Kynurenine Pathway Metabolites as Potential Biomarkers in Chronic Pain
Abstract
:1. Introduction
2. Therapeutic Potential of Chronic Pain Biomarkers
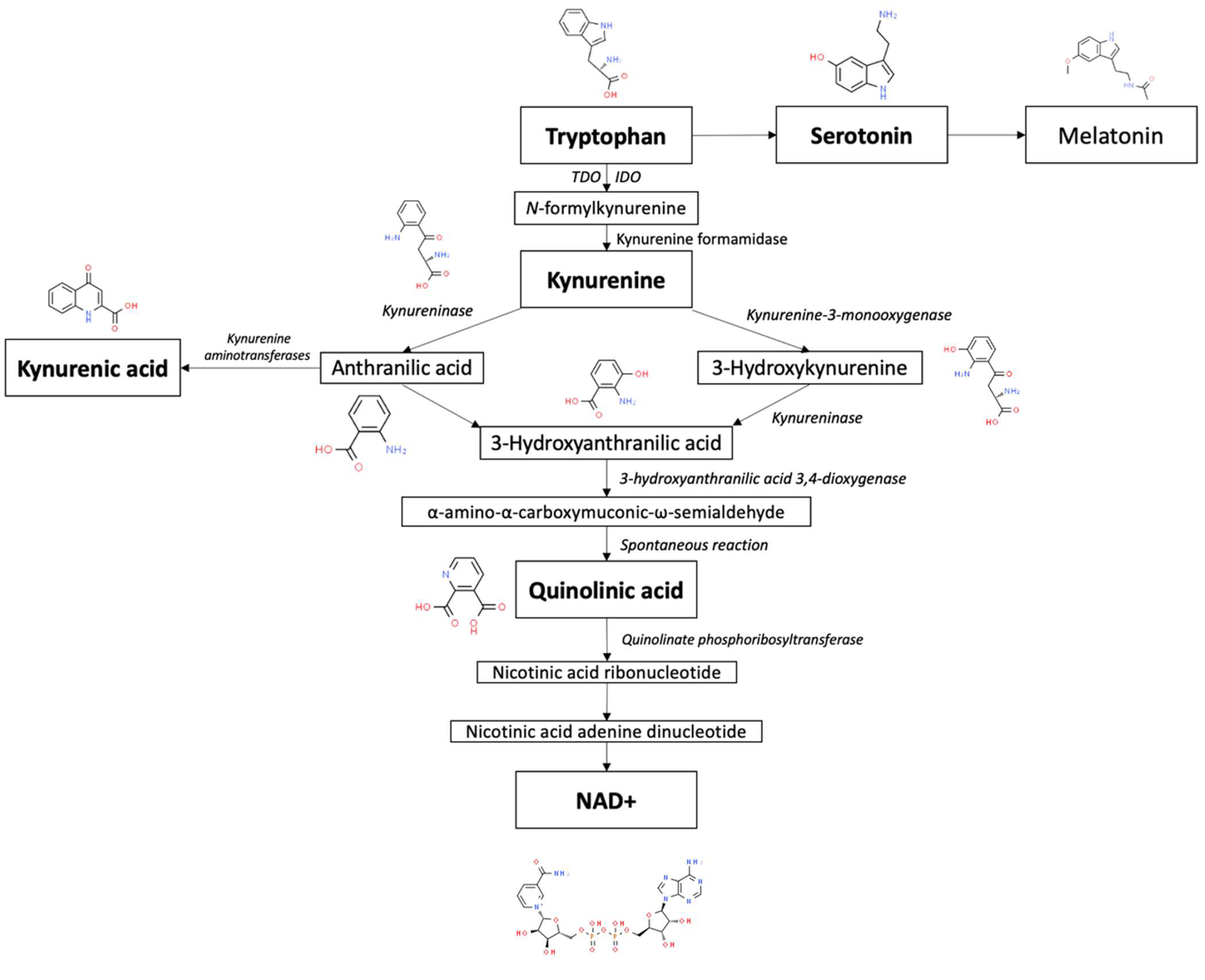
3. The Kynurenine Pathway: An Overview
4. Kynurenine Pathway Metabolite Biomarkers in Chronic Pain
4.1. Quinolinic Acid
4.2. Kynurenic Acid
4.3. Kynurenic Acid/Quinolinic Acid Ratio
4.4. Kynurenine/Tryptophan Ratio
| Metabolite | Clinical Significance | Chronic Pain Implications | Mechanism |
|---|---|---|---|
| Quinolinic acid (QA) | Hyperalgesia development Comorbid chronic pain with neurodegenerative and psychiatric disorders | Excitotoxin via NMDA receptor agonism [39] | |
| Cytokine-mediated chronic inflammation | Self-potentiation of neurotoxicity via interference of glutamate–glutamine cycle [46] | ||
| Elevated levels involved in neuronal cytoskeleton destabilization and apoptosis [40] | |||
| Kynurenic acid (KA) | Cytokine-mediated chronic inflammation | Neuroprotective properties Diminished levels possibly indicate poor inflammatory regulation with subsequent pain exacerbation Elevated levels possibly indicate upregulation for initial response to pain | Anti-excitotoxin via noncompetitive NMDA receptor antagonism [51] |
| Anti-inflammatory via GPR35-mediated agonism [58] Downregulation of PI3K/Akt and MAPK pathways (inflammatory) [59] Upregulation of β-catenin accumulation (anti-inflammatory) [66] | |||
| Kynurenic acid/Quinolinic acid ratio (KA/QA) | Cytokine-mediated chronic inflammation Neurotoxicity | Lower KA/QA ratios indicate a lack of neuroprotection with subsequent pain exacerbation | Inadequate neuroprotective response via overactivity of QA production relative to KA production [68] |
| Kynurenine/Tryptophan ratio (KYN/Trp) | Cytokine-mediated chronic inflammation | Higher KYN/Trp ratios indicate elevated pain intensity | Upregulated IDO levels shunt available Trp away from serotonin production and towards the KP [70] |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yong, R.J.; Mullins, P.M.; Bhattacharyya, N. Prevalence of chronic pain among adults in the United States. Pain 2022, 163, e328–e332. [Google Scholar] [CrossRef] [PubMed]
- Zelaya, C.E.; Dahlhamer, J.M.; Lucas, J.W.; Connor, E.M. Chronic Pain and High-Impact Chronic Pain among U.S. Adults, 2019; NCHS Data Brief; Centers for Disease Control and Prevention: Hyattsville, MD, USA, 2020; pp. 1–8.
- Smith, T.J.; Hillner, B.E. The Cost of Pain. JAMA Netw. Open 2019, 2, e191532. [Google Scholar] [CrossRef] [PubMed]
- Roughan, W.H.; Campos, A.I.; Garcia-Marin, L.M.; Cuellar-Partida, G.; Lupton, M.K.; Hickie, I.B.; Medland, S.E.; Wray, N.R.; Byrne, E.M.; Ngo, T.T.; et al. Comorbid Chronic Pain and Depression: Shared Risk Factors and Differential Antidepressant Effectiveness. Front. Psychiatry 2021, 12, 643609. [Google Scholar] [CrossRef] [PubMed]
- Pizzo, P.A.; Clark, N.M. Alleviating suffering 101--pain relief in the United States. N. Engl. J. Med. 2012, 366, 197–199. [Google Scholar] [CrossRef]
