Pharmacological Activities and Chemical Stability of Natural and Enzymatically Acylated Anthocyanins: A Comparative Review
Abstract
:1. Introduction
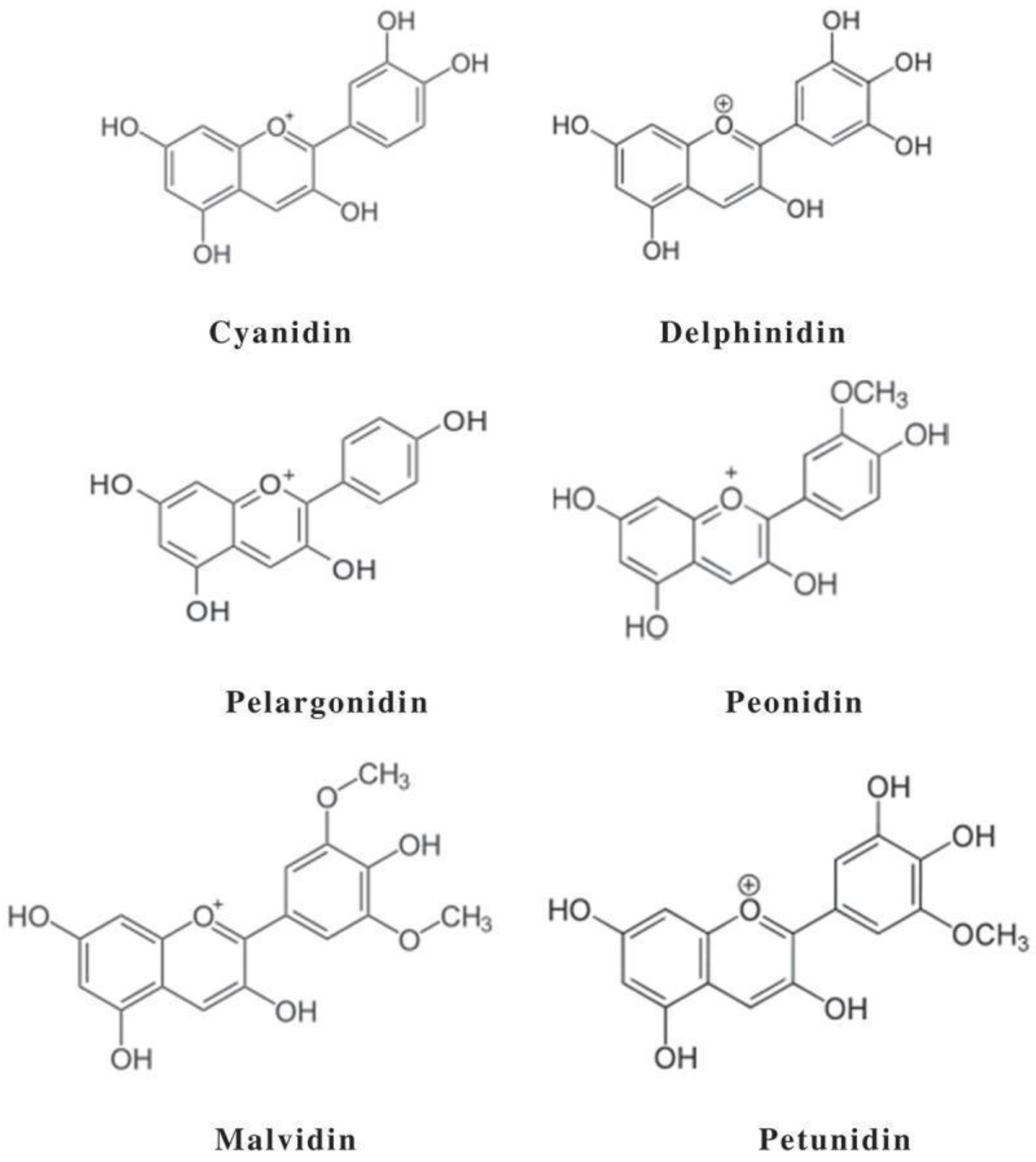
2. Presence of Acylated Anthocyanins in Nature
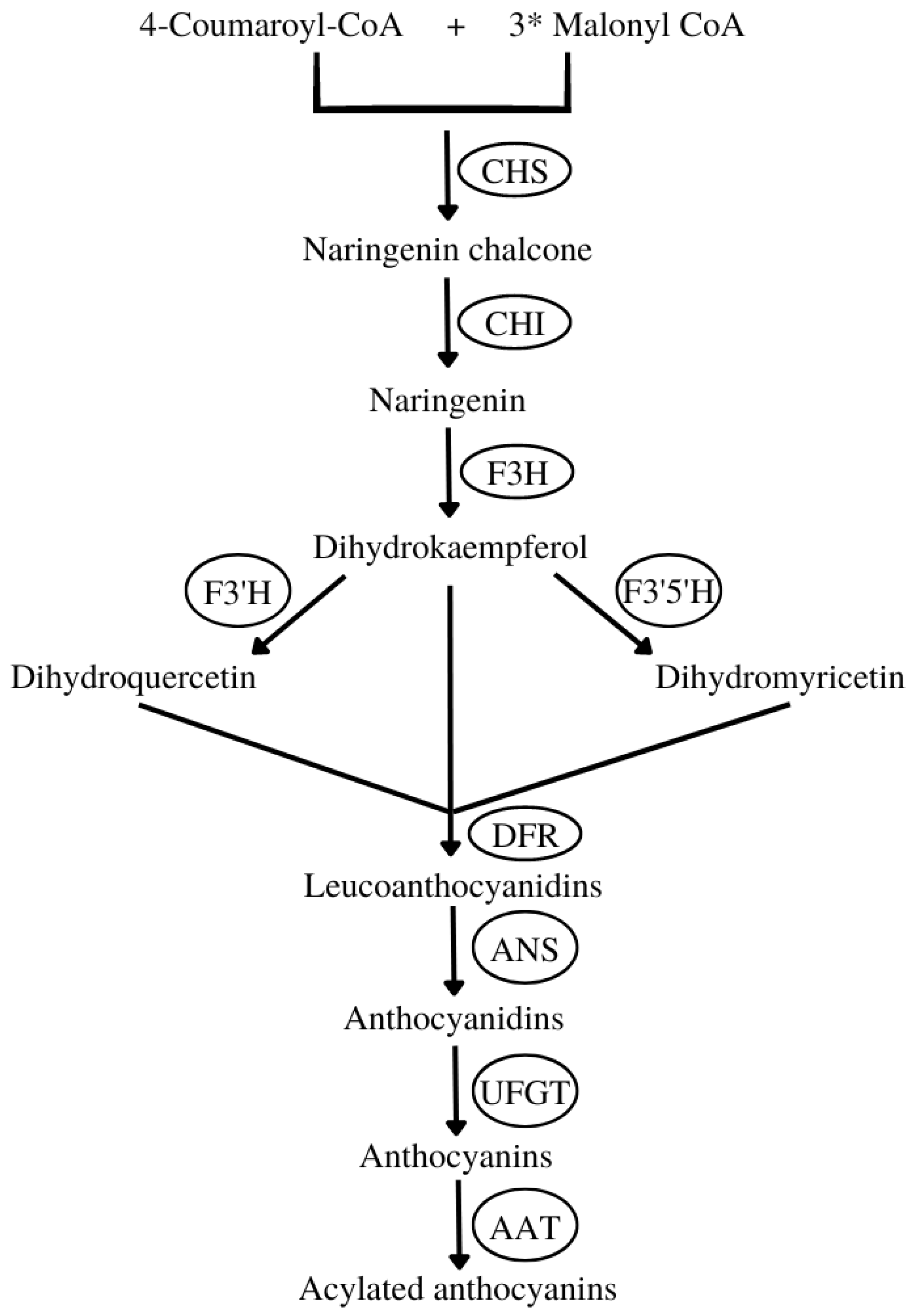
- The synthesis of naringenin chalcone from 4-coumaroyl-CoA and malonyl-CoA mediated by chalcone synthase (CHS).
