 2013, 54, 1–4. [Google Scholar] [CrossRef]
- Loscher, W.; Potschka, H.; Sisodiya, S.M.; Vezzani, A. Drug Resistance in Epilepsy: Clinical Impact, Potential Mechanisms, and New Innovative Treatment Options. Pharmacol. Rev. 2020, 72, 606–638. [Google Scholar] [CrossRef] [PubMed]
- Fricke-Galindo, I.; Jung-Cook, H.; Llerena, A.; Lopez-Lopez, M. Pharmacogenetics of adverse reactions to antiepileptic drugs. Neurologia 2018, 33, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Baker, G.A.; Jacoby, A.; Buck, D.; Stalgis, C.; Monnet, D. Quality of life of people with epilepsy: A European study. Epilepsia 1997, 38, 353–362. [Google Scholar] [CrossRef]
- Tomson, T.; Battino, D.; Perucca, E. Valproic acid after five decades of use in epilepsy: Time to reconsider the indications of a time-honoured drug. Lancet Neurol. 2016, 15, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Landmark, C.J.; Johannessen, S.I.; Patsalos, P.N. Therapeutic drug monitoring of antiepileptic drugs: Current status and future prospects. Expert Opin. Drug Metab. Toxicol. 2020, 16, 227–238. [Google Scholar] [CrossRef]
- Ghodke-Puranik, Y.; Thorn, C.F.; Lamba, J.K.; Leeder, J.S.; Song, W.; Birnbaum, A.K.; Altman, R.B.; Klein, T.E. Valproic acid pathway: Pharmacokinetics and pharmacodynamics. Pharmacogenet. Genom. 2013, 23, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.M.; Li, H.L.; Shi, L.H.; Chen, X.P.; Luo, J.; Zhang, Z.L. The pharmacogenomics of valproic acid. J. Hum. Genet. 2017, 62, 1009–1014. [Google Scholar] [CrossRef]
- Loscher, W.; Klotz, U.; Zimprich, F.; Schmidt, D. The clinical impact of pharmacogenetics on the treatment of epilepsy. Epilepsia 2009, 50, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.H.; Cho, Y.-J.; Kim, W.-J.; Lee, M.G.; Lee, J.H. Genetic Variations of ABCC2 Gene Associated with Adverse Drug Reactions to Valproic Acid in Korean Epileptic Patients. Genom. Inform. 2013, 11, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.X.; Mei, S.H.; Zhu, L.T.; Yu, Y.Z.; Yang, W.L.; Gao, B.Q.; Wu, X.J.; Zhao, Z.G.; Fang, F. Effects of UGT1A6 and GABRA1 on Standardized Valproic Acid Plasma Concentrations and Treatment Effect in Children With Epilepsy in China. Ther. Drug Monit. 2016, 38, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Su, Q.P.; Li, M.; Dayimu, A.; Dai, X.Y.; Wang, Z.H.; Che, F.Y.; Xue, F.Z. Association of SCN1A, SCN2A, and UGT2B7 Polymorphisms with Responsiveness to Valproic Acid in the Treatment of Epilepsy. Biomed Res. Int. 2020, 2020, 8096235. [Google Scholar] [CrossRef]
- Sauna, Z.E.; Kim, I.W.; Ambudkar, S.V. Genomics and the mechanism of P-glycoprotein (ABCB1). J. Bioenerg. Biomembr. 2007, 39, 481–487. [Google Scholar] [CrossRef]
- Tishler, D.M.; Weinberg, K.I.; Hinton, D.R.; Barbaro, N.; Annett, G.M.; Raffel, C. MDR1 gene expression in brain of patients with medically intractable epilepsy. Epilepsia 1995, 36, 1–6. [Google Scholar] [CrossRef]