- Nadeau, S.E.; Wu, J.K.; Lawhern, R.A. Opioids and Chronic Pain: An Analytic Review of the Clinical Evidence. Front. Pain Res. 2021, 2, 721357. [Google Scholar] [CrossRef]
- Amirdelfan, K.; Pope, J.E.; Gunn, J.; Hill, M.M.; Cotten, B.M.; Beresh, J.E.; Dobecki, D.; Miller, N.; Mehta, P.; Girardi, G.; et al. Clinical Validation of a Multi-Biomarker Assay for the Evaluation of Chronic Pain Patients in a Cross-Sectional, Observational Study. Pain Ther. 2020, 9, 511–529. [Google Scholar] [CrossRef]
- Dahlhamer, J.; Lucas, J.; Zelaya, C.; Nahin, R.; Mackey, S.; DeBar, L.; Kerns, R.; Von Korff, M.; Porter, L.; Helmick, C. Prevalence of Chronic Pain and High-Impact Chronic Pain Among Adults—United States, 2016. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 1001–1006. [Google Scholar] [CrossRef]
- Mills, S.E.E.; Nicolson, K.P.; Smith, B.H. Chronic pain: A review of its epidemiology and associated factors in population-based studies. Br. J. Anaesth. 2019, 123, e273–e283. [Google Scholar] [CrossRef]
- Fillingim, R.B. Individual differences in pain: Understanding the mosaic that makes pain personal. Pain 2017, 158 (Suppl. S1), S11–S18. [Google Scholar] [CrossRef]
- Woolf, C.J. Capturing Novel Non-opioid Pain Targets. Biol. Psychiatry 2020, 87, 74–81. [Google Scholar] [CrossRef]
- Carpenedo Mun, C.; Schuler, H.; Baker, R.; Byrne, F.; Bresani, E.; Meyers, K. Rural communities face more than an opioid crisis: Reimagining funding assistance to address polysubstance use, associated health problems, and limited rural service capacity. J. Rural Health 2023. [Google Scholar] [CrossRef]
- Gunn, J.; Hill, M.M.; Cotten, B.M.; Deer, T.R. An Analysis of Biomarkers in Patients with Chronic Pain. Pain Physician 2020, 23, E41–E49. [Google Scholar] [CrossRef]
- Califf, R.M. Biomarker definitions and their applications. Exp. Biol. Med. 2018, 243, 213–221. [Google Scholar] [CrossRef]
- Strimbu, K.; Tavel, J.A. What are biomarkers? Curr. Opin. HIV AIDS 2010, 5, 463–466. [Google Scholar] [CrossRef]
- Hagedorn, J.M.; Gunn, J.; Budwany, R.; D’Souza, R.S.; Chakravarthy, K.; Deer, T.R. How Well Do Current Laboratory Biomarkers Inform Clinical Decision-Making in Chronic Pain Management? J. Pain Res. 2021, 14, 3695–3710. [Google Scholar] [CrossRef]
- Kalso, E. Biomarkers for pain. Pain 2004, 107, 199–201. [Google Scholar] [CrossRef]
- Bäckryd, E. Pain in the Blood? Envisioning Mechanism-Based Diagnoses and Biomarkers in Clinical Pain Medicine. Diagnostics 2015, 5, 84–95. [Google Scholar] [CrossRef]
- Bäckryd, E.; Ghafouri, B.; Larsson, B.; Gerdle, B. Do low levels of beta-endorphin in the cerebrospinal fluid indicate defective top-down inhibition in patients with chronic neuropathic pain? A cross-sectional, comparative study. Pain Med. 2014, 15, 111–119. [Google Scholar] [CrossRef]
- Tanaka, M.; Torok, N.; Toth, F.; Szabo, A.; Vecsei, L. Co-Players in Chronic Pain: Neuroinflammation and the Tryptophan-Kynurenine Metabolic Pathway. Biomedicines 2021, 9, 897. [Google Scholar] [CrossRef]