- Then, naringenin chalcone is isomerized by chalcone isomerase (CHI) to naringenin.
- The naringenin is converted into dihydrokaempferol by flavanone 3-hydroxylase (F3H). This compound can be further hydroxylated by flavonoid 3′-hydroxylase (F3′H) or flavonoid 3′,5′-hydroxylase (F3′5′H) into two other dihydroflavonols, dihydroquercetin or dihydromyricetin, respectively.
- Next, the three dihydroflavonols are converted into colorless leucoanthocyanidins by dihydroflavonol 4-reductase (DFR) and subsequently to colored anthocyanidins by anthocyanidin synthase (ANS).
- Then, sugar molecules are attached to anthocyanidins by various glycosyltransferases, for instance, flavonoid 3-O-glucosyltransferase (UFGT), yielding ANCs.
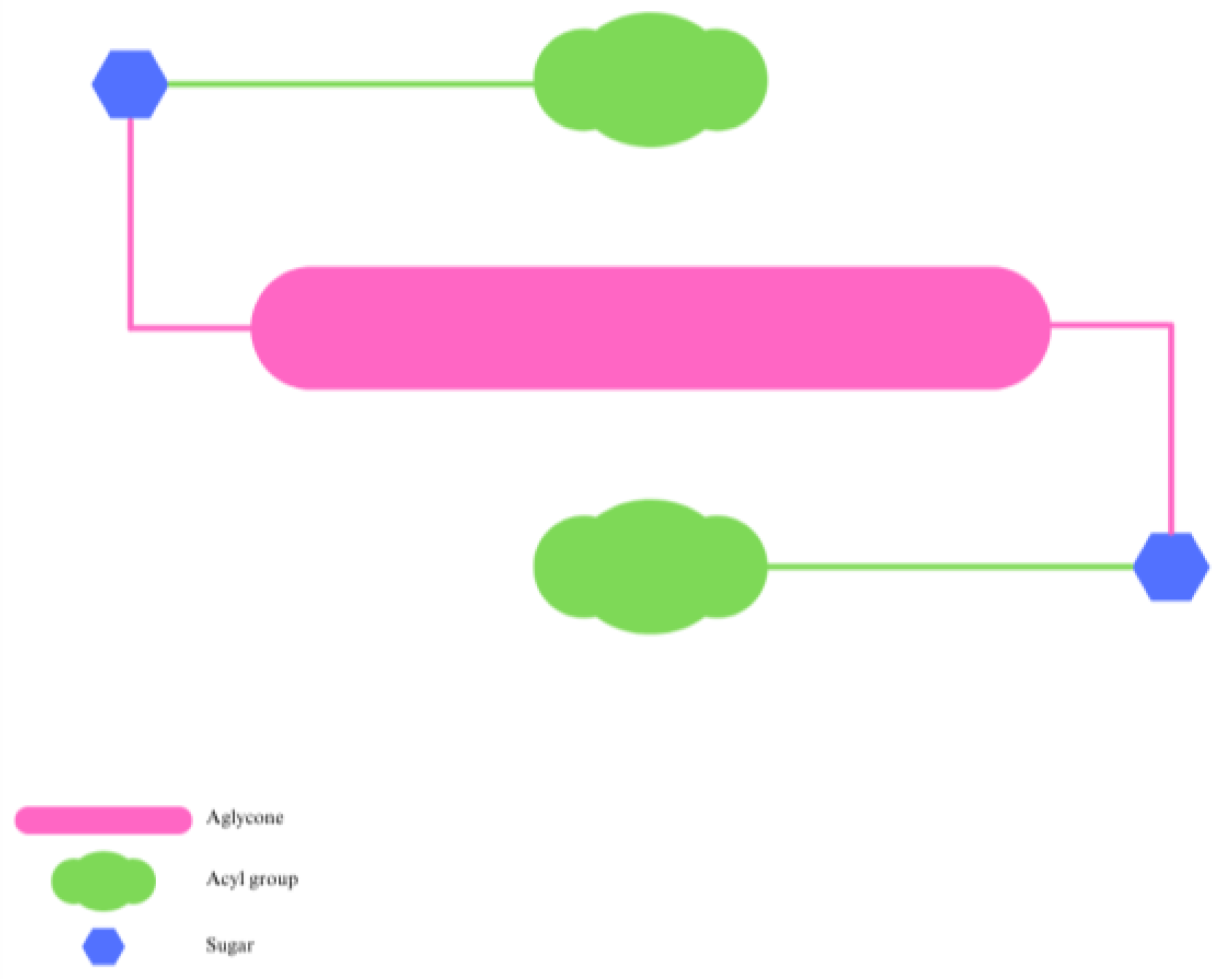
3. Effect of Natural Acylation on Anthocyanin Stability
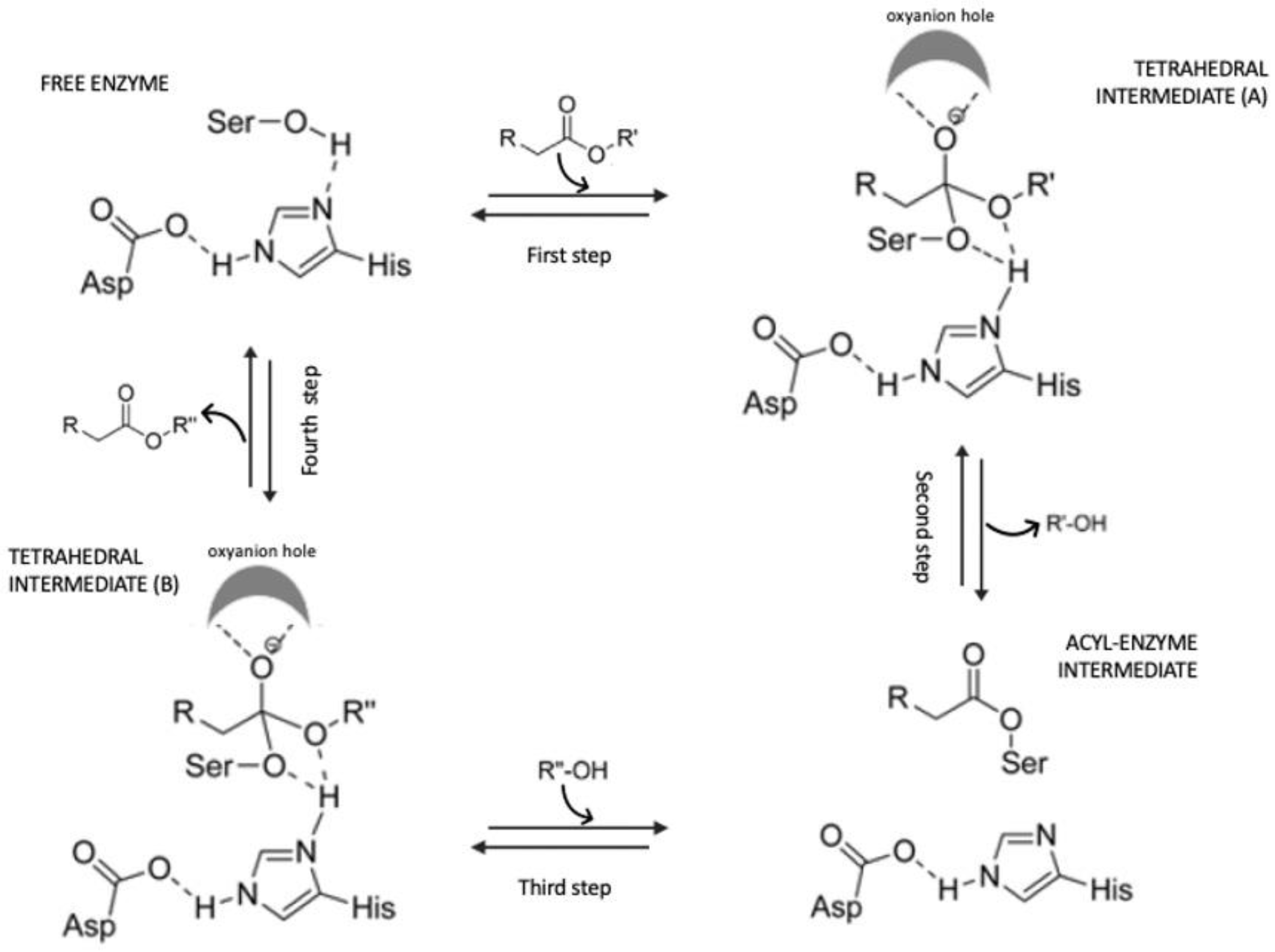
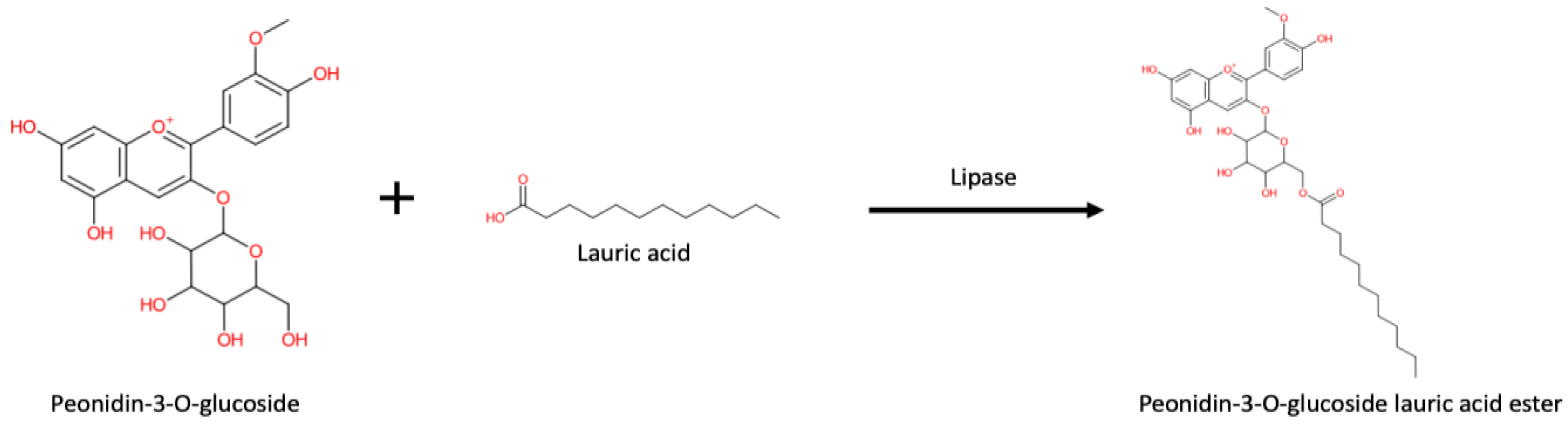
4. Enzymatic Synthesis of Acylated Anthocyanins and Effect on Their Stability
Enzymes Used for Anthocyanin Acylation
5. Relevance of Natural Acylated and Non-Acylated Anthocyanins in Inflammation and Diabetes
6. Green and Sustainable Alternatives for Enzymatic Acylation
7. Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Konczak, I.; Zhang, W. Anthocyanins—More than nature’s colours. J. Biomed. Biotechnol. 2004, 2004, 239. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, M.; Mateus, N.; de Freitas, V.; Cruz, L. Improvement of the color stability of cyanidin-3-glucoside by fatty acid enzymatic acylation. J. Agric. Food Chem. 2018, 66, 10003–10010. [Google Scholar] [CrossRef]
- Cortez, R.; Luna-Vital, D.A.; Margulis, D.; Gonzalez de Mejia, E. Natural pigments: Stabilization methods of anthocyanins for food applications. Compr. Rev. Food Sci. 2017, 16, 180–198. [Google Scholar] [CrossRef] [PubMed]
- Fei, P.; Zeng, F.; Zheng, S.; Chen, Q.; Hu, Y.; Cai, J. Acylation of blueberry anthocyanins with maleic acid: Improvement of the stability and its application potential in intelligent color indicator packing materials. Dye. Pigment. 2021, 184, 108852. [Google Scholar] [CrossRef]
- Cai, D.; Li, X.; Chen, J.; Jiang, X.; Ma, X.; Sun, J.; Tian, L.; Vidyarthi, S.K.; Xu, J.; Pan, Z.; et al. A comprehensive review on innovative and advanced stabilization approaches of anthocyanin by modifying structure and controlling environmental factors. Food Chem. 2022, 366, 130611. [Google Scholar] [CrossRef] [PubMed]
- Eker, M.E.; Aaby, K.; Budic-Leto, I.; Rimac Brnčić, S.; El, S.N.; Karakaya, S.; Simsek, S.; Manach, C.; Wiczkowski, W.; de Pascual-Teresa, S. A review of factors affecting anthocyanin bioavailability: Possible implications for the inter-individual variability. Foods 2019, 9, 2. [Google Scholar] [CrossRef]
- Zhao, C.L.; Yu, Y.Q.; Chen, Z.J.; Wen, G.S.; Wei, F.G.; Zheng, Q.; Wang, C.D.; Xiao, X.L. Stability-increasing effects of anthocyanin glycosyl acylation. Food Chem. 2017, 214, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.Y.; Chen, J.; Wang, Z.Q.; Shen, R.M.; Cui, N.; Sun, A.D. Direct acylation of cyanidin-3-glucoside with lauric acid in blueberry and its stability analysis. Int. J. Food Prop. 2016, 19, 1–12. [Google Scholar] [CrossRef]
- Li, A.; Xiao, R.; He, S.; An, X.; He, Y.; Wang, C.; Yin, S.; Wang, B.; Shi, X.; He, J. Research advances of purple sweet potato anthocyanins: Extraction, identification, stability, bioactivity, application, and biotransformation. Molecules 2019, 24, 3816. [Google Scholar] [CrossRef]
- Zhao, C.L.; Chen, Z.J.; Bai, X.S.; Ding, C.; Long, T.J.; Wei, F.G.; Miao, K.R. Structure–activity relationships of anthocyanidin glycosylation. Mol. Divers. 2014, 18, 687–700. [Google Scholar] [CrossRef]
- Liu, Y.; Tikunov, Y.; Schouten, R.E.; Marcelis, L.F.; Visser, R.G.; Bovy, A. Anthocyanin biosynthesis and degradation mechanisms in Solanaceous vegetables: A review. Front. Chem. 2018, 6, 52. [Google Scholar] [CrossRef]
- Zhang, Y.; Butelli, E.; Martin, C. Engineering anthocyanin biosynthesis in plants. Curr. Opin. 2014, 19, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, N.; Nishizaki, Y.; Ozeki, Y.; Miyahara, T. The role of acyl-glucose in anthocyanin modifications. Molecules 2014, 19, 18747–18766. [Google Scholar] [CrossRef] [PubMed]
- Rinaldo, A.R.; Cavallini, E.; Jia, Y.; Moss, S.M.A.; McDavid, D.A.J.; Hooper, L.C.; Robinson, S.P.; Tornielli, G.B.; Zenoni, S.; Ford, C.M.; et al. A grapevine anthocyanin acyltransferase, transcriptionally regulated by VvMYBA, can produce most acylated anthocyanins present in grape skins. Plant Physiol. 2015, 169, 1897–1916. [Google Scholar] [CrossRef] [PubMed]
- Bakowska-Barczak, A. Acylated anthocyanins as stable, natural food colorants—A review. Polish J. Food Nutr. Sci 2005, 14, 55. [Google Scholar]