- Rizzi, M.; Caccia, S.; Guiso, G.; Richichi, C.; Gorter, J.A.; Aronica, E.; Aliprandi, M.; Bagnati, R.; Fanelli, R.; D’Incalci, M.; et al. Limbic seizures induce P-glycoprotein in rodent brain: Functional implications for pharmacoresistance. J. Neurosci. 2002, 22, 5833–5839. [Google Scholar] [CrossRef]
- Kimchi-Sarfaty, C.; Oh, J.M.; Kim, I.W.; Sauna, Z.E.; Calcagno, A.M.; Ambudkar, S.V.; Gottesman, M.M. A “silent” polymorphism in the MDR1 gene changes substrate specificity. Science 2007, 315, 525–528. [Google Scholar] [CrossRef]
- Siddiqui, A.; Kerb, R.; Weale, M.E.; Brinkmann, U.; Smith, A.; Goldstein, D.B.; Wood, N.W.; Sisodiya, S.M. Association of multidrug resistance in epilepsy with a polymorphism in the drug-transporter gene ABCB1. N. Engl. J. Med. 2003, 348, 1442–1448. [Google Scholar] [CrossRef]
- Kwan, P.; Baum, L.; Wong, V.; Ng, P.W.; Lui, C.H.; Sin, N.C.; Hui, A.C.F.; Yu, E.; Wong, L.K.S. Association between ABCB1 C3435T polymorphism and drug-resistant epilepsy in han chinese. Epilepsy Behav. 2007, 11, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Seo, T.; Ishitsu, T.; Ueda, N.; Nakada, N.; Yurube, K.; Ueda, K.; Nakagawa, K. ABCB1 polymorphisms influence the response to antiepileptic drugs in Japanese epilepsy patients. Pharmacogenomics 2006, 7, 551–561. [Google Scholar] [CrossRef]
- Kwan, P.; Wong, V.; Ng, P.W.; Lui, C.H.T.; Sin, N.C.; Poon, W.S.; Ng, H.K.; Wong, K.S.; Baum, L. Gene-wide tagging study of association between ABCB1 polymorphisms and multidrug resistance in epilepsy in Han Chinese. Pharmacogenomics 2009, 10, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Chouchi, M.; Klaa, H.; Turki, I.B.Y.; Hila, L. ABCB1 Polymorphisms and Drug-Resistant Epilepsy in a Tunisian Population. Dis. Markers 2019, 2019, 1–16. [Google Scholar] [CrossRef]
- Shen, X.H.; Chen, X.Y.; Lu, J.L.; Chen, Q.; Li, W.Z.; Zhu, J.H.; He, Y.D.; Guo, H.J.; Xu, C.S.; Fan, X.M. Pharmacogenetics-based population pharmacokinetic analysis and dose optimization of valproic acid in Chinese southern children with epilepsy: Effect of ABCB1 gene polymorphism. Front. Pharmacol. 2022, 13, 14. [Google Scholar] [CrossRef] [PubMed]
- Adiga, M.N.S.; Nandit, P.B.; Adiga, U.; Shenoy, V. UGT2B7 gene polymorphism and linkage disequilibrium in pediatric epileptic patients and their influence on sodium valproate monotherapy: A cohort study. Front. Pharmacol. 2022, 13, 8. [Google Scholar] [CrossRef]
- Hung, C.C.; Tai, J.J.; Kao, P.J.; Lin, M.S.; Liou, H. Association of polymorphisms in NR1I2 and ABCB1 genes with epilepsy treatment responses. Pharmacogenomics 2007, 8, 1151–1158. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Wu, X.; Yan, H.; Zhang, Y.; He, R.H.; Tang, Y.J.; He, Y.J.; Tan, D.; Mao, X.Y.; et al. Polymorphisms of ABAT, SCN2A and ALDH5A1 may affect valproic acid responses in the treatment of epilepsy in Chinese. Pharmacogenomics 2016, 17, 2007–2014. [Google Scholar] [CrossRef]