- Jovanovic, F.; Jovanovic, V.; Knezevic, N.N. Glucocorticoid Hormones as Modulators of the Kynurenine Pathway in Chronic Pain Conditions. Cells 2023, 12, 1178. [Google Scholar] [CrossRef]
- Gostner, J.M.; Geisler, S.; Stonig, M.; Mair, L.; Sperner-Unterweger, B.; Fuchs, D. Tryptophan Metabolism and Related Pathways in Psychoneuroimmunology: The Impact of Nutrition and Lifestyle. Neuropsychobiology 2020, 79, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Mu, C.L.; Farzi, A.; Zhu, W.Y. Tryptophan Metabolism: A Link between the Gut Microbiota and Brain. Adv. Nutr. 2020, 11, 709–723. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, N.; Lee, H.M.; Kim, J. Oncology Therapeutics Targeting the Metabolism of Amino Acids. Cells 2020, 9, 1904. [Google Scholar] [CrossRef] [PubMed]
- Savitz, J. The kynurenine pathway: A finger in every pie. Mol. Psychiatry 2020, 25, 131–147. [Google Scholar] [CrossRef]
- Schwarcz, R.; Bruno, J.P.; Muchowski, P.J.; Wu, H.Q. Kynurenines in the mammalian brain: When physiology meets pathology. Nat. Rev. Neurosci. 2012, 13, 465–477. [Google Scholar] [CrossRef]
- Liang, Y.; Xie, S.; He, Y.; Xu, M.; Qiao, X.; Zhu, Y.; Wu, W. Kynurenine Pathway Metabolites as Biomarkers in Alzheimer’s Disease. Dis. Markers 2022, 2022, 9484217. [Google Scholar] [CrossRef]
- Modoux, M.; Rolhion, N.; Mani, S.; Sokol, H. Tryptophan Metabolism as a Pharmacological Target. Trends Pharmacol. Sci. 2021, 42, 60–73. [Google Scholar] [CrossRef]
- Palzer, L.; Bader, J.J.; Angel, F.; Witzel, M.; Blaser, S.; McNeil, A.; Wandersee, M.K.; Leu, N.A.; Lengner, C.J.; Cho, C.E.; et al. Alpha-Amino-Beta-Carboxy-Muconate-Semialdehyde Decarboxylase Controls Dietary Niacin Requirements for NAD(+) Synthesis. Cell Rep. 2018, 25, 1359–1370.e1354. [Google Scholar] [CrossRef]
- Liu, C.L.; Cheng, S.P.; Chen, M.J.; Lin, C.H.; Chen, S.N.; Kuo, Y.H.; Chang, Y.C. Quinolinate Phosphoribosyltransferase Promotes Invasiveness of Breast Cancer Through Myosin Light Chain Phosphorylation. Front. Endocrinol. 2020, 11, 621944. [Google Scholar] [CrossRef]
- Ogyu, K.; Kubo, K.; Noda, Y.; Iwata, Y.; Tsugawa, S.; Omura, Y.; Wada, M.; Tarumi, R.; Plitman, E.; Moriguchi, S.; et al. Kynurenine pathway in depression: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2018, 90, 16–25. [Google Scholar] [CrossRef]
- Kawai, J.; Okuno, E.; Kido, R. Organ distribution of rat kynureninase and changes of its activity during development. Enzyme 1988, 39, 181–189. [Google Scholar] [CrossRef]
- Baran, H.; Schwarcz, R. Presence of 3-hydroxyanthranilic acid in rat tissues and evidence for its production from anthranilic acid in the brain. J. Neurochem. 1990, 55, 738–744. [Google Scholar] [CrossRef]
- Athnaiel, O.; Ong, C.; Knezevic, N.N. The Role of Kynurenine and Its Metabolites in Comorbid Chronic Pain and Depression. Metabolites 2022, 12, 950. [Google Scholar] [CrossRef]