- Jokioja, J.; Yang, B.; Linderborg, K.M. Acylated anthocyanins: A review on their bioavailability and effects on postprandial carbohydrate metabolism and inflammation. Compr. Rev. Food Sci. 2021, 20, 5570–5615. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Acylated anthocyanins from edible sources and their applications in food systems. Biochem. Eng. J. 2003, 14, 217–225. [Google Scholar] [CrossRef]
- Goto, T. Structure, Stability and Color Variation of Natural Anthocyanins. In Fortschritte der Chemie Organischer Naturstoffe/Progress in the Chemistry of Organic Natural Products; Springer: Vienna, Austria, 1987; Volume 52. [Google Scholar]
- Montilla, E.C.; Arzaba, M.R.; Hillebrand, S.; Winterhalter, P. Anthocyanin composition of black carrot (Daucus carota ssp. sativus var. atrorubens Alef.) cultivars Antonina, Beta Sweet, Deep Purple, and Purple Haze. J. Agric. Food Chem. 2011, 59, 3385–3390. [Google Scholar] [CrossRef]
- Moreno, D.A.; Pérez-Balibrea, S.; Ferreres, F.; Gil-Izquierdo, Á.; García-Viguera, C. Acylated anthocyanins in broccoli sprouts. Food Chem. 2010, 123, 358–363. [Google Scholar] [CrossRef]
- Xu, J.; Su, X.; Lim, S.; Griffin, J.; Carey, E.; Katz, B.; Tomich, J.; Smith, J.S.; Wang, W. Characterisation and stability of anthocyanins in purple-fleshed sweet potato P40. Food Chem. 2015, 186, 90–96. [Google Scholar] [CrossRef]
- Cevallos-Casals, B.A.; Cisneros-Zevallos, L. Stability of anthocyanin-based aqueous extracts of Andean purple corn and red-fleshed sweet potato compared to synthetic and natural colorants. Food Chem. 2004, 86, 69–77. [Google Scholar] [CrossRef]
- Matsufuji, H.; Kido, H.; Misawa, H.; Yaguchi, J.; Otsuki, T.; Chino, M.; Takeda, M.; Yamagata, K. Stability to light, heat, and hydrogen peroxide at different pH values and DPPH radical scavenging activity of acylated anthocyanins from red radish extract. J. Agric. Food Chem. 2007, 55, 3692–3701. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Lin, C.; Chen, M.H.; Chiang, P.Y. Stability and quality of anthocyanin in purple sweet potato extracts. Foods 2019, 8, 393. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.; Giusti, M.M. Black goji as a potential source of natural color in a wide pH range. Food Chem. 2018, 269, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Zheng, J.; Li, W.; Suo, Y. Isolation, stability, and antioxidant activity of anthocyanins from Lycium ruthenicum Murray and Nitraria tangutorum Bobr of Qinghai-Tibetan plateau. Sep. Sci. Technol. 2014, 49, 2897–2906. [Google Scholar] [CrossRef]
- Lakshan, S.A.T.; Jayanath, N.Y.; Abeysekera, W.P.K.M.; Abeysekera, W.K.S.M. A commercial potential blue pea (Clitoria ternatea L.) flower extract incorporated beverage having functional properties. Evid. Based Complement. Altern. Med. 2019, 2019, 2916914. [Google Scholar] [CrossRef]
- Otera, J.; Nishikido, J. Esterification: Methods, Reactions, and Applications, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2009; p. 47. [Google Scholar]
- Prietto, L.; Mirapalhete, T.C.; Pinto, V.Z.; Hoffmann, J.F.; Vanier, N.L.; Lim, L.T.; Guerra Dias, A.R.; da Rosa Zavareze, E. pH-sensitive films containing anthocyanins extracted from black bean seed coat and red cabbage. LWT 2017, 80, 492–500. [Google Scholar] [CrossRef]
- Gras, C.C.; Bause, K.; Leptihn, S.; Carle, R.; Schweiggert, R.M. Effect of chlorogenic acid on spectral properties and stability of acylated and non-acylated cyanidin-3-O-glycosides. Food Chem. 2018, 240, 940–950. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, S.; Zhao, Z.; You, L.; Harrison, M.D.; Zhang, Z. Enzymatic acylation of cyanidin-3-glucoside with fatty acid methyl esters improves stability and antioxidant activity. Food Chem. 2021, 343, 128482. [Google Scholar] [CrossRef]
- Yang, X.; Sun, H.; Tu, L.; Jin, Y.; Zhang, Z.; Wang, M.; Liu, S.; Wang, Y.; He, S. Kinetics of enzymatic synthesis of cyanidin-3-glucoside lauryl ester and its physicochemical property and proliferative effect on intestinal probiotics. Biology 2020, 9, 205. [Google Scholar] [CrossRef]
- Cruz, L.; Benohoud, M.; Rayner, C.M.; Mateus, N.; de Freitas, V.; Blackburn, R.S. Selective enzymatic lipophilization of anthocyanin glucosides from blackcurrant (Ribes nigrum L.) skin extract and characterization of esterified anthocyanins. Food Chem. 2018, 266, 415–419. [Google Scholar] [CrossRef]
- Fernandez-Aulis, F.; Torres, A.; Sanchez-Mendoza, E.; Cruz, L.; Navarro-Ocana, A. New acylated cyanidin glycosides extracted from underutilized potential sources: Enzymatic synthesis, antioxidant activity and thermostability. Food Chem. 2020, 309, 125796. [Google Scholar] [CrossRef]