- Marchi, N.; Hallene, K.L.; Kight, K.M.; Cucullo, L.; Moddel, G.; Bingaman, W.; Dini, G.; Vezzani, A.; Janigro, D. Significance of MDR1 and multiple drug resistance in refractory human epileptic brain. BMC Med. 2004, 2, 10. [Google Scholar] [CrossRef]
- Potschka, H.; Loscher, W. In vivo evidence for P-glycoprotein-mediated transport of phenytoin at the blood-brain barrier of rats. Epilepsia 2001, 42, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Potschka, H.; Fedrowitz, M.; Loscher, W. P-glycoprotein-mediated efflux of phenobarbital, lamotrigine, and felbamate at the blood-brain barrier: Evidence from microdialysis experiments in rats. Neurosci. Lett. 2002, 327, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Cerveny, L.; Svecova, L.; Anzenbacherova, E.; Vrzal, R.; Staud, F.; Dvorak, Z.; Ulrichova, J.; Anzenbacher, P.; Pavek, P. Valproic acid induces CYP3A4 and MDR1 gene expression by activation of constitutive androstane receptor and pregnane X receptor pathways. Drug Metab. Dispos. 2007, 35, 1032–1041. [Google Scholar] [CrossRef] [PubMed]
- Baltes, S.; Fedrowitz, M.; Tortos, C.L.; Potschka, H.; Loscher, W. Valproic acid is not a substrate for P-glycoprotein or multidrug resistance proteins 1 and 2 in a number of in vitro and in vivo transport assays. J. Pharmacol. Exp. Ther. 2007, 320, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Lovric, M.; Bozina, N.; Hajnsek, S.; Kuzman, M.R.; Sporis, D.; Lalic, Z.; Bozina, T.; Granic, P. Association Between Lamotrigine Concentrations and ABCB1 Polymorphisms in Patients With Epilepsy. Ther. Drug Monit. 2012, 34, 518–525. [Google Scholar] [CrossRef]
- Puranik, Y.G.; Birnbaum, A.K.; Marino, S.E.; Ahmed, G.; Cloyd, J.C.; Remmel, R.P.; Leppik, I.E.; Lamba, J.K. Association of carbamazepine major metabolism and transport pathway gene polymorphisms and pharmacokinetics in patients with epilepsy. Pharmacogenomics 2013, 14, 35–45. [Google Scholar] [CrossRef]
- Tamimi, D.E.; Abduljabbar, R.; Yousef, A.; Saeed, R.M.; Zawiah, M. Association between ABCB1 polymorphisms and response to antiepileptic drugs among Jordanian epileptic patients. Neurol. Res. 2021, 43, 724–735. [Google Scholar] [CrossRef]
- Dong, L.; Luo, R.; Tong, Y.; Cai, X.T.; Mao, M.; Yu, D. Lack of association between ABCB1 gene polymorphisms and pharmacoresistant epilepsy: An analysis in a western Chinese pediatric population. Brain Res. 2011, 1391, 114–124. [Google Scholar] [CrossRef]
- Escalante-Santiago, D.; Feria-Romero, I.A.; Ribas-Aparicio, R.M.; Rayo-Mares, D.; Fagiolino, P.; Vazquez, M.; Escamilla-Nunez, C.; Grijalva-Otero, I.; Lopez-Garcia, M.A.; Orozco-Suarez, S. MDR-1 and MRP2 gene polymorphisms in Mexican epileptic pediatric patients with complex partial seizures. Front. Neurol. 2014, 5, 11. [Google Scholar] [CrossRef]