- Han, Q.; Cai, T.; Tagle, D.A.; Li, J. Structure, expression, and function of kynurenine aminotransferases in human and rodent brains. Cell. Mol. Life Sci. 2010, 67, 353–368. [Google Scholar] [CrossRef]
- Guidetti, P.; Amori, L.; Sapko, M.T.; Okuno, E.; Schwarcz, R. Mitochondrial aspartate aminotransferase: A third kynurenate-producing enzyme in the mammalian brain. J. Neurochem. 2007, 102, 103–111. [Google Scholar] [CrossRef]
- Marx, W.; McGuinness, A.J.; Rocks, T.; Ruusunen, A.; Cleminson, J.; Walker, A.J.; Gomes-da-Costa, S.; Lane, M.; Sanches, M.; Diaz, A.P.; et al. The kynurenine pathway in major depressive disorder, bipolar disorder, and schizophrenia: A meta-analysis of 101 studies. Mol. Psychiatry 2021, 26, 4158–4178. [Google Scholar] [CrossRef]
- Castro-Portuguez, R.; Sutphin, G.L. Kynurenine pathway, NAD(+) synthesis, and mitochondrial function: Targeting tryptophan metabolism to promote longevity and healthspan. Exp. Gerontol. 2020, 132, 110841. [Google Scholar] [CrossRef]
- Hestad, K.; Alexander, J.; Rootwelt, H.; Aaseth, J.O. The Role of Tryptophan Dysmetabolism and Quinolinic Acid in Depressive and Neurodegenerative Diseases. Biomolecules 2022, 12, 998. [Google Scholar] [CrossRef]
- Behl, T.; Kaur, I.; Sehgal, A.; Singh, S.; Bhatia, S.; Al-Harrasi, A.; Zengin, G.; Bumbu, A.G.; Andronie-Cioara, F.L.; Nechifor, A.C.; et al. The Footprint of Kynurenine Pathway in Neurodegeneration: Janus-Faced Role in Parkinson’s Disorder and Therapeutic Implications. Int. J. Mol. Sci. 2021, 22, 6737. [Google Scholar] [CrossRef]
- Moffett, J.R.; Arun, P.; Puthillathu, N.; Vengilote, R.; Ives, J.A.; Badawy, A.A.; Namboodiri, A.M. Quinolinate as a Marker for Kynurenine Metabolite Formation and the Unresolved Question of NAD(+) Synthesis During Inflammation and Infection. Front. Immunol. 2020, 11, 31. [Google Scholar] [CrossRef]
- Mithaiwala, M.N.; Santana-Coelho, D.; Porter, G.A.; O’Connor, J.C. Neuroinflammation and the Kynurenine Pathway in CNS Disease: Molecular Mechanisms and Therapeutic Implications. Cells 2021, 10, 1548. [Google Scholar] [CrossRef] [PubMed]
- Combes, V.; Guillemin, G.J.; Chan-Ling, T.; Hunt, N.H.; Grau, G.E. The crossroads of neuroinflammation in infectious diseases: Endothelial cells and astrocytes. Trends Parasitol. 2012, 28, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Bansal, Y.; Singh, R.; Parhar, I.; Kuhad, A.; Soga, T. Quinolinic Acid and Nuclear Factor Erythroid 2-Related Factor 2 in Depression: Role in Neuroprogression. Front. Pharmacol. 2019, 10, 452. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chang, L.; Song, Y.; Li, H.; Wu, Y. The Role of NMDA Receptors in Alzheimer’s Disease. Front. Neurosci. 2019, 13, 43. [Google Scholar] [CrossRef]
- Ting, K.K.; Brew, B.J.; Guillemin, G.J. Effect of quinolinic acid on human astrocytes morphology and functions: Implications in Alzheimer’s disease. J. Neuroinflamm. 2009, 6, 36. [Google Scholar] [CrossRef]