- Liu, J.; Zhuang, Y.; Hu, Y.; Xue, S.; Li, H.; Chen, L.; Fei, P. Improving the color stability and antioxidation activity of blueberry anthocyanins by enzymatic acylation with p-coumaric acid and caffeic acid. LWT 2020, 130, 109673. [Google Scholar] [CrossRef]
- Marquez-Rodriguez, A.S.; Guimarães, M.; Mateus, N.; de Freitas, V.; Ballinas-Casarrubias, M.L.; Fuentes-Montero, M.E.; Salas, E.; Cruz, L. Disaccharide anthocyanin delphinidin 3-O-sambubioside from Hibiscus sabdariffa L.: Candida antarctica lipase B-catalyzed fatty acid acylation and study of its color properties. Food Chem. 2021, 344, 128603. [Google Scholar] [CrossRef]
- Marathe, S.J.; Shah, N.N.; Bajaj, S.R.; Singhal, R.S. Esterification of anthocyanins isolated from floral waste: Characterization of the esters and their application in various food systems. Food Biosci. 2021, 40, 100852. [Google Scholar] [CrossRef]
- Teng, H.; Mi, Y.; Cao, H.; Chen, L. Enzymatic acylation of raspberry anthocyanin: Evaluations on its stability and oxidative stress prevention. Food Chem. 2022, 372, 130766. [Google Scholar] [CrossRef]
- Zisis, T.; Freddolino, P.L.; Turunen, P.; van Teeseling, M.C.; Rowan, A.E.; Blank, K.G. Interfacial activation of Candida antarctica lipase B: Combined evidence from experiment and simulation. Biochemistry 2015, 54, 5969–5979. [Google Scholar] [CrossRef]
- Zígolo, M.A. Lipasas Como Biocatalizadores en la Síntesis de Derivados de Ácidos Biliares, Fenilacéticos Sustituidos y Glicirretínico. Ph.D. Thesis, Universidad de Buenos Aires, Buenos Aires, Argentina, 2017. [Google Scholar]
- Cha, H.J.; Park, J.B.; Park, S. Esterification of secondary alcohols and multi-hydroxyl compounds by Candida antarctica lipase B and subtilisin. Biotechnol. Bioprocess Eng. 2019, 24, 41–47. [Google Scholar] [CrossRef]
- Chebil, L.; Humeau, C.; Falcimaigne, A.; Engasser, J.M.; Ghoul, M. Enzymatic acylation of flavonoids. Process Biochem. 2006, 41, 2237–2251. [Google Scholar] [CrossRef]
- Zhang, Q.; de Mejia, E.G.; Luna-Vital, D.; Tao, T.; Chandrasekaran, S.; Chatham, L.; Juvik, J.; Singh, V.; Kumar, D. Relationship of phenolic composition of selected purple maize (Zea mays L.) genotypes with their anti-inflammatory, anti-adipogenic and anti-diabetic potential. Food Chem. 2019, 289, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Li, X.; Pan, Z.; Zhu, Y.; Tuo, J.; Meng, Q.; Dai, G.; Yang, G.; Pan, Y. Anthocyanin ameliorates hypoxia and ischemia induced inflammation and apoptosis by increasing autophagic flux in SH-SY5Y cells. Eur. J. Pharmacol. 2020, 883, 173360. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, J.L.; Shen, L.H.; Feng, L.J.; Zhou, Q. Inhibition mechanism of diacylated anthocyanins from purple sweet potato (Ipomoea batatas L.) against α-amylase and α-glucosidase. Food Chem. 2021, 359, 129934. [Google Scholar] [CrossRef]
- Wu, T.; Gao, Y.; Guo, X.; Zhang, M.; Gong, L. Blackberry and blueberry anthocyanin supplementation counteract high-fat-diet-induced obesity by alleviating oxidative stress and inflammation and accelerating energy expenditure. Oxidative Med. Cell. Longev. 2018, 2018, 4051232. [Google Scholar] [CrossRef]
- Bell, L.; Lamport, D.J.; Butler, L.T.; Williams, C.M. A study of glycaemic effects following acute anthocyanin-rich blueberry supplementation in healthy young adults. Food Funct. 2017, 8, 3104–3110. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, Z.; Zhao, H.; Wang, X.; Pang, J.; Li, Q.; Yang, Y.; Ling, W. Anthocyanin supplementation improves anti-oxidative and anti-inflammatory capacity in a dose–response manner in subjects with dyslipidemia. Redox Biol. 2020, 32, 101474. [Google Scholar] [CrossRef] [PubMed]
- Nikbakht, E.; Singh, I.; Vider, J.; Williams, L.T.; Vugic, L.; Gaiz, A.; Kundur, A.R.; Colson, N. Potential of anthocyanin as an anti-inflammatory agent: A human clinical trial on type 2 diabetic, diabetic at-risk and healthy adults. Inflamm. Res. 2021, 70, 275–284. [Google Scholar] [CrossRef]
- Jokioja, J.; Linderborg, K.M.; Kortesniemi, M.; Nuora, A.; Heinonen, J.; Sainio, T.; Viitanen, M.; Kallio, H.; Yang, B. Anthocyanin-rich extract from purple potatoes decreases postprandial glycemic response and affects inflammation markers in healthy men. Food Chem. 2020, 310, 125797. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Wei, X.; Zhang, J.; Pariyani, R.; Jokioja, J.; Kortesniemi, M.; Linderborg, K.M.; Heinonen, J.; Sainio, T.; Zhang, Y.; et al. Effects of anthocyanin extracts from bilberry (Vaccinium myrtillus L.) and purple potato (Solanum tuberosum L. Var.‘Synkea Sakari’) on the plasma metabolomic profile of zucker diabetic fatty rats. J. Agric. Food Chem. 2020, 68, 9436–9450. [Google Scholar] [CrossRef]