- Fromm, M.F. The influence of MDR1 polymorphisms on P-glycoprotein expression and function in humans. Adv. Drug Deliv. Rev. 2002, 54, 1295–1310. [Google Scholar] [CrossRef]
- Salama, N.N.; Yang, Z.P.; Bui, T.; Ho, R.J.Y. MDR1 haplotypes significantly minimize intracellular uptake and transcellular P-gp substrate transport in recombinant LLC-PK1 cells. J. Pharm. Sci. 2006, 95, 2293–2308. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, B.; Chang, C.; Wu, M.H.; Xu, Y.; Jiang, Y.J. The Roles of Variants in Human Multidrug Resistance (MDR1) Gene and Their Haplotypes on Antiepileptic Drugs Response: A Meta-Analysis of 57 Studies. PLoS ONE 2015, 10, e0122043. [Google Scholar] [CrossRef] [PubMed]
- Zimprich, F.; Sunder-Plassmann, R.; Stogmann, E.; Gleiss, A.; Dal-Bianco, A.; Zimprich, A.; Plumer, S.; Baumgartner, C.; Mannhalter, C. Association of an ABCB1 gene haplotype with pharmacoresistance in temporal lobe epilepsy. Neurology 2004, 63, 1087–1089. [Google Scholar] [CrossRef]
- Perucca, P.; Carter, J.; Vahle, V.; Gilliam, F.G. Adverse antiepileptic drug effects Toward a clinically and neurobiologically relevant taxonomy. Neurology 2009, 72, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Ho, G.T.; Soranzo, N.; Nimmo, E.R.; Tenesa, A.; Goldstein, D.B.; Satsangi, J. ABCB1/MDR1 gene determines susceptibility and phenotype in ulcerative colitis: Discrimination of critical variants using a gene-wide haplotype tagging approach. Hum. Mol. Genet. 2006, 15, 797–805. [Google Scholar] [CrossRef]
- He, T.; Mo, A.; Zhang, K.; Liu, L. ABCB1/MDR1 gene polymorphism and colorectal cancer risk: A meta-analysis of case-control studies. Color. Dis. 2013, 15, 12–18. [Google Scholar] [CrossRef]
- Panwala, C.M.; Jones, J.C.; Viney, J.L. A novel model of inflammatory bowel disease: Mice deficient for the multiple drug resistance gene, mdr1a, spontaneously develop colitis. J. Immunol. 1998, 161, 5733–5744. [Google Scholar] [CrossRef]
- Vasku, V.; Machal, J.; Zlamal, F.; Vasku, A. Some New Aspects of Genetic Variability in Patients with Cutaneous T-Cell Lymphoma. Genes 2022, 13, 2401. [Google Scholar] [CrossRef]
- Munisamy, M.; Tripathi, M.; Behari, M.; Raghavan, S.; Jain, D.C.; Ramanujam, B.; Arumugam, K.; Rajakannan, T.; Mallayasamy, S.R.; Subbiah, V. The Effect of Uridine Diphosphate Glucuronosyltransferase (UGT)1A6 Genetic Polymorphism on Valproic Acid Pharmacokinetics in Indian Patients with Epilepsy: A Pharmacogenetic Approach. Mol. Diagn. Ther. 2013, 17, 319–326. [Google Scholar] [CrossRef]
- Zhao, M.M.; Zhang, T.; Li, G.F.; Qiu, F.; Sun, Y.X.; Zhao, L.M. Associations of CYP2C9 and CYP2A6 Polymorphisms with the Concentrations of Valproate and its Hepatotoxin Metabolites and Valproate-Induced Hepatotoxicity. Basic Clin. Pharmacol. Toxicol. 2017, 121, 138–143. [Google Scholar] [CrossRef]