- Guillemin, G.J.; Wang, L.; Brew, B.J. Quinolinic acid selectively induces apoptosis of human astrocytes: Potential role in AIDS dementia complex. J. Neuroinflamm. 2005, 2, 16. [Google Scholar] [CrossRef]
- Pope, J.E.; Fishman, M.A.; Gunn, J.A.; Cotten, B.M.; Hill, M.M.; Deer, T.R. Cross-Validation of the Foundation Pain Index with PROMIS-29 in Chronic Pain Patients. J. Pain Res. 2021, 14, 2677–2685. [Google Scholar] [CrossRef]
- Muneer, A. Kynurenine Pathway of Tryptophan Metabolism in Neuropsychiatric Disorders: Pathophysiologic and Therapeutic Considerations. Clin. Psychopharmacol. Neurosci. 2020, 18, 507–526. [Google Scholar] [CrossRef]
- Feher, E.; Szatmari, I.; Dudas, T.; Zalatnai, A.; Farkas, T.; Lorinczi, B.; Fulop, F.; Vecsei, L.; Toldi, J. Structural Evaluation and Electrophysiological Effects of Some Kynurenic Acid Analogs. Molecules 2019, 24, 3502. [Google Scholar] [CrossRef]
- Stone, T.W. Does kynurenic acid act on nicotinic receptors? An assessment of the evidence. J. Neurochem. 2020, 152, 627–649. [Google Scholar] [CrossRef]
- Ganong, A.H.; Lanthorn, T.H.; Cotman, C.W. Kynurenic acid inhibits synaptic and acidic amino acid-induced responses in the rat hippocampus and spinal cord. Brain Res. 1983, 273, 170–174. [Google Scholar] [CrossRef]
- Rajani, V.; Sengar, A.S.; Salter, M.W. Tripartite signalling by NMDA receptors. Mol. Brain 2020, 13, 23. [Google Scholar] [CrossRef]
- Majlath, Z.; Torok, N.; Toldi, J.; Vecsei, L. Memantine and Kynurenic Acid: Current Neuropharmacological Aspects. Curr. Neuropharmacol. 2016, 14, 200–209. [Google Scholar] [CrossRef]
- Birch, P.J.; Grossman, C.J.; Hayes, A.G. Kynurenic acid antagonises responses to NMDA via an action at the strychnine-insensitive glycine receptor. Eur. J. Pharmacol. 1988, 154, 85–87. [Google Scholar] [CrossRef]
- Lipton, S.A. Failures and successes of NMDA receptor antagonists: Molecular basis for the use of open-channel blockers like memantine in the treatment of acute and chronic neurologic insults. NeuroRx 2004, 1, 101–110. [Google Scholar] [CrossRef]
- Milligan, G. G protein-coupled receptors not currently in the spotlight: Free fatty acid receptor 2 and GPR35. Br. J. Pharmacol. 2018, 175, 2543–2553. [Google Scholar] [CrossRef]
- Wirthgen, E.; Hoeflich, A.; Rebl, A.; Gunther, J. Kynurenic Acid: The Janus-Faced Role of an Immunomodulatory Tryptophan Metabolite and Its Link to Pathological Conditions. Front. Immunol. 2017, 8, 1957. [Google Scholar] [CrossRef]
- Walczak, K.; Wnorowski, A.; Turski, W.A.; Plech, T. Kynurenic acid and cancer: Facts and controversies. Cell. Mol. Life Sci. 2020, 77, 1531–1550. [Google Scholar] [CrossRef]
- Ramprasath, T.; Han, Y.M.; Zhang, D.; Yu, C.J.; Zou, M.H. Tryptophan Catabolism and Inflammation: A Novel Therapeutic Target for Aortic Diseases. Front. Immunol. 2021, 12, 731701. [Google Scholar] [CrossRef]
- Salimi Elizei, S.; Poormasjedi-Meibod, M.S.; Wang, X.; Kheirandish, M.; Ghahary, A. Kynurenic acid downregulates IL-17/1L-23 axis in vitro. Mol. Cell. Biochem. 2017, 431, 55–65. [Google Scholar] [CrossRef]