- Buko, V.; Zavodnik, I.; Kanuka, O.; Belonovskaya, E.; Naruta, E.; Lukivskaya, O.; Kirko, S.; Budryn, G.; Żyżelewicz, D.; Oracz, J.; et al. Antidiabetic effects and erythrocyte stabilization by red cabbage extract in streptozotocin-treated rats. Food Funct. 2018, 9, 1850–1863. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, M.; Mateus, N.; de Freitas, V.; Branco, L.C.; Cruz, L. Microwave-assisted synthesis and ionic liquids: Green and sustainable alternatives toward enzymatic lipophilization of anthocyanin monoglucosides. J. Agric. Food Chem. 2020, 68, 7387–7392. [Google Scholar] [CrossRef]
- da Silva Lacerda, V.; López Sotelo, J.B.; Correa Guimaraes, A.; Martín Ramos, P.; Hernández Navarro, S.; Sánchez Báscones, M.; Navas Gracia, L.M.; Pérez Lebeña, E.; Martín Gil, J. Efficient microwave-assisted acid hydrolysis of lignocellulosic materials into total reducing sugars in ionic liquids. Cellul. Chem. Technol. 2016, 50, 761–770. [Google Scholar]
- Martínez-Palou, R. Ionic liquid and microwave-assisted organic synthesis: A “green” and synergic couple. J. Mex. Chem. Soc. 2007, 51, 252–264. [Google Scholar]
- Cruz, L.; Fernandes, I.; Guimarães, M.; de Freitas, V.; Mateus, N. Enzymatic synthesis, structural characterization and antioxidant capacity assessment of a new lipophilic malvidin-3-glucoside–oleic acid conjugate. Food Funct. 2016, 7, 2754–2762. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Li, C.; Zhang, L.; Liu, Q.; Ou, S.; Zeng, X. Enzymatic acylation of anthocyanin isolated from black rice with methyl aromatic acid ester as donor: Stability of the acylated derivatives. J. Agric. Food Chem. 2016, 64, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Cruz, L.; Guimarães, M.; Araujo, P.; Evora, A.; de Freitas, V.; Mateus, N. Malvidin 3-glucoside–fatty acid conjugates: From hydrophilic toward novel lipophilic derivatives. J. Agric. Food Chem. 2017, 65, 6513–6518. [Google Scholar] [CrossRef]
- Luo, S.Z.; Chen, S.S.; Pan, L.H.; Qin, X.S.; Zheng, Z.; Zhao, Y.Y.; Pang, M.; Jiang, S.T. Antioxidative capacity of crude camellia seed oil: Impact of lipophilization products of blueberry anthocyanin. Int. J. Food Prop. 2017, 20 (Suppl. 2), 1627–1636. [Google Scholar] [CrossRef]
- Yang, W.; Kortesniemi, M.; Yang, B.; Zheng, J. Enzymatic acylation of anthocyanins isolated from alpine bearberry (Arctostaphylos alpina) and lipophilic properties, thermostability, and antioxidant capacity of the derivatives. J. Agric. Food Chem. 2018, 66, 2909–2916. [Google Scholar] [CrossRef]
- Guimarães, M.; Pérez-Gregorio, M.; Mateus, N.; de Freitas, V.; Galinha, C.F.; Crespo, J.G.; Portugal, C.A.M.; Cruz, L. An efficient method for anthocyanins lipophilization based on enzyme retention in membrane systems. Food Chem. 2019, 300, 125167. [Google Scholar] [CrossRef]
- Yang, W.; Kortesniemi, M.; Ma, X.; Zheng, J.; Yang, B. Enzymatic acylation of blackcurrant (Ribes nigrum) anthocyanins and evaluation of lipophilic properties and antioxidant capacity of derivatives. Food Chem. 2019, 281, 189–196. [Google Scholar] [CrossRef]
- Viskupičová, J.; Ondrejovič, M.; Šturdík, E. The potential and practical applications of acylated flavonoids. Pharm. J. Pharm. 2009, 64, 355–360. [Google Scholar]
- Leonarski, E.; Cesca, K.; de Oliveira, D.; Zielinski, A.A. A review on enzymatic acylation as a promising opportunity to stabilizing anthocyanins. Crit. Rev. Food Sci. Nutr. 2022, 1–20. [Google Scholar] [CrossRef]
- Bueno, J.M.; Sáez-Plaza, P.; Ramos-Escudero, F.; Jiménez, A.M.; Fett, R.; Asuero, A.G. Analysis and antioxidant capacity of anthocyanin pigments. Part II: Chemical structure, color, and intake of anthocyanins. Crit. Rev. Anal. Chem. 2012, 42, 126–151. [Google Scholar] [CrossRef]
- Malien-Aubert, C.; Dangles, O.; Amiot, M.J. Color stability of commercial anthocyanin-based extracts in relation to the phenolic composition. Protective effects by intra-and intermolecular copigmentation. J. Agric. Food Chem. 2001, 49, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Netzel, M.; Netzel, G.; Kammerer, D.R.; Schieber, A.; Carle, R.; Simons, L.; Bitsch, I.; Konczak, I. Cancer cell antiproliferation activity and metabolism of black carrot anthocyanins. Innov. Food Sci. Emerg. Technol. 2007, 8, 365–372. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, L.; Huang, L.; Tekliye, M.; Xia, X.; Li, J.; Dong, M. Composition, antioxidant activity, and neuroprotective effects of anthocyanin-rich extract from purple highland barley bran and its promotion on autophagy. Food Chem. 2021, 339, 127849. [Google Scholar] [CrossRef] [PubMed]