- Ghosh, C.; Hossain, M.; Puvenna, V.; Martinez-Gonzalez, J.; Alexopolous, A.; Janigro, D.; Marchi, N. Expression and functional relevance of UGT1A4 in a cohort of human drug-resistant epileptic brains. Epilepsia 2013, 54, 1562–1570. [Google Scholar] [CrossRef]
- Ghosh, C.; Hossain, M.; Solanki, J.; Najm, I.M.; Marchi, N.; Janigro, D. Overexpression of pregnane X and glucocorticoid receptors and the regulation of cytochrome P450 in human epileptic brain endothelial cells. Epilepsia 2017, 58, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, I.E.; Berkovic, S.; Capovilla, G.; Connolly, M.B.; French, J.; Guilhoto, L.; Hirsch, E.; Jain, S.; Mathern, G.W.; Moshe, S.L.; et al. ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017, 58, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.N.; Zhao, L.M.; Liang, M.; Dong, Y.; Yun, W.T.; Qiu, F.; Meng, H.M.; Guo, Y.J. Effects of UGT2B7 Genetic Polymorphisms on Serum Concentrations of Valproic Acid in Chinese Children With Epilepsy Comedicated With Lamotrigine. Ther. Drug Monit. 2016, 38, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Kwan, P.; Arzimanoglou, A.; Berg, A.T.; Brodie, M.J.; Hauser, W.A.; Mathern, G.; Moshe, S.L.; Perucca, E.; Wiebe, S.; French, J. Definition of drug resistant epilepsy: Consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2010, 51, 1069–1077. [Google Scholar] [CrossRef]
- Fan, X.M.; Chen, Y.N.; Li, W.Z.; Xia, H.B.; Liu, B.; Guo, H.J.; Yang, Y.X.; Xu, C.S.; Xie, S.J.; Xu, X.Q. Genetic Polymorphism of ADORA2A Is Associated With the Risk of Epilepsy and Predisposition to Neurologic Comorbidity in Chinese Southern Children. Front. Neurosci. 2020, 14, 590605. [Google Scholar] [CrossRef]
- Lu, J.L.; Xia, H.B.; Li, W.Z.; Shen, X.H.; Guo, H.J.; Zhang, J.P.; Fan, X.M. Genetic Polymorphism of GABRG2 rs211037 is Associated with Drug Response and Adverse Drug Reactions to Valproic Acid in Chinese Southern Children with Epilepsy. Pharmacogn. Pers. Med. 2021, 14, 1141–1150. [Google Scholar] [CrossRef]
- Abe, T.; Seo, T.; Ishitsu, T.; Nakagawa, T.; Hori, M.; Nakagawa, K. Association between SCN1A polymorphism and carbamazepine-resistant epilepsy. Br. J. Clin. Pharmacol. 2008, 66, 304–307. [Google Scholar] [CrossRef]
- Shukla, A.K.; Jhaj, R.; Misra, S.; Ahmed, S.N.; Nanda, M.; Chaudhary, D. Agreement between WHO-UMC causality scale and the Naranjo algorithm for causality assessment of adverse drug reactions. J. Fam. Med. Prim. Care 2021, 10, 3303–3308. [Google Scholar] [CrossRef]
- Fan, X.M.; Chen, Y.A.; Lu, J.L.; Li, W.Z.; Li, X.; Guo, H.J.; Chen, Q.; Yang, Y.X.; Xia, H.B. AS3MT Polymorphism: A Risk Factor for Epilepsy Susceptibility and Adverse Drug Reactions to Valproic Acid and Oxcarbazepine Treatment in Children From South China. Front. Neurosci. 2021, 15, 705297. [Google Scholar] [CrossRef]