- Yang, I.H.; Wong, J.H.; Chang, C.M.; Chen, B.K.; Tsai, Y.T.; Chen, W.C.; Wang, E.T.; Hsu, W.L.; Chang, W.C. Involvement of intracellular calcium mobilization in IL-8 activation in human retinal pigment epithelial cells. Investig. Ophthalmol. Vis. Sci. 2015, 56, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Arthur, J.S.; Ley, S.C. Mitogen-activated protein kinases in innate immunity. Nat. Rev. Immunol. 2013, 13, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.; Yang, H.; Liu, H.; Lu, Y.; Han, L.; Liu, G. Kinase AKT controls innate immune cell development and function. Immunology 2013, 140, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Silva-Garcia, O.; Valdez-Alarcon, J.J.; Baizabal-Aguirre, V.M. The Wnt/beta-catenin signaling pathway controls the inflammatory response in infections caused by pathogenic bacteria. Mediat. Inflamm. 2014, 2014, 310183. [Google Scholar] [CrossRef]
- Walczak, K.; Turski, W.A.; Rajtar, G. Kynurenic acid inhibits colon cancer proliferation in vitro: Effects on signaling pathways. Amino Acids 2014, 46, 2393–2401. [Google Scholar] [CrossRef]
- Zadori, D.; Klivenyi, P.; Vamos, E.; Fulop, F.; Toldi, J.; Vecsei, L. Kynurenines in chronic neurodegenerative disorders: Future therapeutic strategies. J. Neural Transm. 2009, 116, 1403–1409. [Google Scholar] [CrossRef]
- Groven, N.; Reitan, S.K.; Fors, E.A.; Guzey, I.C. Kynurenine metabolites and ratios differ between Chronic Fatigue Syndrome, Fibromyalgia, and healthy controls. Psychoneuroendocrinology 2021, 131, 105287. [Google Scholar] [CrossRef]
- Barjandi, G.; Louca Jounger, S.; Lofgren, M.; Bileviciute-Ljungar, I.; Kosek, E.; Ernberg, M. Plasma tryptophan and kynurenine in females with temporomandibular disorders and fibromyalgia-An exploratory pilot study. J. Oral Rehabil. 2020, 47, 150–157. [Google Scholar] [CrossRef]
- Staats Pires, A.; Tan, V.X.; Heng, B.; Guillemin, G.J.; Latini, A. Kynurenine and Tetrahydrobiopterin Pathways Crosstalk in Pain Hypersensitivity. Front. Neurosci. 2020, 14, 620. [Google Scholar] [CrossRef]
- Ciapala, K.; Mika, J.; Rojewska, E. The Kynurenine Pathway as a Potential Target for Neuropathic Pain Therapy Design: From Basic Research to Clinical Perspectives. Int. J. Mol. Sci. 2021, 22, 1055. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Auyeung, A.; Wang, H.C.; Aravagiri, K.; Knezevic, N.N. Kynurenine Pathway Metabolites as Potential Biomarkers in Chronic Pain. Pharmaceuticals 2023, 16, 681. https://doi.org/10.3390/ph16050681
Auyeung A, Wang HC, Aravagiri K, Knezevic NN. Kynurenine Pathway Metabolites as Potential Biomarkers in Chronic Pain. Pharmaceuticals. 2023; 16(5):681. https://doi.org/10.3390/ph16050681
Chicago/Turabian StyleAuyeung, Andrew, Hank C. Wang, Kannan Aravagiri, and Nebojsa Nick Knezevic. 2023. "Kynurenine Pathway Metabolites as Potential Biomarkers in Chronic Pain" Pharmaceuticals 16, no. 5: 681. https://doi.org/10.3390/ph16050681