- Yañez Apam, J.; Herrera-González, A.; Domínguez Uscanga, A.; Guerrero-Analco, J.A.; Monribot-Villanueva, J.L.; Fragoso-Medina, J.A.; Luna-Vital, D.A. Effect of the Enzymatic Treatment of Phenolic-Rich Pigments from Purple Corn (Zea mays L.): Evaluation of Thermal Stability and Alpha-Glucosidase Inhibition. Food Bioprocess Technol. 2023, 1–15. [Google Scholar] [CrossRef]

| ANC Source | Main ANC | Acyl Donor | Enzyme | ANC Conjugate | Main Findings | Reference |
|---|---|---|---|---|---|---|
| Raspberry (Rubus idaeus) | Cyanidin-3-O-glucoside | Methyl salicylate | Novozym 435 (acrylic-resin-immobilized CALB) | Cyanidin-3-(6-salicyloyl) glucoside | Acylated ANCs showed improved thermal, photo-, and oxidative stabilities and also showed a good protective effect on oxidative stress damage | [37] |
| Red rose petals | Cyanidin-3,5-O-diglucoside | Fatty acids: caprylic acid, lauric acid, palmitic acid | Fermase CALBTM 10,000 (lipase B from C. antarctica immobilized on polyacrylate beads) | ANC lauric ester | The optimized reaction parameters were the following: acetonitrile (reaction medium), 40 °C (reaction temperature), 24 h (reaction time), 1:100 (molar ratio of reactants), 20 mg/mL (enzyme load), 100 mg/mL (molecular sieve load), 150 rpm (rate of shaking). ANC esters improved the thermo-oxidative stability of biscuit cream, also showed enhanced color stability in rice extrudate during thermal processing and storage | [38] |
| Pure C3G | Cyanidin-3-glucoside | Fatty acid methyl esters: methyl butyrate, methyl n-octanoate, methyl laurate, methyl myristate, methyl palmitate, and methyl stearate | Lipozyme 435 (recombinant lipase from C. antarctica) | Cyanidin-3-(6″-n-octanoyl)-glucoside, cyanidin-3-(6″-lauroyl)-glucoside, and cyanidin-3-(6″-myristoyl)-glucoside | C3G-n-octanoate had the highest thermostability and photostability. C3G-laurate had the highest cellular antioxidant capacity | [33] |
| Red wine | Malvidin 3-glucoside | Oleic acid (C18) | Lipase acrylic resin from C. antarctica (≥5000 U/g, recombinant, expressed in Aspergillus niger) | Mv3glc-C18 | Preserved chromatic features (red-violet color) and antioxidant activity. Improved technological applications (potential in lipophilic systems, such as fats, oils, lipid-based food or cosmetic formulations) | [39] |
| Black rice (Oryza sativa L.) | Cyanidin-3-galactoside | Methyl benzoate, methyl salicylate, and methyl cinnamate | Novozym 435 (lipase B from C. antarctica immobilized on acrylic resin) | Cyanidin 3-(6″-benzoyl)-glucoside, cyanidin 3-(6″-salicyloyl)-glucoside, and cyanidin 3-(6″-cinnamoyl)-glucoside | Acylation with aromatic carboxylic acids enhanced thermostability and light-resistivity of ANCs. Cyanidin-3-(6″-cinnamoyl)-glucoside was the most stable | [40] |
| Red wine | Malvidin 3-glucoside | C4 to C16 | Lipase acrylic resin from C. antarctica lipase B (≥5000 84 U/g, recombinant, expressed in Aspergillus niger) | Mv3glc-C4 to Mv3glc-C16 | Increased lipophilicity. The maximum antioxidant activity was achieved when ANC was linked with caprylic acid (C8) | [41] |
| Blueberry (Vaccinium corymbosum) | Cyanidin-3-galactoside | Oleic acid and palmitic acid | Novozym 435 (C. antarctica lipase B 10,000 U/g) | Cyanidin-3-galactoside oleate and cyanidin-3-galactoside palmitate | Lipophilized ANC derivatives with free fatty acids improved oxidative stability under high temperature | [42] |
| Blackcurrant skin (Ribes nigrum L.) | Delphinidin-3-O-rutinoside, cyanidin-3-O-rutinoside, delphinidin-3-O-glucoside, cyanidin-3-O-glucoside | Octanoic acid (C8) | Lipase acrylic resin from C. antarctica lipase B (≥5000 U/g, recombinant, expressed in Aspergillus niger) | Dp3glc-C8 and Cy3glc-C8 | Improved color stability (pH 3–7). Lower thermal degradation. Selective and preferential enzymatic acylation of cyanidin and delphinidin glucosides but not the corresponding rutinosides | [35] |
| Blackberry (Rubus fruticosus L.) | Cyanidin-3-O-glucoside | C4-C12 | CalB immobilized in acrylic resin (≥5000 U/g, recombinant, expressed in Aspergillus niger) | Cy3glc-C4 to Cy3glc-C12 | Improved color stability and lowered sensitivity to thermal degradation in an SDS micellar solution between pH 3 and 7 | [2] |
| Alpine bearberry (Arctostaphylos alpina) | Cyanidin-3-O-galactoside | Lauric acid (C12) | C. antarctica lipase immobilized on acrylic resin (Novozyme 435) (≥5000 U/g, recombinant, expressed in Aspergillus niger) | Cyanidin-3-O-(6″-dodecanoyl) galactoside | Highest conversion yields (73%) obtained by acylation of cy-gal with lauric acid (C12). Improved lipophilicity and thermo-stability. Preserved UV-VIS absorbance and antioxidant properties | [43] |