- Loparev, V.N.; Cartas, M.A.; Monken, C.E.; Velpandi, A.; Srinivasan, A. An efficient and simple method of DNA extraction from whole blood and cell lines to identify infectious agents. J. Virol. Methods 1991, 34, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.Y.; He, L. SHEsis, a powerful software platform for analyses of linkage disequilibrium, haplotype construction, and genetic association at polymorphism loci. Cell Res. 2005, 15, 97–98. [Google Scholar] [CrossRef] [PubMed]


| Demographic Data | Total (n = 170) | Persistent Seizure (n = 61) | Seizure Free (n = 109) | p-Value 3 |
|---|---|---|---|---|
| Age of initial diagnosis | ||||
| 1 month–2 years | 68 (40.0%) | 27 (44.3%) | 41 (37.6%) | 0.40 |
| 2–16 years | 102 (60.0%) | 34 (55.7%) | 68 (62.4%) | |
| Median (quartile 1–3) | 3.42 (1.00–6.44) | 2.50 (1.00–5.17) | 3.58 (1.08–7.04) | 0.11 |
| Gender | ||||
| Male | 102 (60.0%) | 38 (62.3%) | 64 (58.7%) | 0.65 |
| Female | 68 (40.0%) | 23 (37.7%) | 45 (41.3%) | |
| Seizure type | ||||
| Generalized onset | 103 (60.6%) | 36 (59.0%) | 67 (61.5%) | 0.89 |
| Focal onset | 52 (30.6%) | 20 (32.8%) | 32 (29.4%) | |
| Unknown onset | 15 (8.8%) | 5 (8.2%) | 10 (9.2%) | |
| Subtype of epilepsy | ||||
| Generalized tonic-clonic seizure | 63 (37.1%) | 23 (37.7%) | 40 (36.7%) | 0.90 |
| Tonic seizure | 5 (2.9%) | 0 (0.0%) | 5 (4.6%) | 0.16 |
| Clonic seizure | 9 (5.3%) | 1 (1.6%) | 8 (7.3%) | 0.22 |
| Myoclonic seizure | 3 (1.8%) | 2 (3.3%) | 1 (0.9%) | 0.61 |
| Childhood absence epilepsy | 12 (7.1%) | 4 (6.6%) | 8 (7.3%) | 1.00 |
| Focal seizures | 26 (15.3%) | 9 (14.8%) | 17 (15.6%) | 0.88 |
| Benign epilepsy in childhood with centrotemporal spikes | 17 (10.0%) | 5 (8.2%) | 12 (11.0%) | 0.56 |
| Focal to bilateral tonic-clonic seizure | 4 (2.4%) | 2 (3.3%) | 2 (1.8%) | 0.95 |
| Others | 31 (18.2%) | 15 (24.6%) | 16 (14.7%) | 0.11 |
| Serum concentration of VPA (µg/mL) | ||||
| Mean ± SD | 62.68 ± 22.46 | 60.79 ± 23.00 | 63.69 ± 22.15 | 0.22 |
| ASM therapy | ||||
| VPA monotherapy | 91 (53.5%) | 15 (24.6%) | 76 (69.7%) | <0.01 * |
| Polytherapy | 79 (46.5%) 1 | 46 (75.4%) | 33 (30.3%) | |
| Levetiracetam | 45 (26.5%) | 30 (49.2%) | 15 (13.8%) | |
| Oxcarbazepine | 34 (20.0%) | 18 (29.5%) | 16 (14.7%) | |
| Topiramate | 10 (5.9%) | 10 (16.4%) | 0 (0.0%) | |
| Lamotrigine | 7 (4.1%) | 5 (8.2%) | 2 (1.8%) | |
| Clonazepam | 3 (1.8%) | 0 (0.0%) | 3 (2.8%) | |
| Carbamazepine | 2 (1.2%) | 2 (3.3%) | 0 (0.0%) | |
| Adverse drug reactions | ||||
| With ADRs | 123 (72.4%) | 42 (68.9%) | 81 (74.3%) | 0.45 |
| Without ADRs | 47 (27.6%) | 19 (31.1%) | 28 (25.7%) | |
| Gastrointestinal ADRs | 63 (31.8%) 2 | |||
| Neurological ADRs | 60 (30.3%) | |||
| Weight gain | 48 (24.3%) | |||
| Rash | 27 (13.6%) |
| Demographic Data | ABCB1 rs1128503 | p-Value | ABCB1 rs3789243 | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| CC | CT | TT | CC | CT | TT | |||
| Age of initial diagnosis | ||||||||
| 1 month–2 years | 8 (11.8%) | 30 (44.1%) | 30 (44.1%) | 0.445 | 30 (44.1%) | 32 (47.1%) | 6 (8.8%) | 0.668 |
| 2–16 years | 9 (8.8%) | 55 (53.9%) | 38 (37.3%) | 40 (39.2%) | 49 (48.0%) | 13 (12.7%) | ||
| Gender | ||||||||
| Male | 11 (10.8%) | 46 (45.1%) | 45 (44.1%) | 0.292 | 42 (41.2%) | 50 (49.0%) | 10 (9.8%) | 0.767 |
| Female | 6 (8.8%) | 39 (57.4%) | 23 (33.8%) | 28 (41.2%) | 31 (45.6%) | 9 (13.2%) | ||
| Seizure type | ||||||||
| Generalized onset | 11 (10.7%) | 47 (45.6%) | 45 (43.7%) | 0.298 | 45 (43.7%) | 45 (43.7%) | 13 (12.6%) | 0.363 |
| Focal onset | 3 (5.8%) | 30 (57.7%) | 19 (36.5%) | 18 (34.6%) | 29 (55.8%) | 5 (9.6%) | ||
| ASM therapy | ||||||||
| VPA monotherapy | 11 (12.1%) | 45 (49.5%) | 35 (38.5%) | 0.612 | 37 (40.7%) | 43 (47.3%) | 11 (12.1%) | 0.921 |
| Polytherapy | 6 (7.6%) | 40 (50.6%) | 33 (41.8%) | 33 (41.8%) | 38 (48.1%) | 8 (10.1%) | ||
| ADRs | ||||||||
| With ADRs | 13 (10.6%) | 64 (52.0%) | 46 (37.4%) | 0.534 | 52 (42.3%) | 57 (46.3%) | 14 (11.4%) | 0.858 |
| Without ADRs | 4 (8.5%) | 21 (44.7%) | 22 (46.8%) | 18 (38.3%) | 24 (51.1%) | 5 (10.6%) | ||
| Genetic Model | Genotype | Persistent Seizure (n = 61) | Seizure-Free (n = 109) | OR (95% CI) | p-Value |
|---|---|---|---|---|---|
| Allele contrast | C vs. T | 34 (27.9%) | 85 (39.0%) | 1.00 | 0.039 * |
| 88 (72.1%) | 133 (61.0%) | 0.60 (0.37–0.98) | |||
| Codominant | CC vs. CT vs. TT | 5 (8.2%) | 12 (11.0%) | 1.00 | 0.047 * |
| 24 (39.3%) | 61 (56.0%) | 1.06 (0.34–3.33) | |||
| 32 (52.5%) | 36 (33.0%) | 0.47 (0.15–1.48) | |||
| Dominant | CC vs. CT + TT | 5 (8.2%) | 12 (11.0%) | 1.00 | 0.550 |
| 56 (91.8%) | 97 (89.0%) | 0.72 (0.24–2.16) | |||
| Recessive | CC + CT vs. TT | 29 (47.5%) | 73 (67.0%) | 1.00 | 0.013 * |
| 32 (52.5%) | 36 (33.0%) | 0.45 (0.24–0.85) | |||
| Overdominant | CC + TT vs. CT | 37 (60.7%) | 48 (44.0%) | 1.00 | 0.037 * |
| 24 (39.3%) | 61 (56.0%) | 1.96 (1.04–3.71) | |||
| Log-additive | CC vs. TT | 5 (8.2%) | 12 (11.0%) | 1.00 | 0.028 * |
| 32 (52.5%) | 36 (33.0%) | 0.57 (0.34–0.95) |
| Gene | Haplotypes | Persistent Seizure (Freq) | Seizure-Free (Freq) | OR (95% CI) | p-Value |
|---|---|---|---|---|---|
| ABCB1 | TT 1 | 0.244 | 0.243 | 1.00 (0.60–1.68) | 0.989 |
| TC | 0.478 | 0.367 | 1.58 (1.00–2.47) | 0.047 * | |
| CT | 0.117 | 0.101 | 1.18 (0.58–2.39) | 0.648 | |
| CC | 0.162 | 0.289 | 0.48 (0.27–0.84) | 0.009 * |
| Genetic Model | Genotype | Patients with gADRs (n = 56) | Patients without gADRs (n = 114) | OR (95% CI) | p-Value |
|---|---|---|---|---|---|
| Allele contrast | C vs. T | 64 (57.1%) | 157 (68.9%) | 1.00 | 0.033 * |
| 48 (42.9%) | 71 (31.1%) | 0.60 (0.38–0.96) | |||
| Codominant | CC vs. CT vs. TT | 16 (28.6%) | 54 (47.4%) | 1.00 | 0.059 |
| 32 (57.1%) | 49 (43.0%) | 0.45 (0.22–0.93) | |||
| 8 (14.3%) | 11 (9.7%) | 0.41 (0.14–1.19) | |||