| Blackberry (Rubus fruticosus L.) | Cyanidin-3-O-glucoside | Octanoic acid (C8) | Lipase B, powder form from C. antarctica (CalB) retained in composite membranes | Cy3glc-C8 | Increased enzymatic activity of CalB-rich extract without enzyme purification. Improved yield of lipophilization reaction by 2.5-fold. Reusability of the membrane for three consecutive reaction cycles with the same ester conversion yield | [44] |
| Black rice (Oryza sativa L.) | Cyanidin-3-O-glucoside | Lauric acid (C12) | CalB, Novozym 435 (≥10,000 U/g, recombinant, expressed in Aspergillus niger) | Cy3glc-C12 | Improved liposolubility, pH resistivity, and thermostability. Cy3glc-C12 promoted the proliferation of Bifidobacteria and Lactobacillus in the middle and later log phase and metabolization into phenolic acids | [34] |
| Blackcurrant (Ribes nigrum) | Delphinidin-3-O-glucoside, delphinidin-3-O-rutinoside, cyanidin-3-O-glucoside, and cyanidin-3-O-rutinoside | Lauric acid (C12) | Lipase acrylic resin from C. antarctica (≥5000 U/g, recombinant, expressed in Aspergillus niger) | Dp-glu-lauric acid, dp-rut-lauric acid, cy-glu-lauric acid, and cy-rut-lauric acid | Enhanced lipophilicity. Improved thermostability and capacity to inhibit lipid peroxidation | [45] |
| Tiliapo (Sideroxylon palmeri), trueno fruit (Ligustrum japonicum), bottlebrush flower (Callistemon citrinus), plum, and corn husks | Cyanidin-3-O-glucoside, cyanidin-3-O-rutinoside, cyanidin-3,5-diglucoside | Vinyl cinnamate, dihydrocinnamic acid, and cinnamic acid | Immobilized lipase from C. antarctica (≥5000 U/g, recombinant, expressed in Aspergillus niger) | Cy3-(4″- cinnamoyl) rutinoside, cy3,5-(6″-cinnamoyl) diglucoside, cy3-(6″-dihydrocinnamoyl) glucoside, cy3-(6″-dihydroferuloyl) glucoside, and cy3-(6″-dihydrosinapoyl) glucoside | Improved antioxidant activity and thermostability. Optimal reaction conditions involved tert-butanol as reaction media | [36] |
| Blueberry (Vaccinium corymbosum) | Cyanidin-3-O-glucoside | p-coumaric acid and caffeic acid | Lipase (Novozyme 435, ≥10,000 U/g, recombinant, expressed in Aspergillus niger) | ANCs acylated with p-coumaric acid (Co-An) and caffeic acid (Ca-An) | Stronger antioxidant activity and higher color stability during storage. p-coumaric and caffeic acids prevented ANCs from oxidation and breakdown | [46] |
| Hibiscus flower (Hibiscus sabdariffa L.) | Delphinidin 3-O-sambubioside | Octanoic acid (C8) | Lipase acrylic resin from C. antarctica lipase B (≥5000 U/g, recombinant, expressed in Aspergillus niger) | Dp3sam-C8 | Stabilization of the quinoidal base (blue color) at neutral or moderate alkaline pH. Improved lipophilicity | [47] |
| Parameter or Characteristic | Natural Acylation | Synthetic Acylation |
|---|---|---|
| Site of acylation | Glycosidic residues | Glycosidic residues |
| Regioselectivity | 6″-O, 4‴-O and 6‴-O depending on the species | 6″-O (glucose and galactose) and 4″-O (rhamnose) |
| Enzyme | AATs | Lipase CalB from Candida antarctica. Just one study used a lipase from Candida cylindrical [34] |
| Acylating agents | Hydroxycinnamic acids (caffeic, p-coumaric, ferulic, sinapic), hydroxybenzoic acids (p-hydroxybenzoic and gallic), and aliphatic acids (acetic, malic, malonic, oxalic, succinic, tartaric, erucic, glutaric) | Methyl salicylate, methyl benzoate, methyl cinnamate, methyl butyrate, methyl laurate, methyl myristate, methyl palmitate, methyl stearate, n-octanoate, polyoxyethylene stearate, vinyl cinnamate, dihydrocinnamic acid, dihydroferuloyl acid, dihydrosinapic acid, cinnamic acid, p-coumaric acid, caffeic acid, butyric acid, hexanoic acid, octanoic acid, decanoic acid, lauric acid |
| Preferential acylation | Cinnamic acids | Aliphatic acids (lauric and octanoic acids). Long-chain fatty acids are better acyl donors than short-chain fatty acids |
| Polyacylation | Aromatic and aliphatic acylation may occur in the same molecule | Only one type of acylation is reported in enzymatic acylation studies |
| Enzyme immobilization | - | Lipase immobilization advantages: improved thermal and chemical stability, easy recycling and reuse, lower operating costs, and more prolonged enzyme survival |
| Sugars preferred | - | Mainly monosaccharide ANCs have easy access to the active site of the enzyme |
| Polarity | Decreases | Decreases |
| Water solubility | Decreases | Decreases |
| Oxidation | Changes in the ring orientation of ANC molecules influence the ease by which the hydrogen atoms from –OH groups are donated to free radicals, as well as the capacity of ANCs to support unpaired electrons | Acylation promotes a stronger resistance to H2O2 oxidation |
| Color stability | Increases | Increases |
| Resistance to pH increase | Higher | Higher |
| Photo-stability | Increases | Increases |
| Thermostability | Increases | Increases |