| Dominant | CC vs. CT + TT | 16 (28.6%) | 54 (47.4%) | 1.00 | 0.018 * |
| 40 (71.4%) | 60 (52.6%) | 0.44 (0.22–0.88) | |||
| Recessive | CC + CT vs. TT | 48 (85.7%) | 103 (90.3%) | 1.00 | 0.380 |
| 8 (14.3%) | 11 (9.7%) | 0.64 (0.24–1.70) | |||
| Overdominant | CC + TT vs. CT | 24 (42.9%) | 65 (57.0%) | 1.00 | 0.082 |
| 32 (57.1%) | 49 (43.0%) | 0.57 (0.30–1.08) | |||
| Log-additive | CC vs. TT | 16 (28.6%) | 54 (47.4%) | 1.00 | 0.030 * |
| 8 (14.3%) | 11 (9.7%) | 0.58 (0.36–0.95) |
| Genetic Model | Genotype | Patients with cADRs (n = 19) | Patients without cADRs (n = 151) | OR (95% CI) | p-Value |
|---|---|---|---|---|---|
| Allele contrast | C vs. T | 30 (78.9%) | 191 (63.2%) | 1.00 | 0.056 |
| 8 (21.1%) | 111 (36.8%) | 2.18 (0.97–4.92) | |||
| Codominant | CC vs. CT vs. TT | 13 (68.4%) | 57 (37.8%) | 1.00 | 0.027 * |
| 4 (21.1%) | 77 (51.0%) | 4.39 (1.36–14.17) | |||
| 2 (10.5%) | 17 (11.3%) | 1.94 (0.40–9.45) | |||
| Dominant | CC vs. CT + TT | 13 (68.4%) | 57 (37.8%) | 1.00 | 0.011 * |
| 6 (31.6%) | 94 (62.2%) | 3.57 (1.29–9.93) | |||
| Recessive | CC + CT vs. TT | 17 (89.5%) | 134 (88.7%) | 1.00 | 0.920 |
| 2 (10.5%) | 17 (11.3%) | 1.08 (0.23–5.08) | |||
| Overdominant | CC + TT vs. CT | 15 (78.9%) | 74 (49.0%) | 1.00 | 0.011 * |
| 4 (21.1%) | 77 (51.0%) | 3.90 (1.24–12.30) | |||
| Log-additive | CC vs. TT | 13 (68.4%) | 57 (37.8%) | 1.00 | 0.042 * |
| 2 (10.5%) | 17 (11.3%) | 2.27 (0.98–5.26) |
| Gene | SNP | Genotype | VPA Concentration (μg/mL) | Adjusted VPA Concentration (μg/mL)/(mg/kg) |
|---|---|---|---|---|
| ABCB1 | rs1128503 | CC (10.0%) | 67.19 ± 19.51 1 | 2.55 (2.16–3.90) 3 |
| CT (50.0%) | 62.79 ± 22.81 | 2.56 (1.99–3.58) | ||
| TT (40.0%) | 61.60 ± 22.60 | 2.57 (1.99–3.48) | ||
| p-value | 0.417 2 | 0.642 4 | ||
| rs3789243 | CC (41.2%) | 60.98 ± 22.09 | 2.53 (1.89–3.44) | |
| CT (47.6%) | 64.14 ± 23.35 | 2.61 (1.99–3.67) | ||
| TT (11.1%) | 62.95 ± 20.48 | 2.59 (2.24–3.31) | ||
| p-value | 0.422 2 | 0.518 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Lu, J.; He, Y.; Shen, X.; Xia, H.; Li, W.; Zhang, J.; Fan, X. Association of ABCB1 Polymorphisms with Efficacy and Adverse Drug Reactions of Valproic Acid in Children with Epilepsy. Pharmaceuticals 2023, 16, 1536. https://doi.org/10.3390/ph16111536
Zhu J, Lu J, He Y, Shen X, Xia H, Li W, Zhang J, Fan X. Association of ABCB1 Polymorphisms with Efficacy and Adverse Drug Reactions of Valproic Acid in Children with Epilepsy. Pharmaceuticals. 2023; 16(11):1536. https://doi.org/10.3390/ph16111536
Chicago/Turabian StyleZhu, Jiahao, Jieluan Lu, Yaodong He, Xianhuan Shen, Hanbing Xia, Wenzhou Li, Jianping Zhang, and Xiaomei Fan. 2023. "Association of ABCB1 Polymorphisms with Efficacy and Adverse Drug Reactions of Valproic Acid in Children with Epilepsy" Pharmaceuticals 16, no. 11: 1536. https://doi.org/10.3390/ph16111536