| References | [7,17,49] | [36,37,50] |
| Major Acylated ANCs | Study Design | Main Findings | Reference |
|---|---|---|---|
| Cyanidin-3-caffeoylferuloylsophoroside-5-glucoside isolated from red cabbage | Streptozotocin-induced diabetic Wistar male rats, 130–150 g, n = 8. Three groups = Control, Diabetes, Diabetes + Red cabbage extract (RCE). Rats were given RCE daily (800 mg/kg) for 4 weeks | RCE lowered blood glucose and glycated hemoglobin concentrations, improved glucose tolerance, and raised serum insulin, proinsulin, and C-peptide levels. Increased the number of pancreatic β-cells in diabetic animals | [56] |
| Petunidin-3-O-rutinoside (p-coumaroyl)-5-O-glucoside isolated from black goji berry | SH-SY5Y cells. Cells were pre-protected with ANCs at concentrations of 50, 100, and 200 μg/mL for 12 h | Increased the autophagic flux, inhibited oxidative stress, and reduced inflammatory response and neuronal apoptosis with oxygen and glucose deprivation | [57] |
| Petunidin-coumaryl-rutinoside-glucoside isolated from purple potato | Zucker diabetic fatty rats (ZDF, fa/fa), 3 weeks old, n = 8. Rats were fed non-acylated ANC extract from bilberries (NAAB) or acylated ANC extract from purple potatoes (AAPP). Daily doses of 25 mg/kg (low dose) and 50 mg/kg (high dose) for 8 weeks | NAAB and AAPP improved lipid profiles. AAPP increased the glutamine/glutamate ratio and decreased levels of glycerol and improved insulin sensitivity, gluconeogenesis, and glycolysis. AAPP decreased the hepatic TBC1D1 and G6PC messenger RNA level, suggesting the regulation of gluconeogenesis and lipogenesis | [58] |
| Petunidin-coumaroyl-rutinoside-glucoside and peonidin-coumaroyl-rutinoside-glucoside isolated from purple potato | 17 healthy subjects, 30 mL purple potato extract containing 152 mg of ANCs and 140 mg of other phenolics. Blood samples were taken in a range of 20–240 min | Suppressed postprandial plasma glucose and insulin peaks. Decreased plasma glucose and insulin at 20–60 min. Upregulation of postprandial level of insulin-like hormone FGF-19 after a high-carbohydrate meal | [59] |
| Diacylated ANCs cyanidin 3-dicaffeoyl sophoroside-5-glc and peonidin 3-dicaffeoyl sophoroside-5-glc isolated from purple sweet potato | Male Sprague–Dawley rats, 140–160 g, 6-weeks-old, n = 8. Three groups: normal control group (water), low-dose diacylated AF-PSP group (80 mg/kg), high-dose diacylated AF-PSP group (160 mg/kg). Blood samples collected from tail vein at 0, 15, 30, 60, 90, and 120 min | Low dose diacylated AF-PSP and high dose diacylated AF-PSP significantly decreased (p < 0.05 and p < 0.01, respectively) postprandial blood glucose levels after 30 min | [60] |
| Cya3SXylGlcGal, Cya3FXylGlcGal, and Cya3pCXylGlcGal isolated from black carrot | Colorectal adenocarcinoma (HT-29) and promyelocytic leukemia (HL-60) cells. BC-ARE concentrations: 0.0–2.0 mg/mL for 24 h | BC-ARE at 2.0 mg/mL suppressed about 80% of the growth of HT-29 and HL-60 cells | [61] |
| C3G-Mal, Pr3G-Mal, and P3G-Mal isolated from purple maize | Inhibitory effect on α-amylase and dipeptidyl peptidase-4 (DPP-IV). PMW concentrations: 0.05–1.0 mg/mL | PMW inhibited α-amylase with an IC50 from 109.5 to 172.7 μg/mL. PMW repressed DPP-IV activity with an IC50 from 65.5 to 702.7 μg/mL | [55] |
| Cyanidin succinyl glucoside and cyanidin malonyl glucoside isolated from purple highland barley | PC12 cells were exposed to CoCl2 for 12 h to mimic hypoxic conditions and treated with various concentrations of PAE (25–400 μg/mL) | PAE at 400 μg/mL showed the highest protective effect on PC12 cells against the hypoxic treatment, retaining 76.1% of the cell viability | [62] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yañez-Apam, J.; Domínguez-Uscanga, A.; Herrera-González, A.; Contreras, J.; Mojica, L.; Mahady, G.; Luna-Vital, D.A. Pharmacological Activities and Chemical Stability of Natural and Enzymatically Acylated Anthocyanins: A Comparative Review. Pharmaceuticals 2023, 16, 638. https://doi.org/10.3390/ph16050638
Yañez-Apam J, Domínguez-Uscanga A, Herrera-González A, Contreras J, Mojica L, Mahady G, Luna-Vital DA. Pharmacological Activities and Chemical Stability of Natural and Enzymatically Acylated Anthocyanins: A Comparative Review. Pharmaceuticals. 2023; 16(5):638. https://doi.org/10.3390/ph16050638
Chicago/Turabian StyleYañez-Apam, Jimena, Astrid Domínguez-Uscanga, Azucena Herrera-González, Jonhatan Contreras, Luis Mojica, Gail Mahady, and Diego A. Luna-Vital. 2023. "Pharmacological Activities and Chemical Stability of Natural and Enzymatically Acylated Anthocyanins: A Comparative Review" Pharmaceuticals 16, no. 5: 638. https://doi.org/10.3390/ph16050638






